Genetic Links between Endometriosis and Endometriosis-Associated Ovarian Cancer—A Narrative Review (Endometriosis-Associated Cancer)
Abstract
:1. Introduction
2. Pathogenesis of Ovarian Cancer
3. Methods
3.1. Epidemiologic Studies
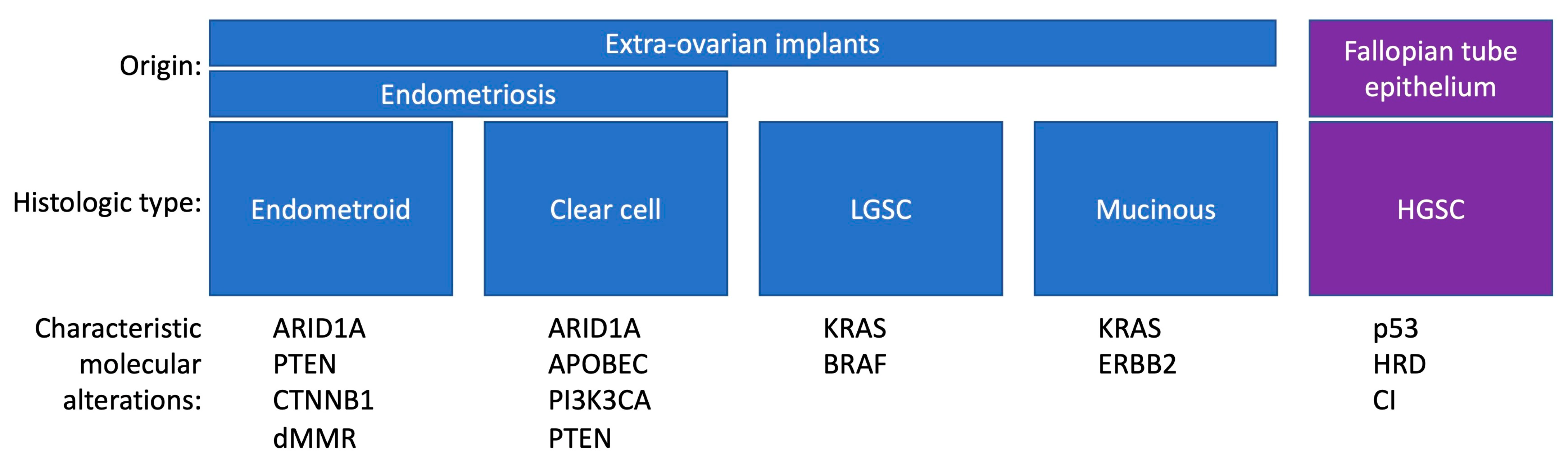
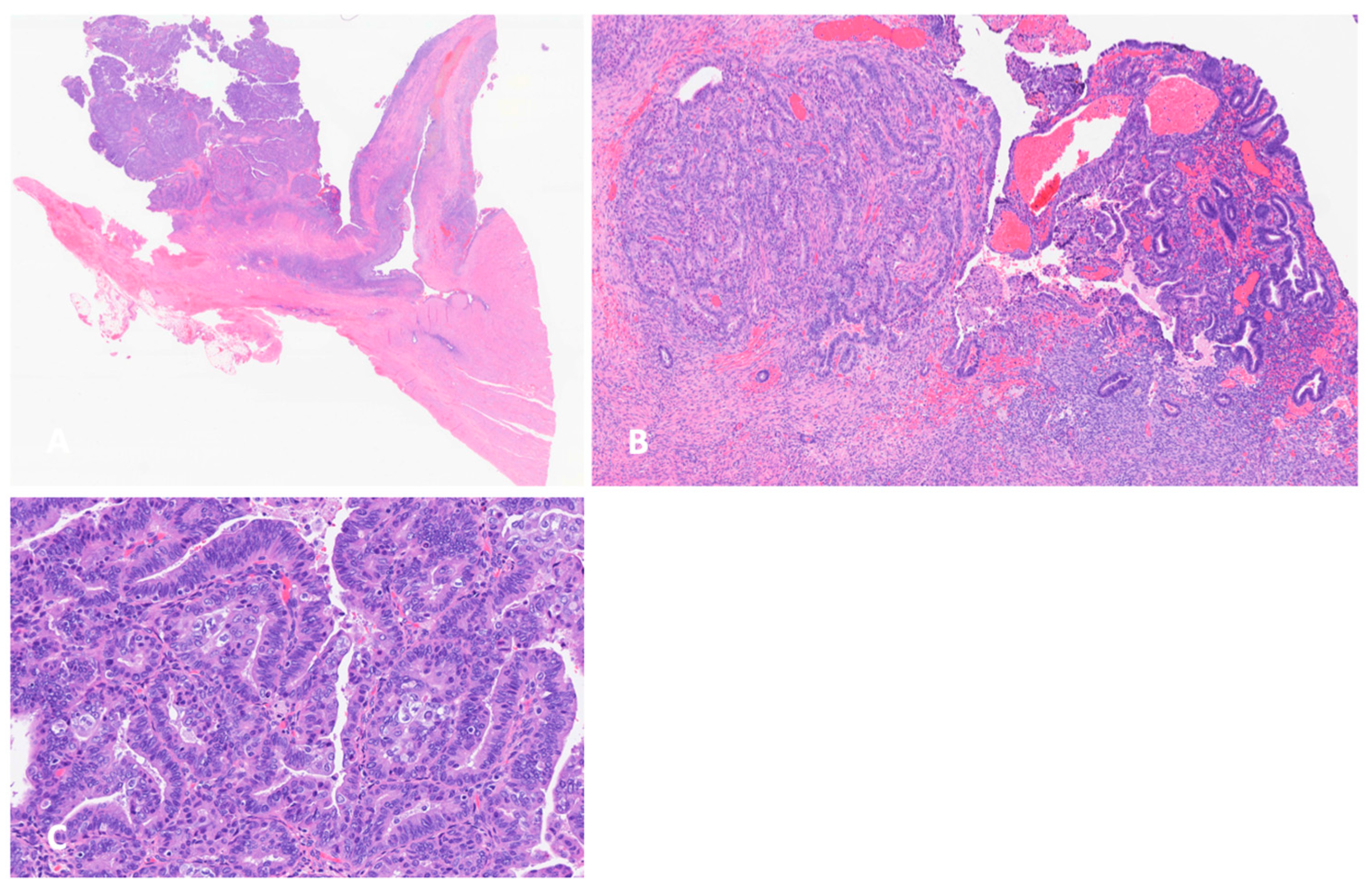
3.2. Histology and Pathogenesis of Endometriosis-Associated Ovarian Cancer
3.3. ARID1A Mutations and Other Genetic Alterations
3.4. Microenvironment of Endometriosis and EAOC
3.5. The Role of Micro RNAs
| Study | Type | |
|---|---|---|
| Lee et al., 2016 [103] | GWAS | SNP Rs7515106 (chr 1p36) |
| Sapkota et al., 2017 [104] | GWAS | SNPs at FN1, CCDC170, ESR1, SYNE1, FSHB (sex steroid hormone pathway) |
| Phelan et al., 2017 [105] | GWAS | SNP at 5q12.3 |
| Lu et al., 2015 [41] | SNP | |
| Mortlock et al., 2022 [40] | GWAS | 28 SNPs associated with both endometriosis and ovarian cancer (chr 1, 2, 4, 5–10, 1217, 18) |
4. Conclusions and Clinical Implications
Author Contributions
Funding
Conflicts of Interest
References
- Giudice, L.C. Clinical practice: Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Labidi-Galy, S.I.; Moschetta, M.; Uccello, M.; Kalaitzopoulos, D.R.; Perez-Fidalgo, J.A.; Boussios, S. Endometriosis-associated ovarian carcinomas: Insights into pathogenesis, diagnostics, and therapeutic targets—A narrative review. Ann. Transl. Med. 2020, 8, 1712. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Pettersson, H.J.; Svedberg, P.; Olovsson, M.; Bergqvist, A.; Marions, L.; Tornvall, P.; Kuja-Halkolka, R. Heritability of endometriosis. Fertil. Steril. 2015, 104, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, U.; Rhiem, K.; Kaminski, M.; Wardelmann, E.; Trog, D.; Valter, M.; Richter, O.N. Parametrial and rectovaginal adenocarcinoma arising from endometriosis. Int. J. Gynecol. Cancer 2005, 15, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Mechsner, S.; Weichbrodt, M.; Riedlinger, W.F.J.; Kaufmann, A.M.; Schneider, A.; Köhler, C. Immunohistochemical evaluation of endometriotic lesions and disseminated endometriosis-like cells in incidental lymph nodes of patients with endometriosis. Fertil. Steril. 2010, 94, 457–463. [Google Scholar] [CrossRef]
- Keichel, S.; Barcena de Arellano, M.-L.; Reichelt, U.; Riedlinger, W.F.J.; Schneider, A.; Köhler, C.; Mechsner, S. Lymphangiogenesis in deep infiltrating endometriosis. Hum. Reprod. 2011, 26, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, N.; Banys-Paluchowski, M.; Schmidt, D.; Ulrich, U.; Fehm, T. Endometriosis-associated Malignancy. Geburtshilfe Grauenheilkd 2016, 76, 176–181. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef]
- Gadducci, A.; Multinu, F.; Cosio, S.; Carinelli, S.; Ghioni, M.; Aletti, G.D. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol. Oncol. 2021, 162, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-J.; William, J.; Bulun, S. Endometriosis and Ovarian Cancer: A Review of Clinical, Pathologic, and Molecular Aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. JAMA 1925, 10, 1–72. [Google Scholar]
