Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Evaluation of Arteriosclerotic Plaque
2.3. Anthropometry and Serum Arteriosclerotic Markers Analysis
2.4. Statistical Analysis
3. Results
3.1. Study and Control Group Characteristics
3.2. Biochemical Effect after GLP1-RA Treatment
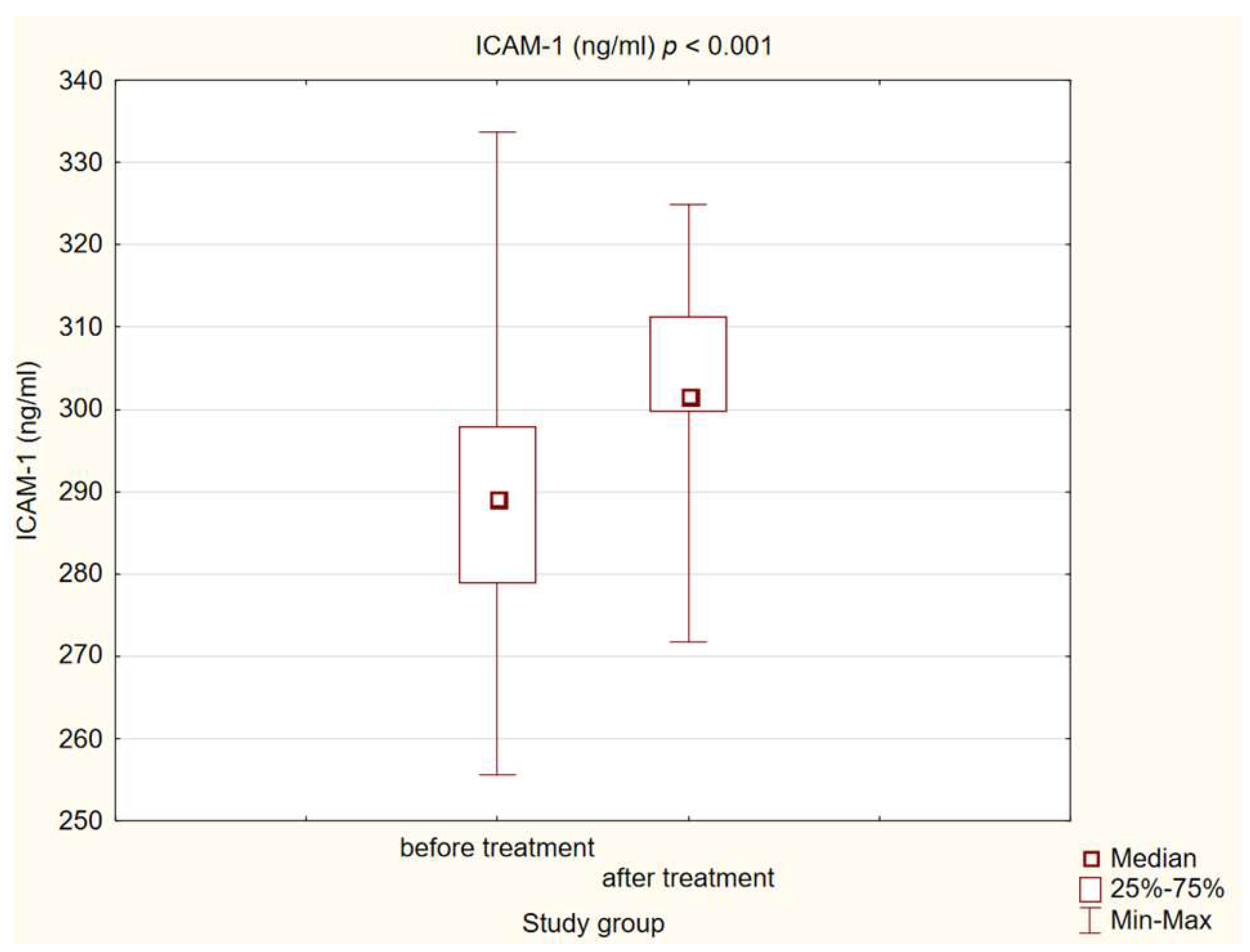
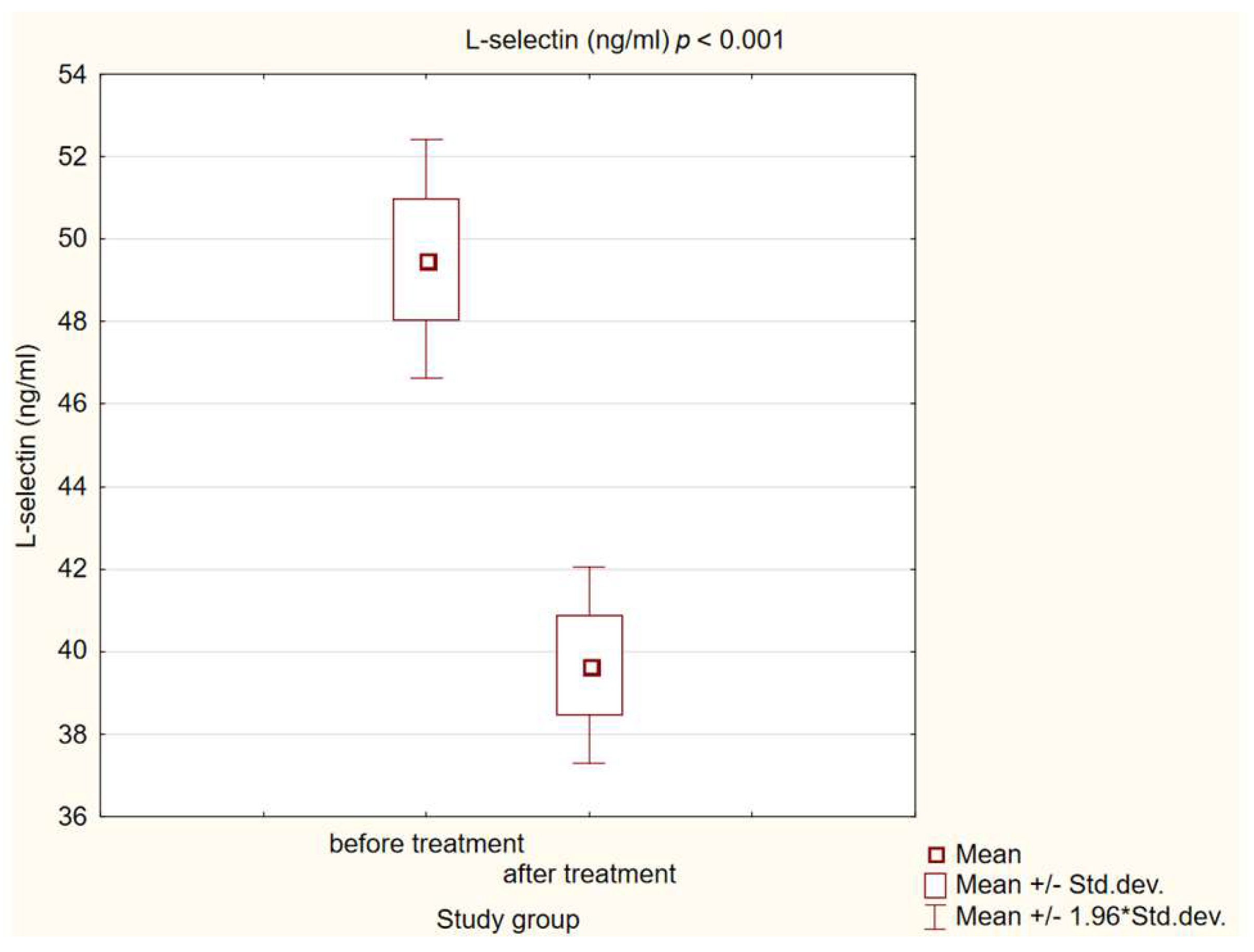
3.3. Chemokine and Adhesion Molecules Level Analysis
3.4. Correlation between Changes in Biochemical Parameters and Chemokine and Adhesion Molecules Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018, 9, 334630. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Mauersberger, C.; Hinterdobler, J.; Schunkert, H.; Kessler, T.; Sager, H.B. Where the Action Is-Leukocyte Recruitment in Atherosclerosis. Front. Cardiovasc. Med. 2022, 11, 813984. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Tscharre, M.; Vogel, B.; Tentzeris, I.; Freynhofer, M.K.; Rohla, M.; Wojta, J.; Weiss, T.W.; Ay, C.; Huber, K.; Farhan, S. Prognostic Impact of Soluble P-Selectin on Long-Term Adverse Cardiovascular Outcomes in Patients Undergoing Percutaneous Coronary Intervention. Thromb. Haemost. 2019, 119, 340–347. [Google Scholar] [CrossRef]
- Tvaroška, I.; Selvaraj, C.; Koča, J. Selectins—The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules—A Review. Molecules 2020, 25, 2835. [Google Scholar] [CrossRef]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef] [PubMed]
- Hoke, M.; Winter, M.P.; Wagner, O.; Exner, M.; Schillinger, M.; Arnold, Z.; Mlekusch, W.; Maurer, G.; Koppensteiner, R.; Minar, E.; et al. The impact of selectins on mortality in stable carotid atherosclerosis. Thromb. Haemost. 2015, 114, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Bernhagen, J.; Heitman, L.H.; Weber, C.; Dichgans, M. Targeting the CCL2-CCR2 axis for atheroprotection. Eur. Heart J. 2022, 43, 1799–1808, Erratum in Eur. Heart J. 2022, 43, 2424. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.; Nusca, A.; Coletti, F.; La Porta, Y.; Piscione, M.; Vespasiano, F.; Mangiacapra, F.; Ricottini, E.; Melfi, R.; Cavallari, I.; et al. Incretins-Based Therapies and Their Cardiovascular Effects: New Game-Changers for the Management of Patients with Diabetes and Cardiovascular Disease. Pharmaceutics 2023, 15, 1858. [Google Scholar] [CrossRef]
