The Complete Mitochondrial Genome Sequence of Eimeria kongi (Apicomplexa: Coccidia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites
2.2. Genomic DNA Extraction and PCR Amplification
2.2.1. Oocyst Purification
2.2.2. Genomic DNA Extraction
2.2.3. PCR Amplification
2.3. Genome Annotation and Sequence Analysis
2.4. Clustal Alignment and Phylogenetic Analyses
3. Results and Discussion
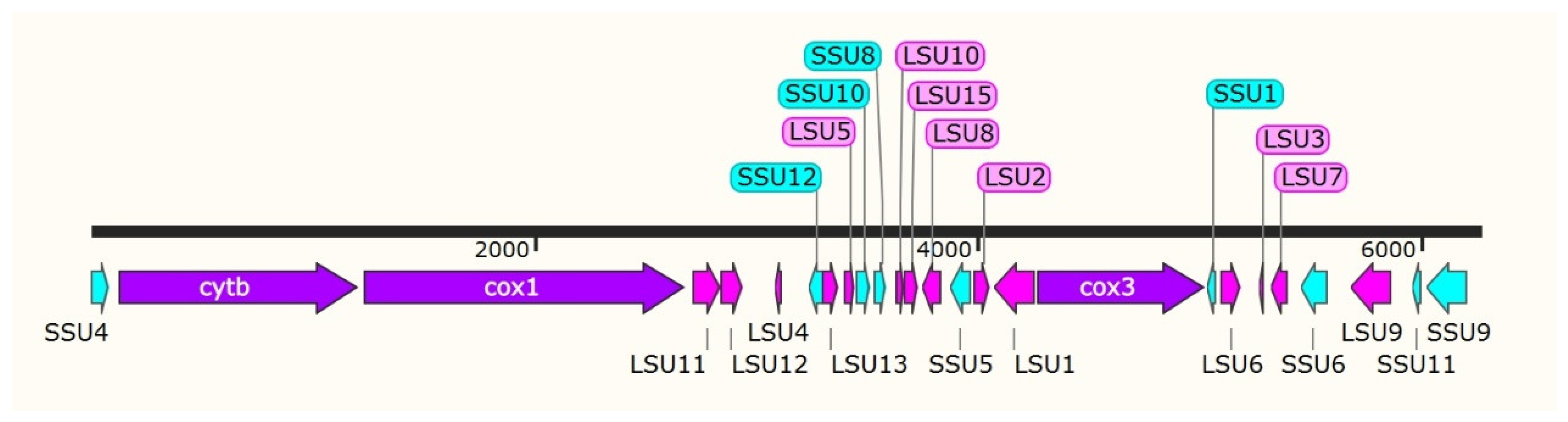
3.1. Analyzing the mtDNA Composition of E. kongi
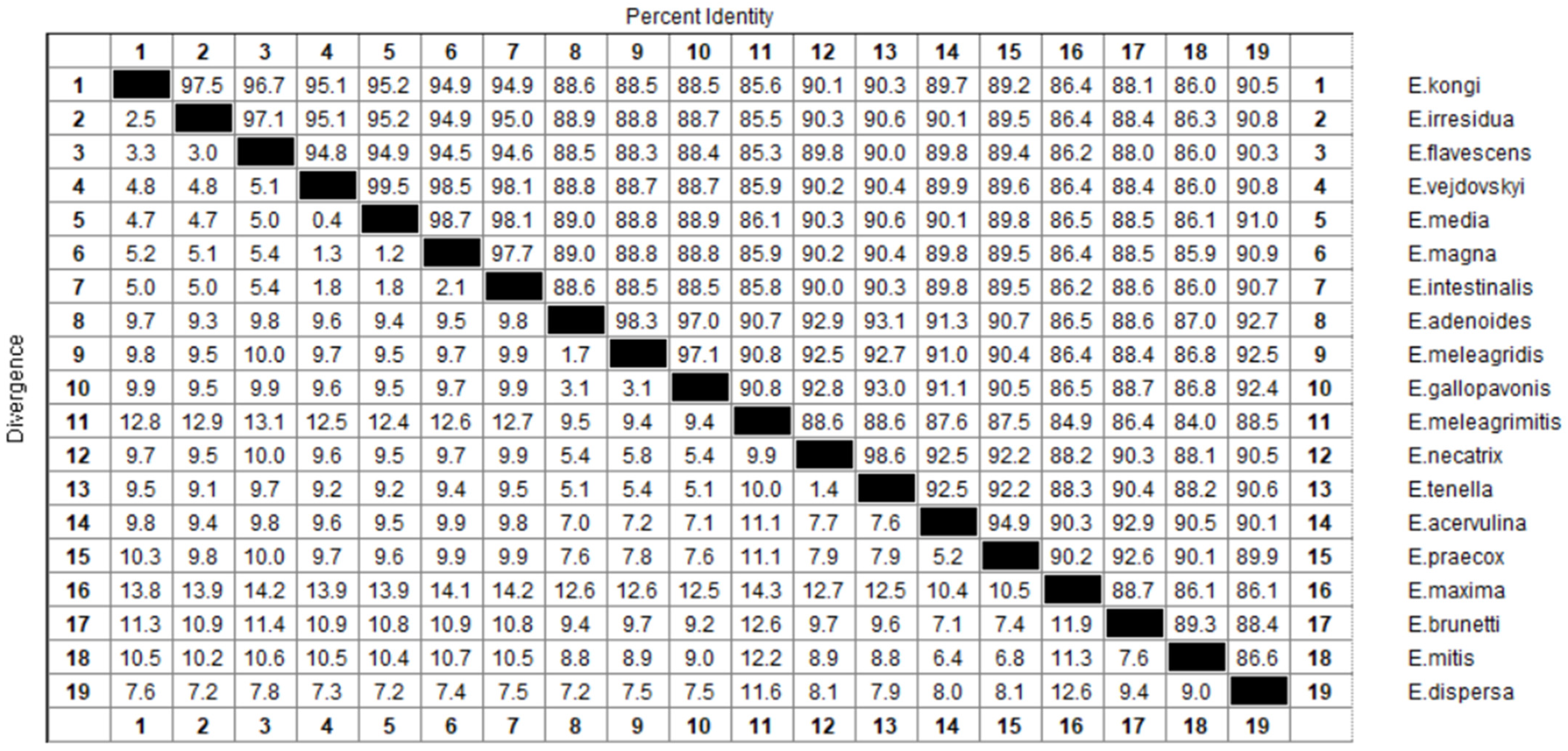
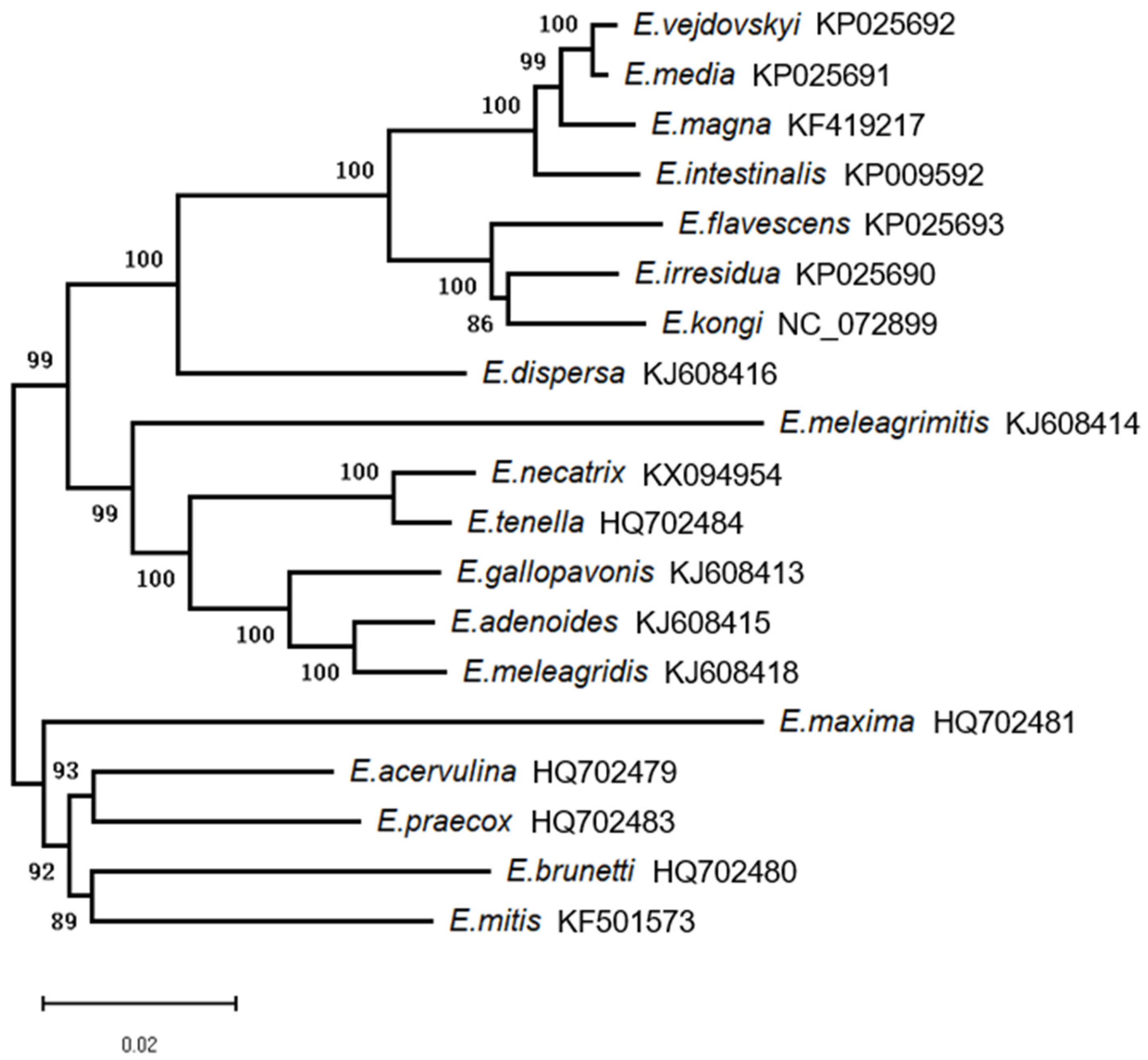
3.2. Clustal Alignment and Phylogenetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eckert, J.; Taylor, M.; Catchpole, J. Guidelines on Techniques in Coccidiosis Research; Directorate-General XII, Science, Research and Development Environment Research Programme; European Commission: Brussels, Belgium, 1995; pp. 103–119. [Google Scholar]
- Cui, P.; Liu, H.; Fang, S.; Gu, X.; Wang, P.; Liu, C.; Tao, G.; Liu, X.; Suo, X. A new species of Eimeria (Apicomplexa: Eimeriidae) from Californian rabbits in Hebei Province, China. Parasitol. Int. 2017, 66, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Lopez, D.L. Complete mitochondrial DNA sequence of the important honey bee pest, Varroa destructor (Acari: Varroidae). Exp. Appl. Acarol. 2002, 27, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Virji, A.Z.; Thekkiniath, J.; Ma, W.; Lawres, L.; Knight, J.; Swei, A.; Roch, K.L.; Mamoun, C. Insights into the evolution and drug susceptibility of Babesia duncani from the sequence of its mitochondrial and apicoplast genomes. Int. J. Parasitol. 2019, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.D.C.; Pereira, P.H.O.; Vilela, D.A.D.R.; Landau, I.; Andreína Pacheco, M.; Escalante, A.A.; Ferreira, F.C.; Braga, É.M. Leucocytozoon cariamae n. sp. and Haemoproteus pulcher coinfection in Cariama cristata (Aves: Cariamiformes): First mitochondrial genome analysis and morphological description of a