- Scott, R.B. Malignant changes in endometriosis. Obstet. Gynecol. 1953, 2, 283–289. [Google Scholar]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.E. Pathology of ovarian cancer precursors. J. Cell. Biochem. 1995, 59, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Partida, G.; Lu, Y.; Dixon, S.C.; Australian Ovarian Cancer Study; Fasching, P.A.; Hein, A.; Burghaus, S.; Beckmann, M.W.; Lambrechts, D.; Nieuwenhuysen, E.V.; et al. Assessing the genetic architecture of epithelial ovarian cancer histological subtypes. Hum. Genet. 2016, 135, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.-L.; Shih, I.-M.; Mao, T.-L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.J.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef]
- Hao, D.; Li, J.; Jia, S.; Meng, Y.; Zhang, C.; Wang, L.; Di, L.-J. Integrated Analysis Reveals Tubal- and Ovarian-Originated Serous Ovarian Cancer and Predicts Differential Therapeutic Responses. Clin. Cancer Res. 2017, 23, 7400–7411. [Google Scholar] [CrossRef]
- Lawrenson, K.; Fonseca, M.A.S.; Liu, A.Y.; Dezem, F.S.; Lee, J.M.; Lin, X.; Corona, R.I.; Abbasi, F.; Vavra, K.C.; Dinh, H.Q.; et al. A Study of High-Grade Serous Ovarian Cancer Origins Implicates the SOX18 Transcription Factor in Tumor Development. Cell 2019, 29, 3726–3735.e3724. [Google Scholar] [CrossRef] [PubMed]
- Colvin, E.K.; Howell, V.M. Why the dual origins of high grade serous ovarian cancer matter. Nat. Commun. 2020, 11, 1200. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Buggio, L.; Makieva, S.; Scarfone, G.; Cribiù, F.M.; Parazzini, F.; Somigliana, E. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 151–168. [Google Scholar] [CrossRef]
- Brinton, L.A.; Gridley, G.; Persson, I.; Baron, J.; Bergqvist, A. Cancer risk after a hospital discharge diagnosis of endometriosis. Americn J. Obstet. Gynecol. 1997, 176, 572–579. [Google Scholar] [CrossRef]
- Melin, A.; Sparén, P.; Persson, I.; Bergqvist, A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum. Reprod. 2006, 21, 1237–1242. [Google Scholar] [CrossRef]
- Brinton, L.A.; Lamb, E.J.; Moghissi, K.S.; Scoccia, B.; Althuis, M.D.; Mabie, J.E.; Westhoff, C.L. Ovarian cancer risk associated with varying causes of infertility. Fertil. Steril. 2003, 82, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Sakoda, L.C.; Sherman, M.E.; Frederiksen, K.; Kjaer, S.K.; Graubard, B.I.; Olsen, J.H.; Mellemkjaer, L. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2929–2935. [Google Scholar] [CrossRef]
- Rossing, M.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; Doherty, J.A.; Weiss, N.S. Risk of Epithelial Ovarian Cancer in Relation to Benign Ovarian Conditions and Ovarian Surgery. Cancer Causes Control 2008, 19, 1357–1364. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Y.; Li, G.; Dai, S.; Cao, M.; Meng, Z.; Ren, S. Endometriosis and epithelial ovarian cancer: A two-sample Mendelian randomization analysis. Sci. Rep. 2023, 13, 21992. [Google Scholar] [CrossRef] [PubMed]