- Hu, E.-H.; Tsai, M.-L.; Lin, Y.; Chou, T.-S.; Chen, T.-H. A Review and Meta-Analysis of the Safety and Efficacy of Using Glucagon-like Peptide-1 Receptor Agonists. Medicina 2024, 60, 357. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337, Erratum in Eur. Heart J. 2022, 44, 4468. [Google Scholar] [CrossRef]
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S124–S138. [Google Scholar] [CrossRef]
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Tummala, R.; Ghosh, R.K.; Blumenthal, C.; Philip, K.; Bandyopadhyay, D.; Ventura, H.; Deedwania, P. An update on pharmacotherapies in diabetic dyslipidemia. Prog. Cardiovasc. Dis. 2019, 62, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, F.; Mastroberardino, S.; Nusca, A.; Frau, L.; Guarino, L.; Napoli, N.; Ussia, G.P.; Grigioni, F. Novel Antidiabetic Agents and Their Effects on Lipid Profile: A Single Shot for Several Cardiovascular Targets. Int. J. Mol. Sci. 2023, 24, 10164. [Google Scholar] [CrossRef] [PubMed]
- Ripa, R.S.; Zobel, E.H.; von Scholten, B.J.; Jensen, J.K.; Binderup, T.; Diaz, L.J.; Curovic, V.R.; Hansen, T.W.; Rossing, P.; Kjaer, A. Effect of Liraglutide on Arterial Inflammation Assessed as [18F]FDG Uptake in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Circ. Cardiovasc. Imaging 2021, 14, e012174. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.; Wan, J.; Yuan, C.S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clin. Sci. 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Méndez-Barbero, N.; Pello Lázaro, A.M.; Aceña, Á.; Tarín, N.; Cristóbal, C.; Martínez-Milla, J.; González-Lorenzo, Ó.; Martín-Ventura, J.L.; Huelmos, A.; et al. MCP-1 Predicts Recurrent Cardiovascular Events in Patients with Persistent Inflammation. J. Clin. Med. 2021, 10, 1137. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Lipton, B.A.; Rosenfeld, M.E.; Särkioja, T.; Yoshimura, T.; Leonard, E.J.; Witztum, J.L.; Steinberg, D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1991, 88, 5252–5256. [Google Scholar] [CrossRef]
- Ikeda, U.; Matsui, K.; Murakami, Y.; Shimada, K. Monocyte chemoattractant protein-1 and coronary artery disease. Clin. Cardiol. 2002, 25, 143–147. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, W.Q.; Ji, X.P.; Zhang, M.; Zhao, Y.X.; Yao, G.H.; Zhang, P.F.; Zhang, C.; Zhang, Y. Dominant-negative mutation of monocyte chemoattractant protein-1 prevents vulnerable plaques from rupture in rabbits independent of serum lipid levels. J. Cell. Mol. Med. 2008, 12, 2362–2371. [Google Scholar] [CrossRef]
