From Zero to Hero: The Cyanide-Free Formation of Amino Acids and Amides from Acetylene, Ammonia and Carbon Monoxide in Aqueous Environments in a Simulated Hadean Scenario
Abstract
1. Introduction
2. Materials and Methods
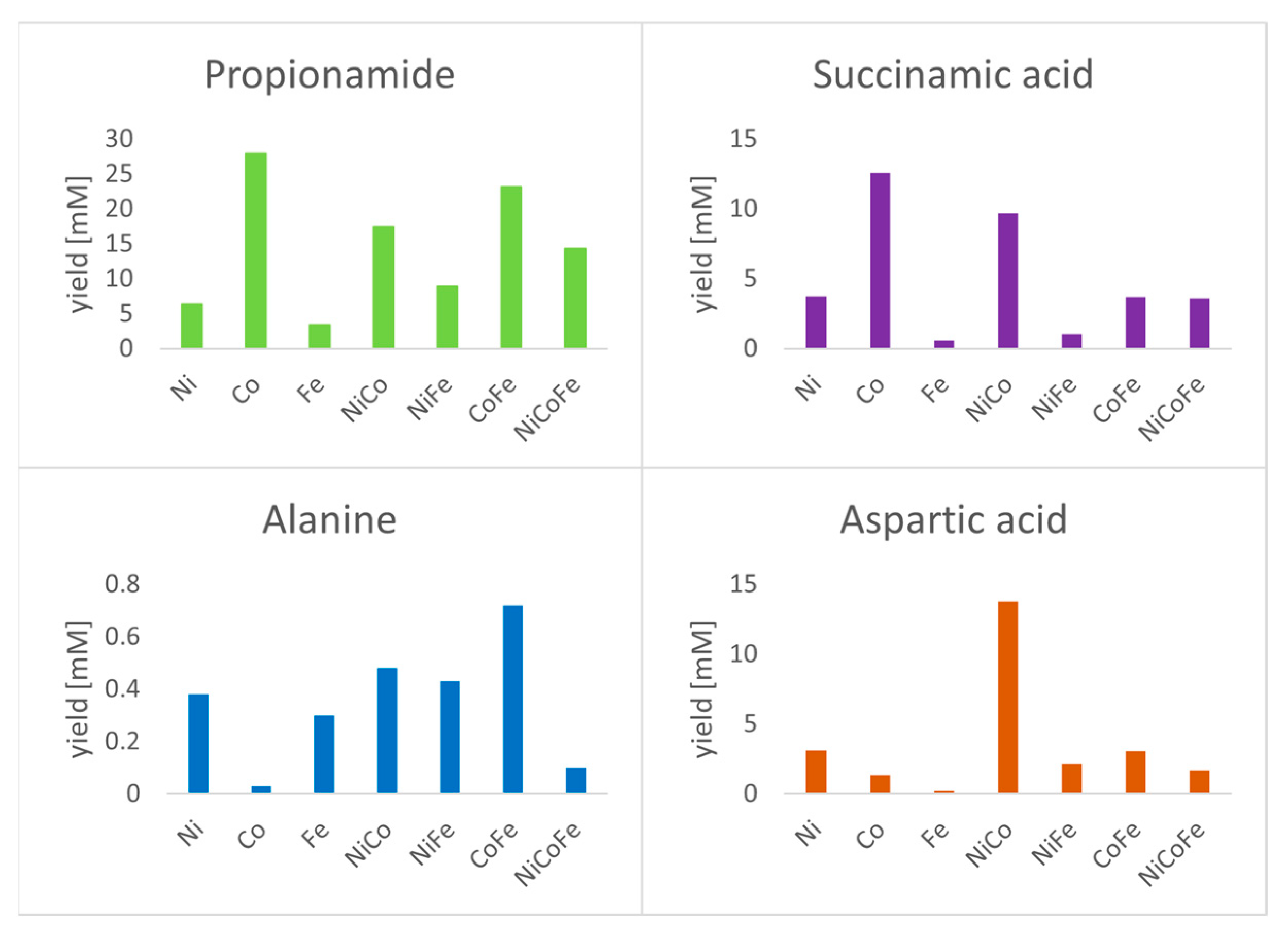
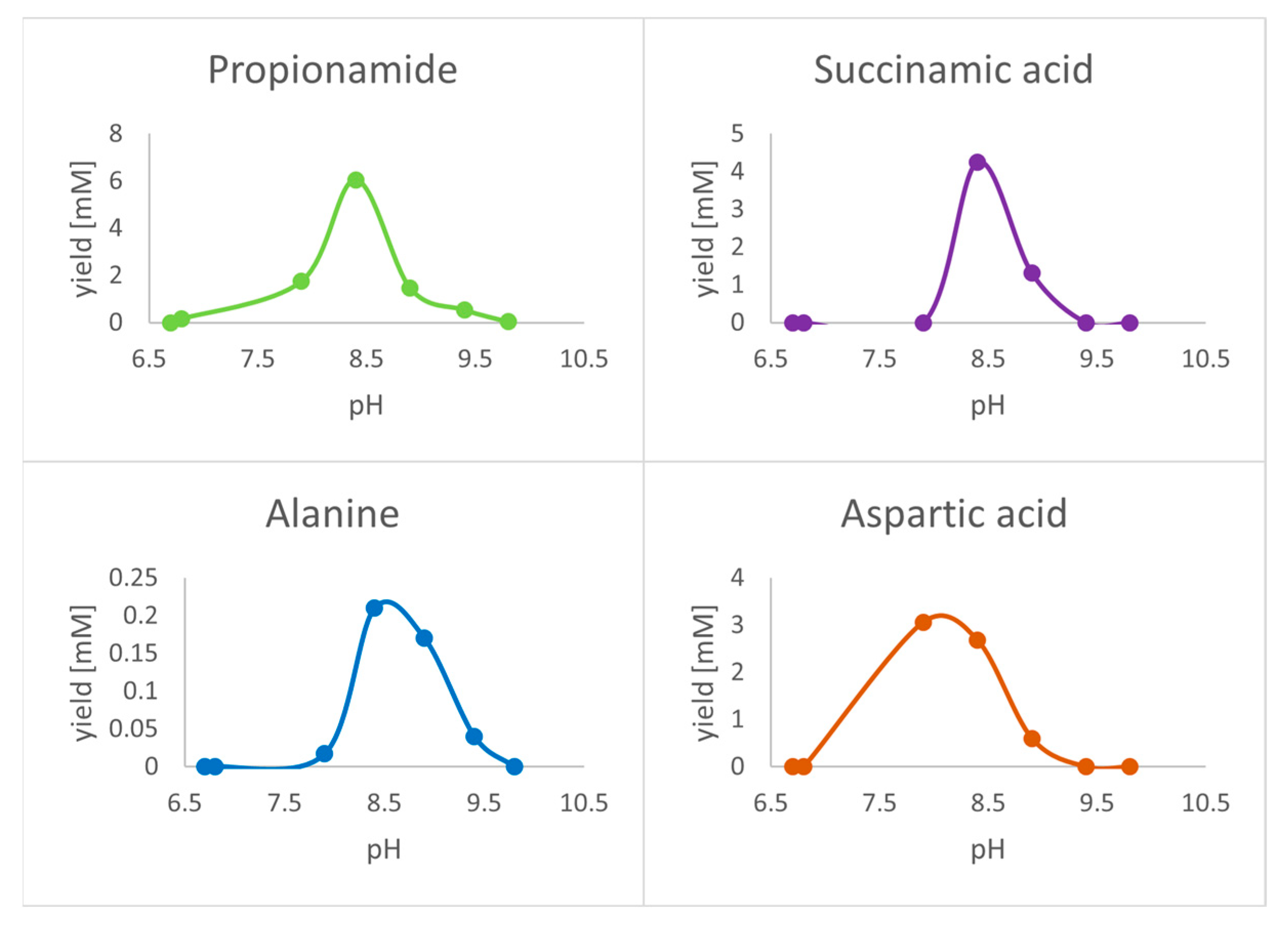
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, S.L. A Production of Amino Acids under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Urey, H.C. On the Early Chemical History of the Earth and the Origin of Life. Proc. Natl. Acad. Sci. USA 1952, 38, 351–363. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef]
- Sagan, C.; Khare, B.N. Long-wavelength ultraviolet photoproduction of amino acids on the primitive Earth. Science 1971, 173, 417–420. [Google Scholar] [CrossRef]
- Sutherland, J.D. The Origin of Life--Out of the Blue. Angew. Chem. Int. Ed. Engl. 2016, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Harkiss, A.H.; Nobis, D.; Malcolm, E.; Knuhtsen, A.; Wellaway, C.R.; Jamieson, A.G.; Magennis, S.W.; Sutherland, A. Conformationally rigid pyrazoloquinazoline α-amino acids: One- and two-photon induced fluorescence. Chem. Commun. 2020, 56, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Harkiss, A.H.; Bell, J.D.; Knuhtsen, A.; Jamieson, A.G.; Sutherland, A. Synthesis and Fluorescent Properties of β-Pyridyl α-Amino Acids. J. Org. Chem. 2019, 84, 2879–2890. [Google Scholar] [CrossRef]
- Steinman, G.; Smith, A.E.; Silver, J.J. Synthesis of a sulfur-containing amino acid under simulated prebiotic conditions. Science 1968, 159, 1108–1109. [Google Scholar] [CrossRef]