- Saavalainen, L.; Lassus, H.; But, A.; Tiitinen, A.; Härkki, P.; Gissler, M.; Pukkala, E.; Heikinheimo, O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet. Gynecol. 2018, 131, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Monnin, N.; Fattet, A.J.; Koscinski, I. Endometriosis: Update of Pathophysiology, (Epi) Genetic and Environmental Involvement. Biomedicines 2023, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, A.; Maksym, R.B.; Paskal, W.; Machnicki, M.; Rak, B.; Pępek, M.; Garbicz, F.; Pełka, K.; Kuśmierczyk, Z.; Jacko, J.; et al. Epithelial Cells of Deep Infiltrating Endometriosis Harbor Mutations in Cancer Driver Genes. Cells 2021, 10, 759. [Google Scholar] [CrossRef]
- Nezhat, F.; Nezhat, C.; Allan, C.J.; Metzger, D.A.; Sears, D.L. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J. Reprod. Med. 1992, 37, 771–776. [Google Scholar] [PubMed]
- Nezhat, C.; Nezhat, F.; Nezhat, C.; Seidman, D.S. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum. Reprod. 1994, 9, 2212–2213. [Google Scholar] [CrossRef]
- Nilbert, M.; Pejovic, T.; Mandahl, N.; Iosif, S.; Willén, H.; Mitelman, F. Monoclonal origin of endometriotic cysts. Int. J. Gynecol. Cancer 1995, 5, 61–63. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sumimoto, K.; Kitanaka, T.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; et al. Ovarian endometrioma--risks factors of ovarian cancer development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 187–193. [Google Scholar] [CrossRef]
- Worley, M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.-W. Endometriosis-associated ovarian cancer: A review of pathogenesis. Int. J. Mol. Sci. 2013, 14, 5367–5379. [Google Scholar] [CrossRef]
- Mortlock, S.; Corona, R.I.; Kho, P.F.; Pharaoh, P.; Seo, J.-H.; Freedman, M.L.; Gayther, S.A.; Siedhoff, M.T.; Rogers, P.A.W.; Leuchter, R.; et al. A multi-level investigation of the genetic relationship between endometriosis and ovarian cancer histotypes. Cell Rep. Med. 2022, 3, 100542. [Google Scholar] [CrossRef]
- Lu, Y.; Cuellar-Partida, G.; Painter, J.N.; Nyholt, D.R.; Australian Ovarian Cancer Study; The International Endogene Consortium (IEC); Morris, A.P.; Fasching, P.A.; Hein, A.; Burghaus, S.; et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum. Mol. Genet. 2015, 24, 5955–5964. [Google Scholar] [CrossRef] [PubMed]
- Czernobilsky, B.; Morris, W.J. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet. Gynecol. 1979, 53, 318–323. [Google Scholar] [PubMed]