- Georgakis, M.K.; van der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; de Jager, S.C.A.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.G.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate With Plaque Vulnerability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef]
- Georgakis, M.K.; de Lemos, J.A.; Ayers, C.; Wang, B.; Björkbacka, H.; Pana, T.A.; Thorand, B.; Sun, C.; Fani, L.; Malik, R.; et al. Association of Circulating Monocyte Chemoattractant Protein–1 Levels with Cardiovascular Mortality: A Meta-analysis of Population-Based Studies. JAMA Cardiol. 2021, 6, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz-Wujec, M.; Henzel, J.; Kępka, C.; Kruk, M.; Wardziak, Ł.; Trochimiuk, P.; Parzonko, A.; Dzielińska, Z.; Demkow, M.; Kozłowska-Wojciechowska, M. Usefulness of MCP-1 Chemokine in the Monitoring of Patients with Coronary Artery Disease Subjected to Intensive Dietary Intervention: A Pilot Study. Nutrients 2021, 13, 3047. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Lappegård, K.T.; Claudi, T.; Damås, J.K.; Mørkrid, L.; Brendberg, R.; Mollnes, T.E. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur. Heart J. 2004, 25, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Diomede, L.; Sironi, M.; Massimiliano, L.; Sottocorno, M.; Polentarutti, N.; Guglielmotti, A.; Albani, D.; Bruno, A.; Fruscella, P. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Investig. 2000, 80, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A.; Winiarska, H.; Owoc, J.; Borowska, M.; Domagała, J.; Mikołajczak, P.Ł.; Iskakova, S.; Dworacki, G.; Dworacka, M. Effects of Low-Dose Atorvastatin on the Peripheral Blood Mononuclear Cell Secretion of Angiogenic Factors in Type 2 Diabetes. Biomolecules 2021, 11, 1885. [Google Scholar] [CrossRef]
- Wang, S.; Ran, Y.; Chen, X.; Li, C.; Cheng, S.; Liu, J. Pleiotropic Effects of Simvastatin on the Regulation of Potassium Channels in Monocytes. Front. Pharmacol. 2020, 21, 101. [Google Scholar] [CrossRef]