- Takahashi, J.; Hosokawa, T.; Masuda, H.; Kaneko, T.; Kobayashi, K.; Saito, T.; Utsumi, Y. Abiotic synthesis of amino acids by x-ray irradiation of simple inorganic gases. Appl. Phys. Lett. 1999, 74, 877–879. [Google Scholar] [CrossRef]
- Utsumi, Y.; Takahashi, J. Synthesis of Amino Acids from N2, H2O Vapor and CO2 Gas Mixture by Synchrotron Radiation Induced Photochemical Reactions at Atmospheric Pressure. Jpn. J. Appl. Phys. 1998, 37, L1268. [Google Scholar] [CrossRef]
- Kobayashil, K.; Kaneko, T.; Saito, T. Characterization of complex organic compounds formed in simulated planetary atmospheres by the action of high energy particels. Adv. Space Res. 1999, 24, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tsuchiya, M.; Oshima, T.; Yanagawa, H. Abiotic synthesis of amino acids and imidazole by proton irradiation of simulated primitive earth atmospheres. Orig. Life Evol. Biosph. 1990, 20, 99–109. [Google Scholar] [CrossRef]
- Lowe, C.U.; Rees, M.W.; Markham, R. Synthesis of complex organic compounds from simple precursors: Formation of amino-acids, amino-acid polymers, fatty acids and purines from ammonium cyanide. Nature 1963, 199, 219–222. [Google Scholar] [CrossRef]
- Islam, N.; Kaneko, T.; Kobayashi, K. Determination of Amino Acids Formed in a Supercritical Water Flow Reactor Simulating Submarine Hydrothermal Systems. Anal. Sci. 2001, 17, 1631–1634. [Google Scholar]
- Marshall, W.L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 1994, 58, 2099–2106. [Google Scholar] [CrossRef]
- Yanagawa, H.; Kobayashi, K. Chapter 8 An experimental approach to chemical evolution in submarine hydrothermal systems. Orig. Life Evol. Biosph. 1992, 22, 147–159. [Google Scholar] [CrossRef]