- LaGrenade, A.; Silverberg, S.G. Ovarian tumors associated with atypical endometriosis. Hum. Pathol. 1988, 19, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Reviczká, A.; Háková, H.; Švajdler, P.; Rabajdová, M.; Ostró, A. Predictive factors of endometriosis progression into ovarian cancer. J. Ovarian Res. 2022, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Nomura, K.; Ishikawa, E.; Ushigome, S. Ovarian atypical endometriosis: Its close association with malignant epithelial tumours. Histopathology 1997, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Kaku, T.; Amada, S.; Kobayashi, H.; Hirakawa, T.; Ariyoshi, K.; Kamura, T.; Nakano, H. Ovarian endometriosis associated with ovarian carcinoma: A clinicopathological and immunohistochemical study. Gynecol. Oncol. 2000, 77, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Tanase, Y.; Furukawa, N.; Kobayashi, H.; Matsumoto, T. Malignant Transformation from Endometriosis to Atypical Endometriosis and Finally to Endometrioid Adenocarcinoma within 10 Years. Case Rep. Oncol. 2013, 6, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Bartiromo, L.; Schimberni, M.; Villanacci, R.; Mangili, G.; Ferrari, S.; Ottolina, J.; Salmeri, N.; Dolci, C.; Tandoi, I.; Candiani, M. A Systematic Review of Atypical Endometriosis-Associated Biomarkers. Int. J. Mol. Sci. 2022, 23, 4425. [Google Scholar] [CrossRef]
- Wepy, C.; Nucci, M.R.; Parra-Herran, C. Atypical Endometriosis: Comprehensive Characterization of Clinicopathologic, Immunohistochemical, and Molecular Features. Int. J. Gynecol. Pathol. 2024, 43, 70–77. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Tessier-Cloutier, B.; Lawrence, K.M.; Nazeran, T.; Karnezis, A.N.; Salamanca, C.; Cheng, A.S.; McAlpine, J.N.; Hoang, L.N.; Gilks, C.B.; et al. Clear cell and endometrioid carcinomas: Are their differences attributable to distinct cells of origin? J. Pathol. 2017, 243, 26–36. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.L.; Albert, A.; Liu, Y.D.; Lum, A.; Hong, J.; Ionescu, C.L.; Senz, J.; Nazeran, T.M.; Lee, A.F.; Noga, H.; et al. KRAS mutations and endometriosis burden of disease. J. Pathol. Soc. Clin. Res. 2023, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.G.; Roberts, C.W.M. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 2011, 11, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L.; Brennan, J.; Schisler, J.C.; Serber, D.; Patterson, C.; Magnuson, T. ARID1a-DNA Interactions Are Required for Promoter Occupancy by SWI/SNF. Mol. Cell. Biol. 2013, 33, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.N.; Roberts, C.W.M. ARID1A mutations in cancer: Another epigenetic tumor suppressor? Cancer Discov. 2013, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Shi, X.; Zhang, Z.; Chen, Y.; Wu, J.I. Dual role of Brg chromatin remodeling factor in Sonic hedgehog signaling during neural development. Proc. Natl. Acad. Sci. USA 2011, 108, 12758–12763. [Google Scholar] [CrossRef] [PubMed]