- Bułdak, Ł.; Machnik, G.; Bułdak, R.J.; Łabuzek, K.; Bołdys, A.; Okopień, B. Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFκB signaling. Naunyn Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1103–1115. [Google Scholar] [CrossRef]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.; Li, J.; Mao, Q.; He, J. Effects of Liraglutide Combined with Insulin on Oxidative Stress and Serum MCP-1 and NF-kB Levels in Type 2 Diabetes. J. Coll. Physicians Surg. Pak. 2019, 29, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Beckman, J.A.; Nian, H.; Garner, E.M.; Mayfield, D.; Devin, J.K.; Koethe, J.R.; Brown, J.D.; Cahill, K.N.; Yu, C.; et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: A randomized controlled trial. Diabetes Obes. Metab. 2023, 25, 570–580. [Google Scholar] [CrossRef]
- Santos, J.C.D.; Cruz, M.S.; Bortolin, R.H.; Oliveira, K.M.; Araújo, J.N.G.; Duarte, V.H.R.; Silva, A.M.G.D.; Santos, I.C.C.D.; Dantas, J.M.O.; Paiva, M.S.M.O. Relationship between circulating VCAM-1, ICAM-1, E-selectin and MMP9 and the extent of coronary lesions. Clinics 2018, 73, e203. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiao, Y.; Zhang, L.; Pan, Q. Protective Role of Glucagon-Like Peptide-1 against High-Glucose-Induced Endothelial Oxidative Damage. Medicine 2015, 94, e2055. [Google Scholar] [CrossRef]
- Dorecka, M.; Siemianowicz, K.; Francuz, T.; Garczorz, W.; Chyra, A.; Klych, A.; Romaniuk, W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol. Rep. 2013, 65, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dear, A.E.; Knudsen, L.B.; Simpson, R.W. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J. Endocrinol. 2009, 201, 59–66, Epub 2009 Jan 9. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Liu, H.; Welungoda, I.; Hu, Y.; Widdop, R.E.; Knudsen, L.B.; Simpson, R.W.; Dear, A.E. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diabetes Vasc. Dis. Res. 2011, 8, 117–124. [Google Scholar] [CrossRef]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef]
- Galkina, E.; Kadl, A.; Sanders, J.; Varughese, D.; Sarembock, I.J.; Ley, K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 2006, 203, 1273–1282. [Google Scholar] [CrossRef]
- Rozenberg, I.; Sluka, S.H.; Mocharla, P.; Hallenberg, A.; Rotzius, P.; Borén, J.; Kränkel, N.; Landmesser, U.; Borsig, L.; Lüscher, T.F.; et al. Deletion of L-selectin increases atherosclerosis development in ApoE-/- mice. PLoS ONE 2011, 6, e21675. [Google Scholar] [CrossRef]