leucocytozoid in Brazil. Parasitology 2023, 150, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Guo, X.; Guo, C.; Wang, X.; Wang, Y.; Yan, F. Complete mitochondrial genome of Malenka flexura (Plecoptera: Nemouridae) and phylogenetic analysis. Genes 2022, 13, 911. [Google Scholar] [CrossRef] [PubMed]
- Aurongzeb, M.; Rashid, Y.; Habib Ahmed Naqvi, S.; Muhammad Talha Malik, H.; Kamran Azim, M.; Hassan, S.S.; Yasir, M.; Karim, A. Insights into genome evolution, pan-genome, and phylogenetic implication through mitochondrial genome sequence of Naegleria fowleri species. Sci. Rep. 2022, 12, 13152. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, X.; Li, A.; Fan, A.; He, L.; Sun, Z.; Niu, Y.; Qiao, Y. Assembly and analysis of the complete mitochondrial genome of Forsythia suspensa (Thunb.) Vahl. BMC Genom. 2023, 24, 708. [Google Scholar] [CrossRef]
- Kim, N.Y.; Ahn, S.J.; Seo, J.S.; Jeon, E.J.; Cho, M.Y.; Choi, H.S. Characterization of the complete mitochondrial genome of Miamiensis avidus causing flatfish scuticociliatosis. Genetica 2022, 150, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Ogedengbe, M.E.; El-Sherry, S.; Whale, J.; Barta, J.R. Complete mitochondrial genome sequences from five Eimeria species (Apicomplexa; Coccidia; Eimeriidae) infecting domestic turkeys. Parasit. Vectors 2014, 7, 335. [Google Scholar] [CrossRef]
- Gray, M.W.; Lang, B.F.; Cedergren, R.; Golding, G.B.; Lemieux, C.; Sankoff, D.; Turmel, M.; Brossard, N.; Delage, E.; Littlejohn, T.G.; et al. Genome structure and gene content in protist mitochondrial DNAs. Nucleic. Acids. Res. 1998, 26, 865–878. [Google Scholar] [CrossRef]
- Osman, S.A.M.; Nishibori, M.; Yonezawa, T. Complete mitochondrial genome sequence of Tosa-Jidori sheds light on the origin and evolution of Japanese native chickens. Anim. Biosci. 