- Scheu, B.; Dingwell, D.B.; Cimarelli, C.; Bada, J.; Chalmers, J.H.; Burton, A.S. Prebiotic Synthesis in Volcanic Discharges: Exposing Ash to Volcanic/Primordial Gas Atmospheres. In AGU Fall Meeting Abstracts; AGU: Washington, DC, USA, 2017; p. B53A-1946. [Google Scholar]
- Springsklee, C.; Steiner, T.; Geisberger, T.; Scheu, B.; Huber, C.; Eisenreich, W.; Cimarelli, C.; Dingwell, D.B. Prebiotic Synthesis in Volcanic Discharges: Lightning, Porous Ash and Volcanic Gas Atmospheres; Copernicus GmbH: Göttingen, Germany, 2020. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 1992, 58, 85–201. [Google Scholar] [CrossRef]
- Huber, C.; Kraus, F.; Hanzlik, M.; Eisenreich, W.; Wächtershäuser, G. Elements of metabolic evolution. Chemistry 2012, 18, 2063–2080. [Google Scholar] [CrossRef]
- Huber, C.; Eisenreich, W.; Hecht, S.; Wächtershäuser, G. A possible primordial peptide cycle. Science 2003, 301, 938–940. [Google Scholar] [CrossRef]
- Herrera, A.L. A new theory of the origin and nature of life. Science 1942, 96, 14. [Google Scholar] [CrossRef]
- Botta, O.; Martins, Z.; Ehrenfreund, P. Amino acids in Antarctic CM1 meteorites and their relationship to other carbonaceous chondrites. Meteorit. Planet. Sci. 2007, 42, 81–92. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 1948–1972. [Google Scholar] [CrossRef]
- Burton, A.S.; Grunsfeld, S.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. The effects of parent-body hydrothermal heating on amino acid abundances in CI-like chondrites. Polar Sci. 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Oro, J.; Gibert, J.; Lichtenstein, H.; Wikstrom, S.; Flory, D.A. Amino-acids, Aliphatic and Aromatic Hydrocarbons in the Murchison Meteorite. Nature 1971, 230, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef]