- Khalique, S.; Naidoo, K.; Attygale, A.D.; Kriplani, D.; Daley, F.; Lowe, A.; Campbell, J.; Jones, T.; Hubank, M.; Fenwick, K.; et al. Optimised ARID1A immunohistochemistry is an accurate predictor of ARID1A mutational status in gynaecological cancers. J. Pathol. Soc. Clin. Res. 2018, 4, 154–166. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Abrão, M.S.; Taube, E.T.; Darb-Esfahani, S.; Köhler, C.; Chiantera, V.; Mechsner, S. (Partial) Loss of BAF250a (ARID1A) in rectovaginal deep-infiltrating endometriosis, endometriomas and involved pelvic sentinel lymph nodes. Mol. Hum. Reprod. 2016, 22, 329–337. [Google Scholar] [CrossRef]
- Yachida, N.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Ueda, H.; Sugino, K.; Yamaguchi, M.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. ARID1A protein expression is retained in ovarian endometriosis with ARID1A loss-of-function mutations: Implication for the two-hit hypothesis. Sci. Rep. 2020, 10, 14260. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Samartzis, N.; Noske, A.; Fedier, A.; Caduff, R.; Dedes, K.J.; Fink, D.; Imesch, P. Loss of ARID1A/BAF250a-expression in endometriosis: A biomarker for risk of carcinogenic transformation? Mod. Pathol. 2012, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 2012, 25, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef]
- Wang, Y.; Hoang, L.; Ji, J.X.; Huntsman, D.G. SWI/SNF Complex Mutations in Gynecologic Cancers: Molecular Mechanisms and Models. Annu. Rev. Pathol. 2020, 15, 467–492. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Ono, R.; Sanuki, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; et al. Affinity-purified DNA-based mutation profiles of endometriosis-related ovarian neoplasms in Japanese patients. Oncotarget 2018, 9, 14754–14763. [Google Scholar] [CrossRef]
- Vercellini, P.; Eskenazi, B.; Consonni, D.; Somigliana, E.; Parazzini, F.; Abbiati, A.; Fedele, L. Oral contraceptives and risk of endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 159–170. [Google Scholar] [CrossRef]
- Lac, V.; Nazeran, T.M.; Tessier-Cloutier, B.; Aguirre-Hernandez, R.; Albert, A.; Lum, A.; Khattra, J.; Praetorius, T.; Mason, M.; Chiu, D.; et al. Oncogenic mutations in histologically normal endometrium: The new normal? J. Pathol. 2019, 249, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Xiang, H.; Ding, P.; Wu, T.; Ji, G. The biochemical and clinical implications of phosphatase and tensin homolog deleted on chromosome ten in different cancers. Am. J. Cancer Res. 2021, 11, 5833–5855. [Google Scholar]
- Russo, A.; Czarnecki, A.A.; Dean, M.; Modi, D.A.; Lantvit, D.D.; Hardy, L.; Baligod, S.; Davis, D.A.; Wei, J.J.; Burdette, J.E. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 2018, 37, 1976–1990. [Google Scholar] [CrossRef]
- McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; Yang, W.; Senz, J.; Chow, C.; Heravi-Moussavi, A.; Morin, G.B.; Mes-Masson, A.M.; Carey, M.S.; et al. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J. Pathol. 2011, 223, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Shih Ie, M.; Panuganti, P.K.; Kuo, K.T.; Mao, T.L.; Kuhn, E.; Jones, S.; Velculescu, V.E.; Kurman, R.J.; Wang, T.L. Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am. J. Pathol. 2011, 178, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.; Westin, S.N.; Herzog, T.J. State of the science: Contemporary front-line treatment of advanced ovarian cancer. Gynecol. Oncol. 2022, 166, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Teplinsky, E.; Muggia, F. Targeting HER2 in ovarian and uterine cancers: Challenges and future directions. Gynecol. Oncol. 2014, 135, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Katsumata, N.; Tsuda, H.; Uchi, N.; Miyazaki, S.; Hidaka, T.; Sakai, M.; Saito, S. HER2 is frequently over-expressed in ovarian clear cell adenocarcinoma: Possible novel treatment modality using recombinant monoclonal antibody against HER2, trastuzumab. Jpn. J. Cancer Res. 2002, 93, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Pal, T.; Permuth-Wey, J.; Kumar, A.; Sellers, T.A. Systematic review and meta-analysis of ovarian cancers: Estimation of microsatellite-high frequency and characterization of mismatch repair deficient tumor histology. Clin. Cancer Res. 2008, 14, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Murali, K.; Orbegoso, C.; Vroobel, K.; Banerjee, S.N.; Gore, M.E.; Attygalle, A.; George, A. Prevalence and clinical implications of mismatch repair (MMR) deficiency in unselected non-serous epithelial ovarian cancer (EOC) patients (pts). J. Clin. Oncol. 2019, 37 (Suppl. 15), 1521. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, S.; Zhong, S.; Shan, B.; Jiang, W.; Yang, W.; Cai, X.; Yang, H. The frequency and clinical implication of mismatch repair protein deficiency in Chinese patients with ovarian clear cell carcinoma. BMC Cancer 2022, 22, 449. [Google Scholar] [CrossRef]
- Helder-Woolderink, J.M.; Blok, E.A.; Vasen, H.F.A.; Hollema, H.; Mourits, M.J.; De Bock, G.H. Ovarian cancer in Lynch syndrome; a systematic review. Eur. J. Cancer 2016, 55, 65–73. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhong, Z.; Wei, C.; Liu, Y.; Zhu, X. Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Front. Immunol. 2023, 14, 1134663. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Wan, Y.; Matei, D. Epithelial Mutations in Endometriosis: Link to Ovarian Cancer. Endocrinology 2019, 160, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Chang, N.; Lin, S.C.; Li, Y.H.; Wu, M.H. Inhibition of dual specificity phosphatase-2 by hypoxia promotes interleukin-8-mediated angiogenesis in endometriosis. Hum. Reprod. 2014, 29, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Becker, C.M. Endometriosis and angiogenesis. Minerva Ginecol 2008, 60, 245–254. [Google Scholar] [PubMed]