2021, 34, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Nakai, Y.; Watanabe, Y.; Tachibana, S.I.; Arisue, N.; Palacpac, N.M.Q.; Toyama, T.; Honma, H.; Horii, T.; Kita, K.; et al. Concatenated mitochondrial DNA of the coccidian parasite Eimeria tenella. Mitochondrion 2011, 11, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.R.; Sigsgaard, E.E.; Liu, S.; Manica, A.; Bach, S.S.; Hansen, M.M.; Møller, P.R.; Thomsen, P.F. Genome-scale target capture of mitochondrial and nuclear environmental DNA from water samples. Mol. Ecol. Resour. 2021, 21, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Wakeman, K.C.; Keeling, P.J. Parallel functional reduction in the mitochondria of apicomplexan parasites. Curr. Biol. 2021, 31, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Conrad, I.; Craft, A.; Thurman, C.L.; Baeza, J.A. The complete mitochondrial genome of the red-jointed brackish-water fiddler crab Minuca minax (LeConte 1855) (Brachyura: Ocypodidae): New family gene order, and purifying selection and phylogenetic informativeness of protein coding genes. Genomics 2021, 113, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shi, Y.; Wang, P.; Guan, C.; Gu, X.; Guan, L.; Cui, P.; Suo, X. Study on the pathogenicity, immunogenicity, endogenous development and drug sensitivity of Eimeria kongi. Front. Vet. Sci. 2023, 10, 1134193. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Barta, J.R.; Beveridge, I.; Duszynski, D.W.; Mehlhorn, H.; Morrison, D.A.; Thompson, R.C.A.; Conrad, P.A. The conceptual basis for a new classification of the coccidia. Int. J. Parasitol. 2002, 32, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Dyachenko, V.; Aupperle, H.; Pees, M.; Krautwald-Junghanns, M.E.; Daugschies, A. Case report of systemic coccidiosis in a radiated tortoise (Geochelone radiata). Parasitol. Res. 2008, 102, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, J.; Zhang, K.; Lang, J.; Wang, N.; Li, J.; Zhang, L. Morphological and molecular characteristics of a single oocyst for the identification of Eimeria species in domestic rabbits (Oryctolagus cuniculus f. domesticus). Vet. Parasitol. 2023, 321, 109986. [Google Scholar] [CrossRef]