- Burton, A.S.; Elsila, J.E.; Hein, J.E.; Glavin, D.P.; Dworkin, J.P. Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit. Planet. Sci. 2013, 48, 390–402. [Google Scholar] [CrossRef]
- Krasnokutski, S.A.; Jäger, C.; Henning, T.; Geffroy, C.; Remaury, Q.B.; Poinot, P. Formation of extraterrestrial peptides and their derivatives. Sci. Adv. 2024, 10, eadj7179. [Google Scholar] [CrossRef]
- Masamba, W. Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis. Molecules 2021, 26, 1707. [Google Scholar] [CrossRef]
- Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Chem. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Jakubke, H.-D.; Kuhl, P.; Könnecke, A. Grundprinzipien der proteasekatalysierten Knüpfung der Peptidbindung. Angew. Chem. 1985, 97, 79–87. [Google Scholar] [CrossRef]
- Forsythe, J.G.; Yu, S.-S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. Int. Ed. Engl. 2015, 54, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Harada, K. The Thermal Copolymerization of Amino Acids Common to Protein 1. J. Am. Chem. Soc. 1960, 82, 3745–3751. [Google Scholar] [CrossRef]
- Keller, M.; Blochl, E.; Wächtershäuser, G.; Stetter, K.O. Formation of amide bonds without a condensation agent and implications for origin of life. Nature 1994, 368, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Scheidler, C.; Sobotta, J.; Eisenreich, W.; Wächtershäuser, G.; Huber, C. Unsaturated C3,5,7,9-Monocarboxylic Acids by Aqueous, One-Pot Carbon Fixation: Possible Relevance for the Origin of Life. Sci. Rep. 2016, 6, 27595. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, T.; Diederich, P.; Kaiser, C.J.O.; Vogele, K.; Ruf, A.; Seitz, C.; Simmel, F.; Eisenreich, W.; Schmitt-Kopplin, P.; Huber, C. Formation of vesicular structures from fatty acids formed under simulated volcanic hydrothermal conditions. Sci. Rep. 2023, 13, 15227. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, J.; Geisberger, T.; Moosmann, C.; Scheidler, C.M.; Eisenreich, W.; Wächtershäuser, G.; Huber, C. A Possible Primordial Acetyleno/Carboxydotrophic Core Metabolism. Life 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.; Eisenreich, W.; Huber, C. The Abiotic Formation of Pyrrole under Volcanic, Hydrothermal Conditions-An Initial Step towards Life’s First Breath? Life 2021, 11, 980. [Google Scholar] [CrossRef]
- Sillen, L.G.; Martell, A.E. Stability Constants of Metal-Ion Complexes, 2nd ed.; Chemical Society: London, UK, 1964. [Google Scholar]
- Huang, S.; Lopez-Capel, E.; Manning, D.A.; Rickard, D. The composition of nanoparticulate nickel sulfide. Chem. Geol. 2010, 277, 207–213. [Google Scholar] [CrossRef]
- Huber, C.; Wächtershäuser, G. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: Implications for the origin of life. Science 1998, 281, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Wächtershäuser, G. alpha-Hydroxy and alpha-amino acids under possible Hadean, volcanic origin-of-life conditions. Science 2006, 314, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [CrossRef] [PubMed]