- Styer, A.K.; Sullivan, B.T.; Puder, M.; Arsenault, D.; Petrozza, J.C.; Serikawa, T.; Chang, S.; Hasan, T.; Gonzalez, R.R.; Rueda, B.R. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology 2008, 149, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Ahmad, S.F.; Brown, J.K.; Duncan, W.C.; Horne, A.W. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci. Rep. 2015, 5, 16859. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Garrett, L.; Henretta, M.; Ferriss, J.S.; Lee, L.; Horowitz, N. When ‘never-events’ occur despite adherence to clinical guidelines: The case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol. Oncol. 2010, 116, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Heinze, K.; Cairns, E.S.; Thornton, S.; Harris, B.; Milne, K.; Grube, M.; Meyer, C.; Karnezis, A.N.; Fereday, S.; Garsed, D.W.; et al. The Prognostic Effect of Immune Cell Infiltration Depends on Molecular Subtype in Endometrioid Ovarian Carcinomas. Clin. Cancer Res. 2023, 29, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol. Oncol. 2018, 151, 381–389. [Google Scholar] [CrossRef]
- Devlin, M.-J.; Miller, R.; Laforets, F.; Kotantaki, P.; Garsed, D.W.; Kristeleit, R.; Bowtell, D.D.; McDermott, J.; Maniati, E.; Balkwill, F.R. The Tumor Microenvironment of Clear-Cell Ovarian Cancer. Cancer Immunol. Res. 2022, 10, 1326–1339. [Google Scholar] [CrossRef]
- Willis, B.C.; Sloan, E.A.; Atkins, K.A.; Stoler, M.H.; Mills, A.M. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod. Pathol. 2017, 30, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, A.; Rekowska, A.; Kuryło, W.; Pańczyszyn, A.; Kotarski, J.; Wertel, I. Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 10859. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; D’Andrea, A.D.; Matulonis, U.A.; Konstantinopoulos, P.A. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology 2017, 6, e1277308. [Google Scholar] [CrossRef] [PubMed]
- Driva, T.S.; Schatz, C.; Haybaeck, J. Endometriosis-Associated Ovarian Carcinomas: How PI3K/AKT/mTOR Pathway Affects Their Pathogenesis. Biomolecules 2023, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, L.; Yang, R.; Xu, T. New insights about endometriosis-associated ovarian cancer: Pathogenesis, risk factors, prediction and diagnosis and treatment. Front. Oncol. 2024, 14, 1329133. [Google Scholar] [CrossRef] [PubMed]
- Teague, E.M.; Print, C.G.; Hull, M.L. The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update 2010, 16, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.K.; Goga, A.; Bhaumik, D.; Berger, C.E.; Sullivan, C.S.; Benz, C.C. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J. Biol. Chem. 2007, 282, 1479–1486. [Google Scholar] [CrossRef]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef]
- Sarkar, S.; Dubaybo, H.; Ali, S.; Goncalves, P.; Kollepara, S.L.; Sethi, S.; Philip, P.A.; Li, Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am. J. Cancer Res. 2013, 3, 465–477. [Google Scholar] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Templeman, C.; Stram, D.A.; Beesley, J.; Tyrer, J.; Berchuck, A.; Pharoah, P.P.; Chenevix-Trench, G.; Pearce, C.L. Ovarian Cancer Association Consortium. Evidence of a genetic link between endometriosis and ovarian cancer. Fertil. Steril. 2016, 105, 35–43. e10. [Google Scholar]