- Šlapeta, J.R.; Modrý, D.; Votýpka, J.; Jirků, M.; Oborník, M.; Lukeš, J.; Koudela, B. Eimeria telekii n.sp. (Apicomplexa: Coccidia) from Lemniscomys striatus (Rodentia: Muridae): Morphology, pathology and phylogeny. Parasitology 2001, 122, 133–143. [Google Scholar] [CrossRef]
- Modrý, D.; Slapeta, J.R.; Jirků, M.; Oborník, M.; Lukeš, J.; Koudela, B. Phylogenetic position of a renal coccidium of the European green frogs, ‘Isospora’ lieberkuehni Labbé, 1894 (Apicomplexa: Sarcocystidae) and its taxonomic implications. Int. J. Syst. Evol. Microbiol. 2001, 53, 767–772. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, J.P.; Lozano-Aguirre, L.F.; Medrano-Félix, J.A.; Chaidez, C.; Gerba, C.P.; Betancourt, W.Q.; Castro-del Campo, N. Evaluation of nuclear and mitochondrial phylogenetics for the subtyping of Cyclospora cayetanensis. Parasitol. Res. 2023, 122, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.; Williams, T.A.; Gibson, W. Mitochondrial DNAs provide insight into trypanosome phylogeny and molecular evolution. BMC Evol. Biol. 2020, 20, 161. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Zhu, P.; Gu, X.; He, R.; Xu, J.; Jing, B.; Wang, L.; Chen, S.; Xie, Y. Eimeria zuernii (Eimeriidae: Coccidia): Mitochondrial genome and genetic diversity in the Chinese yak. Parasit. Vectors 2023, 16, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Zhu, P.; Yang, Z.; Wang, Z.; Chen, Y.; Gu, X.; He, R.; Xu, J.; Jing, B.; et al. Comprehensive molecular characterization of complete mitogenome assemblies of 33 Eimeria isolates infecting domestic chickens. Parasit. Vectors 2023, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Sun, C.; Bah, A.B.; Oberstaller, J.; Pierre-Louis, E.; Etheridge, R.D.; Feschotte, C.; Pritham, E.J.; Kissinger, J.C. Massive invasion of organellar DNA drives nuclear genome evolution in Toxoplasma. Proc. Natl. Acad. Sci. USA 2023, 120, e2308569120. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Brice, B.; Oskam, C.; Zhang, Y.; Brigg, F.; Berryman, D.; Ryan, U. Characterization of two complete Isospora mitochondrial genomes from passerine birds: Isospora serinuse in a domestic canary and Isospora manorinae in a yellow-throated miner. Vet. Parasitol. 2017, 237, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.Q.; Cui, P.; Fang, S.F.; Liu, G.H.; Wang, C.R.; Zhu, X.Q. The complete mitochondrial genome sequence of Eimeria magna (Apicomplexa: Coccidia). Mitochondrial DNA 2015, 26, 714–715. [Google Scholar] [CrossRef]
- Liu, G.H.; Tian, S.Q.; Cui, P.; Fang, S.F.; Wang, C.R.; Zhu, X.Q. The complete mitochondrial genomes of five Eimeria species infecting domestic rabbits. Exp. Parasitol. 2015, 159, 67–71. [Google Scholar] [CrossRef]
| Species | Accession Number | Species | Accession Number |
|---|---|---|---|
| E. kongi | NC_072899 | E. gallopavonis | KJ608413 |
| E. vejdovskyi | KP025692 | E. meleagrimitis | KJ608414 |
| E. media | KP025691 | E. necatrix | KX094954 |
| E. magna | KF419217 | E. tenella | HQ702484 |
| E. intestinalis | KP009592 | E. acervulina | HQ702479 |
| E. flavescens | KP025693 | E. praecox | HQ702483 |
| E. irresidua | KP025690 | E. maxima | HQ702481 |
| E. dispersa | KJ608416 | E. brunetti | HQ702480 |
| E. adenoides | KJ608415 | E. mitis | KF501573 |
| E. meleagridis | KJ608418 |
| Genes | Nucleotide No. | Size (bp) | Start Codon | Stop Codon |
|---|---|---|---|---|
| SSU4 | 1–82 | 82 | ||
| cytb | 128–1207 | 1080 | ATG | TAA |
| cox1 | 1240–2682 | 1443 | ATG | TAA |
| LSU11 | 2722–2849 | 128 | ||
| LSU12 | 2851–2949 | 99 | ||
| LSU4 | 3090–3114 | 24 | ||
| SSU12 | 3244–3304 | 61 | ||
| LSU13 | 3305–3379 | 75 | ||
| LSU5 | 3403–3451 | 49 | ||
| SSU10 | 3460–3524 | 65 | ||
| SSU8 | 3538–3596 | 59 | ||
| LSU10 | 3637–3669 | 33 | ||
| LSU15 | 3678–3733 | 56 | ||
| LSU8 | 3757–3837 | 81 | ||
| SSU5 | 3883–3974 | 92 | ||
| LSU2 | 3992–4057 | 66 | ||
| LSU1 | 4083–4261 | 179 | ||
| cox3 | 4279–5034 | 756 | TTA | TAA |