- Matreux, T.; Aikkila, P.; Scheu, B.; Braun, D.; Mast, C.B. Heat flows enrich prebiotic building blocks and enhance their reactivity. Nature 2024, 628, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Surman, A.J.; Cooper, G.J.T.; Suárez-Marina, I.; Hosni, Z.; Lee, M.P.; Cronin, L. Formation of oligopeptides in high yield under simple programmable conditions. Nat. Commun. 2015, 6, 8385. [Google Scholar] [CrossRef] [PubMed]
- Canavelli, P.; Islam, S.; Powner, M.W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 2019, 571, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.; Haas, M.; Sydow, C.; Siegle, A.F.; Lauer, C.A.; Trapp, O. From amino acid mixtures to peptides in liquid sulphur dioxide on early Earth. Nat. Commun. 2021, 12, 7182. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1787–1806; discussion 1806-8. [Google Scholar] [CrossRef]
- Baross, J.A.; Hoffman, S.E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Huber, C.; Wächtershäuser, G. Primordial reductive amination revisited. Tetrahedron Lett. 2003, 44, 1695–1697. [Google Scholar] [CrossRef]
- Toxvaerd, S. Origin of Homochirality: The Formation and Stability of Homochiral Peptides in Aqueous Prebiological Environment in the Earth’s Crust. Symmetry 2023, 15, 155. [Google Scholar] [CrossRef]
- Mukhin, L.M. Volcanic processes and synthesis of simple organic compounds on primitive earth. Orig. Life 1976, 7, 355–368. [Google Scholar] [CrossRef]
- Wiberg, E.; Wiberg, N. Anorganische Chemie; 103. Auflage; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- McCarthy, M.C.; Gottlieb, C.A.; Cernicharo, J. Building Blocks of Dust: A Coordinated Laboratory and Astronomical Study of AGB Stars. J. Mol. Spectrosc. 