- Sapkota, Y.; Steinthorsdottir, V.; Morris, A.P.; Fassbender, A.; Rahmioglu, N.; De Vivo, I.; Buring, J.E.; Zhang, F.; Edwards, T.L.; Jones, S. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Comm. 2017, 8, 15539. [Google Scholar]
- Phelan, C.M.; Kuchenbaecker, K.B.; Tyrer, J.P.; Kar, S.P.; Lawrenson, K.; Winham, S.J.; Dennis, J.; Pirie, A.; Riggan, M.; Chornokur, G. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017, 49, 680–691. [Google Scholar]
- Orezzoli, J.P.; Russell, A.H.; Oliva, E.; Del Carmen, M.G.; Eichhorn, J.; Fuller, A.F. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol. Oncol. 2008, 110, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Munkarah, A.; Arabi, H.; Bandyopadhyay, S.; Semaan, A.; Hayek, K.; Garg, G.; Morris, R.; Ali-Fehmi, R. Prognostic analysis of ovarian cancer associated with endometriosis. Am. J. Obstet. Gynecol. 2011, 204, 63.e1–63.e7. [Google Scholar] [CrossRef]
| Endometriosis | Ovarian Cancer | |
|---|---|---|
| Early menarche | yes | yes |
| Infertility | yes | yes |
| Estrogen exposure | yes | yes |
| Family history | yes | yes |
| Nulliparity | yes | yes |
| BTL (bilateral tubal ligation) | protective | protective |
| Hysterectomy | protective | protective |
| Progesterone exposure | protective | protective |
| Symptoms | yes | yes |
| Elevated CA125 | yes | yes |
| Invasion/infiltration of tissues | yes | yes |
| Spreading | yes | yes |
| Neoangiogenesis | yes | yes |
| Genetic alterations | yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejovic, T.; Cathcart, A.M.; Alwaqfi, R.; Brooks, M.N.; Kelsall, R.; Nezhat, F.R. Genetic Links between Endometriosis and Endometriosis-Associated Ovarian Cancer—A Narrative Review (Endometriosis-Associated Cancer). Life 2024, 14, 704. https://doi.org/10.3390/life14060704
Pejovic T, Cathcart AM, Alwaqfi R, Brooks MN, Kelsall R, Nezhat FR. Genetic Links between Endometriosis and Endometriosis-Associated Ovarian Cancer—A Narrative Review (Endometriosis-Associated Cancer). Life. 2024; 14(6):704. https://doi.org/10.3390/life14060704
Chicago/Turabian StylePejovic, Tanja, Ann M. Cathcart, Rofieda Alwaqfi, Marjorie N. Brooks, Rachel Kelsall, and Farr R. Nezhat. 2024. "Genetic Links between Endometriosis and Endometriosis-Associated Ovarian Cancer—A Narrative Review (Endometriosis-Associated Cancer)" Life 14, no. 6: 704. https://doi.org/10.3390/life14060704
APA StylePejovic, T., Cathcart, A. M., Alwaqfi, R., Brooks, M. N., Kelsall, R., & Nezhat, F. R. (2024). Genetic Links between Endometriosis and Endometriosis-Associated Ovarian Cancer—A Narrative Review (Endometriosis-Associated Cancer). Life, 14(6), 704. https://doi.org/10.3390/life14060704