- Berardi, C.; Decker, P.A.; Kirsch, P.S.; de Andrade, M.; Tsai, M.Y.; Pankow, J.S.; Sale, M.M.; Sicotte, H.; Tang, W.; Hanson, N.; et al. Plasma and serum L-selectin and clinical and subclinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Transl. Res. 2014, 163, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Shouzu, A.; Omoto, S.; Inami, N.; Tanaka, A.; Nanba, M.; Shouda, Y.; Takahashi, N.; Kimura, Y.; Iwasaka, T. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb. Res. 2008, 122, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; George, T.P.; Mujammami, M.; Isnani, A.; Alfadda, A.A. The association of cell adhesion molecules and selectins (VCAM-1, ICAM-1, E-selectin, L-selectin, and P-selectin) with microvascular complications in patients with type 2 diabetes: A follow-up study. Front. Endocrinol. 2023, 14, 1072288. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Systematic Review and Meta-Analysis of the Effect of Statins on Circulating E-Selectin, L-Selectin, and P-Selectin. Biomedicines 2021, 9, 1707. [Google Scholar] [CrossRef] [PubMed]

| Diabetes Treatment, n (%) | |
| Metformin | 49 (98%) |
| Sulfonylurea | 22 (44%) |
| Insulin | 14 (28%) |
| SGLT2 inhibitors | 10 (20%) |
| DPP-4 inhibitors | 4 (8%) |
| Other treatment, n (%) | |
| HMG-CoA reductase inhibitor | 42 (84%) |
| ACEI/ARB | 36 (72%) |
| Bblokers | 21 (42%) |
| Indapamide | 12 (24%) |
| Fibrates | 10 (20%) |
| Thiazide diuretics | 10 (20%) |
| Acetylsalicylic acid | 10 (20%) |
| Loop diuretics | 8 (16%) |
| Study Group | Control Group | |
|---|---|---|
| Number of patients, n | 50 | 26 |
| Age, years | 60.8 | 33.1 |
| Women, n (%) | 27 (54%) | 13 (50%) |
| Men, n (%) | 23 (46%) | 13 (50%) |
| Body mass, kg | 99.9 | 69.7 |
| Height, cm | 168.7 | 175.3 |
| BMI, kg/m2 | 35 | 22.5 |
| Overweight n(%) | 12 (24%) | 5 (19%) |
| Obese, n (%) | 35 (70%) | 0 |
| SBP, mmHg | 135 | 122 |
| WHO guidelines on physical activity, n (%) | 20 (40%) | 14 (54%) |
| Smokers, n (%) | ||
| Active | 9 (18%) | 1 (4%) |
| Past | 11 (22%) | 12 (46%) |
| Alcohol abuse, % | 0 | 0 |
| Co-morbidity, n (%) | ||
| Hypertension | 40 (80%) | 0 |
| Chronic kidney diseases | 9 (18%) | 0 |
| Thyroid diseases | 8 (16%) | 0 |