| SSU1 | 5044–5073 | 30 | ||
| LSU6 | 5102–5195 | 94 | ||
| LSU3 | 5272–5295 | 24 | ||
| LSU7 | 5331–5403 | 73 | ||
| SSU6 | 5467–5582 | 116 | ||
| LSU9 | 5684–5871 | 188 | ||
| SSU11 | 5970–6006 | 37 | ||
| SSU9 | 6032–6212 | 181 |
| Species | A (%) | T (%) | G (%) | C (%) | Total |
|---|---|---|---|---|---|
| E. mitis | 30.9 | 36.5 | 16.3 | 16.3 | 6408 |
| E. irresidua | 29.8 | 35.6 | 17 | 17.6 | 6259 |
| E. kongi | 29.8 | 35.6 | 17 | 17.6 | 6258 |
| E. flavescens | 30 | 35.5 | 16.9 | 17.6 | 6258 |
| E. magna | 29.7 | 35.4 | 17.1 | 17.7 | 6249 |
| E. intestinalis | 29.7 | 35.5 | 17.1 | 17.7 | 6247 |
| E. media | 29.6 | 35.5 | 17.1 | 17.7 | 6245 |
| E. vejdovskyi | 29.6 | 35.5 | 17.2 | 17.7 | 6240 |
| E. dispersa | 29.9 | 35.6 | 16.8 | 17.6 | 6238 |
| E. gallopavonis | 30.1 | 34.8 | 16.9 | 18.3 | 6215 |
| E. tenella | 29.8 | 35.2 | 16.9 | 18.1 | 6213 |
| E. meleagridis | 29.8 | 34.9 | 17 | 18.3 | 6212 |
| E. necatrix | 29.8 | 35.2 | 16.9 | 18.2 | 6212 |
| E. adenoides | 29.8 | 34.8 | 17 | 18.4 | 6211 |
| E. acervulina | 30.1 | 35.3 | 17 | 17.6 | 6179 |
| E. praecox | 30.1 | 35.5 | 17.1 | 17.3 | 6172 |
| E. maxima | 30 | 34.5 | 17.5 | 17.9 | 6167 |
| E. meleagrimitis | 30 | 33.5 | 17.3 | 19.2 | 6165 |
| E. brunetti | 29.8 | 35.8 | 17.1 | 17.4 | 6156 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Fang, S.; Gu, X.; Hao, C.; Du, F.; Cui, P.; Tang, X. The Complete Mitochondrial Genome Sequence of Eimeria kongi (Apicomplexa: Coccidia). Life 2024, 14, 699. https://doi.org/10.3390/life14060699
Shi Y, Fang S, Gu X, Hao C, Du F, Cui P, Tang X. The Complete Mitochondrial Genome Sequence of Eimeria kongi (Apicomplexa: Coccidia). Life. 2024; 14(6):699. https://doi.org/10.3390/life14060699
Chicago/Turabian StyleShi, Yubo, Sufang Fang, Xiaolong Gu, Chengyu Hao, Fangchen Du, Ping Cui, and Xinming Tang. 2024. "The Complete Mitochondrial Genome Sequence of Eimeria kongi (Apicomplexa: Coccidia)" Life 14, no. 6: 699. https://doi.org/10.3390/life14060699