| Heart failure | 4 (8%) | 0 |
| Control Group | Study Group before Treatment | Study Group after Treatment | |
|---|---|---|---|
| Mean | Mean | Mean | |
| BMI kg/m2 | 22.46 # | 35.02 *,# | 33.21 * |
| HbA1C % | 5.5 # | 8.68 *,# | 7.56 * |
| GFR mL/min/1.73 m2 | 96.5 # | 69.76 # | 73.1 |
| Median | Median | Median | |
| Glucose mg/dL | 89 # | 160.5 *,# | 138.3 * |
| TC mg/dL | 173.8 | 166.55 | 169.1 |
| LDL mg/dL | 90.5 | 83 | 80.5 |
| HDL mg/dL | 64.3 # | 48.95 # | 50.65 |
| non-HDL mg/dL | 109 | 113.7 | 107.8 |
| TG mg/dL | 86.5 # | 167.95 # | 146.5 |
| Creatinine mg/dL | 0.93 # | 1.06 # | 1.04 |
| ALT U/I | 17 # | 25.5 # | 29 |
| AST U/I | 18.5 # | 28 # | 25 |
| De Ritis ratio (ALT/ASP) | 1.11 | 0.98 ** | 0.85 ** |
| GGTP U/I | 18 # | 37 # | 35 |
| FIB-4 | 0.66 # | 1.5 # | 1.3 * |
| ΔHbA1C | ΔWeight | ΔBMI | |
|---|---|---|---|
| ΔICAM-1 | ρ = −0.31, p < 0.05 | ρ = −0.24, p > 0.05 | ρ = 0.20, p > 0.05 |
| ΔL-selectine | ρ = 0.13, p > 0.05 | ρ = 0.13, p > 0.05 | ρ = 0.15, p > 0.05 |
| ΔMCP-1 | ρ = 0.15, p > 0.05 | ρ = −0.014, p > 0.05 | ρ = 0.01, p > 0.05 |
| ΔTCh | ΔLDL | Δnon-HDL | ΔHDL | ΔTG | |
|---|---|---|---|---|---|
| ΔICAM-1 | ρ = −0.11, p > 0.05 | ρ = −0.06, p > 0.05 | ρ = −0.06, p > 0.05 | ρ = −0.001, p > 0.05 | ρ = −0.16, p > 0.05 |
| ΔL-selectine | ρ = −0.004, p > 0.05 | ρ = −0.21, p > 0.05 | ρ = 0.004, p > 0.05 | ρ = −0.27, p > 0.05 | ρ = −0.002, p > 0.05 |
| ΔMCP-1 | ρ = 0.15, p > 0.05 | ρ = 0.11, p > 0.05 | ρ = 0.16, p > 0.05 | ρ = −0.034, p > 0.05 | ρ = 0.26, p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachuła, M.; Basiak, M.; Kosowski, M.; Okopień, B. Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis. Life 2024, 14, 690. https://doi.org/10.3390/life14060690
Hachuła M, Basiak M, Kosowski M, Okopień B. Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis. Life. 2024; 14(6):690. https://doi.org/10.3390/life14060690
Chicago/Turabian StyleHachuła, Marcin, Marcin Basiak, Michał Kosowski, and Bogusław Okopień. 2024. "Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis" Life 14, no. 6: 690. https://doi.org/10.3390/life14060690
APA StyleHachuła, M., Basiak, M., Kosowski, M., & Okopień, B. (2024). Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis. Life, 14(6), 690. https://doi.org/10.3390/life14060690