2019, 356, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfreund, P.; Spaans, M.; Holm, N.G. The evolution of organic matter in space. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 538–554. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Murga, M.S.; Ananikov, V.P. Role of Acetylene in the Chemical Evolution of Carbon Complexity. ACS Earth Space Chem. 2024, 8, 798–856. [Google Scholar] [CrossRef]
- Cox, P.A. Elements: Their Origin, Abundance, and Distribution; Reprinted with Corr.; Oxford Science Publications: Oxford, UK, 1989. [Google Scholar]
- Wedepohl, H.K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef]
- de Graaf, R.; de Decker, Y.; Sojo, V.; Hudson, R. Quantifying Catalysis at the Origin of Life. Chemistry 2023, 29, e202301447. [Google Scholar] [CrossRef]
- Li, Y.; Kitadai, N.; Nakamura, R. Chemical Diversity of Metal Sulfide Minerals and Its Implications for the Origin of Life. Life 2018, 8, 46. [Google Scholar] [CrossRef]
- Henderson-Sellers, A. The Earth’s environment? A uniquely stable system? Geophys. Surv. 1981, 4, 297–329. [Google Scholar] [CrossRef]
- Kasting, J.F. Earth’s early atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, E.; Keller, M.; Wachtershäuser, G.; Stetter, K.O. Reactions depending on iron sulfide and linking geochemistry with biochemistry. Proc. Natl. Acad. Sci. USA 1992, 89, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.L.; McKay, C.P. The evolution of nitrogen cycling. Orig. Life Evol. Biosph. 1988, 18, 311–325. [Google Scholar] [CrossRef]

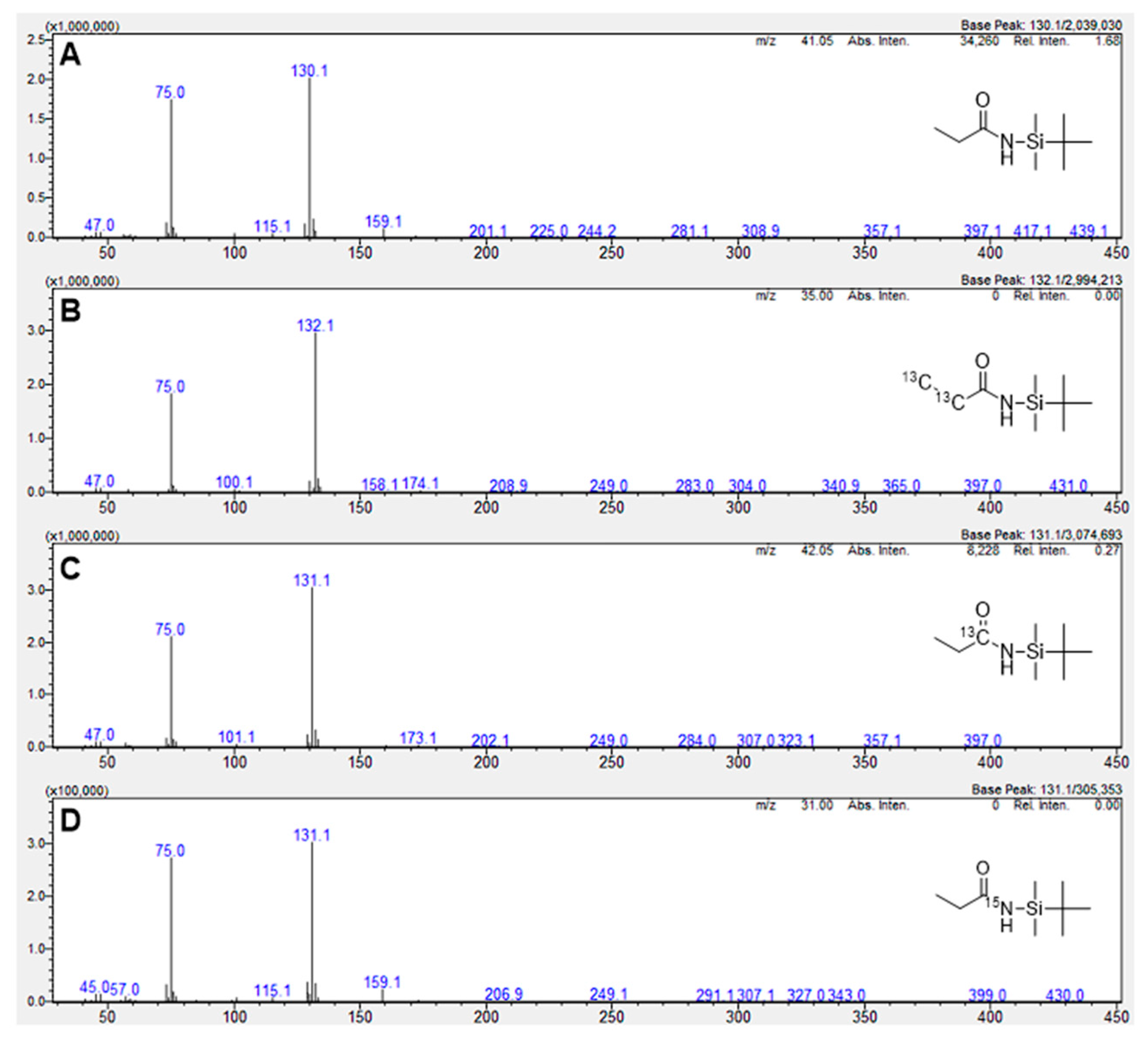



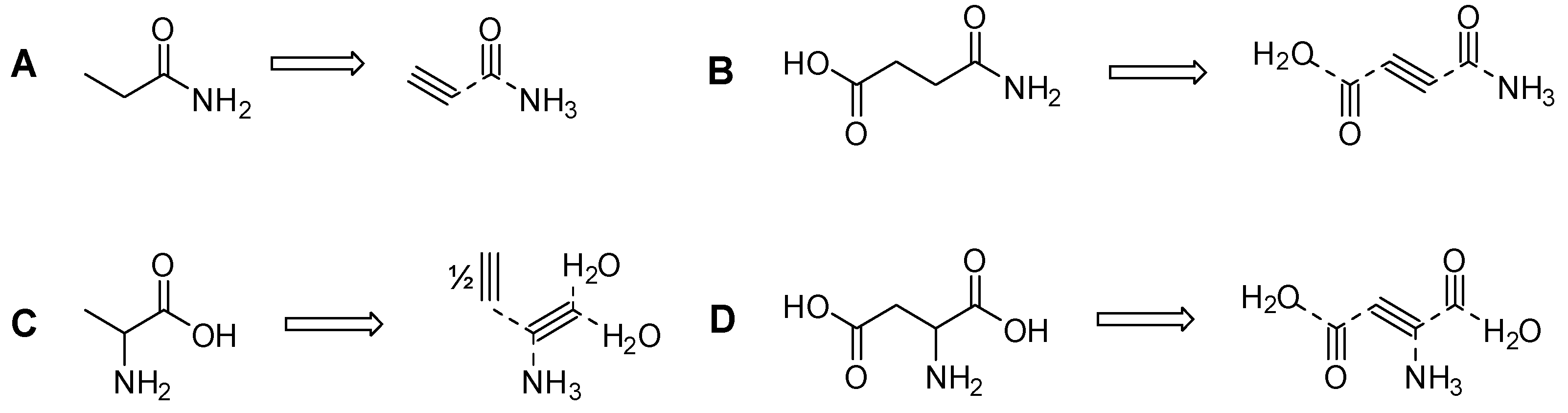
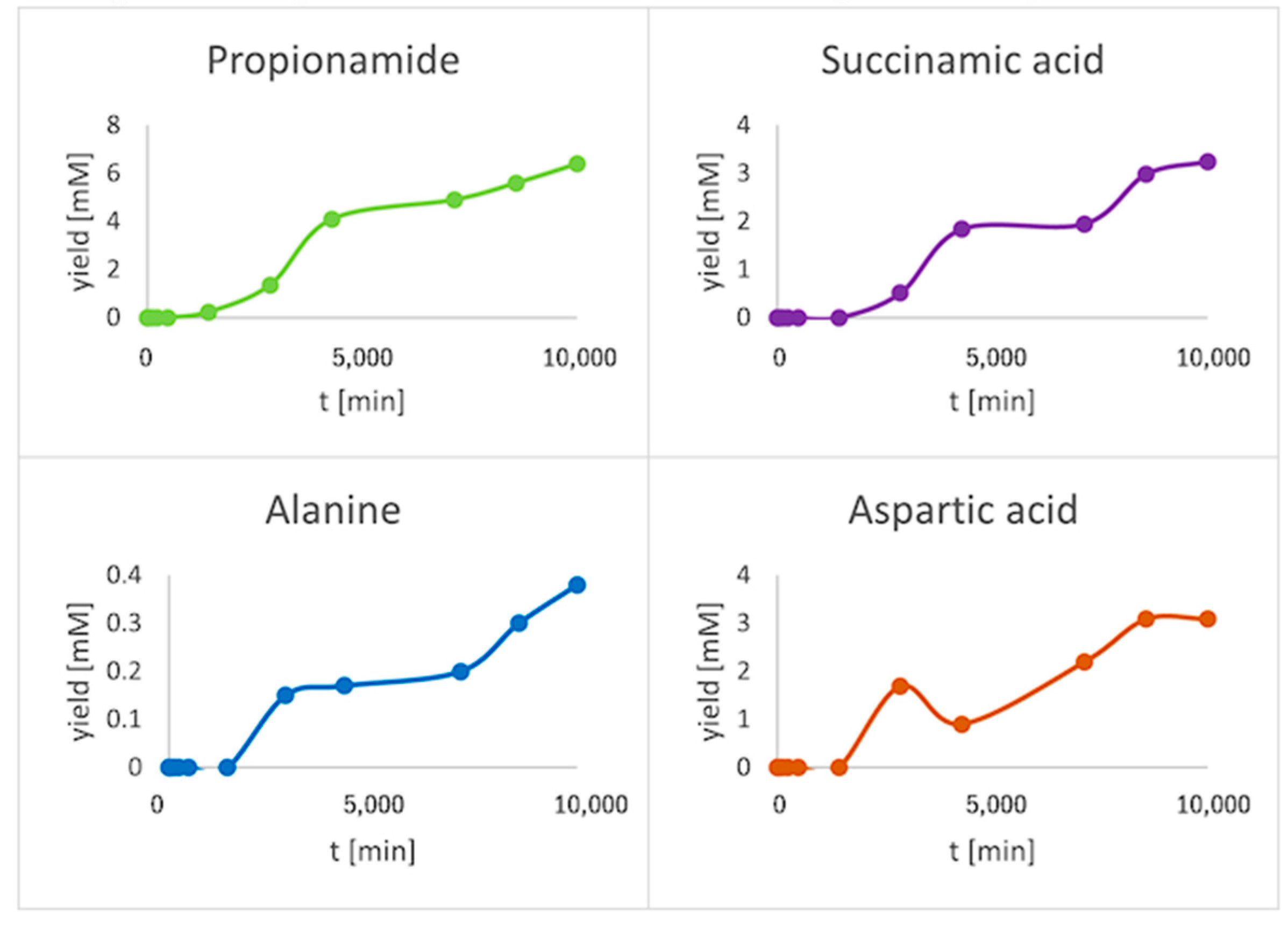
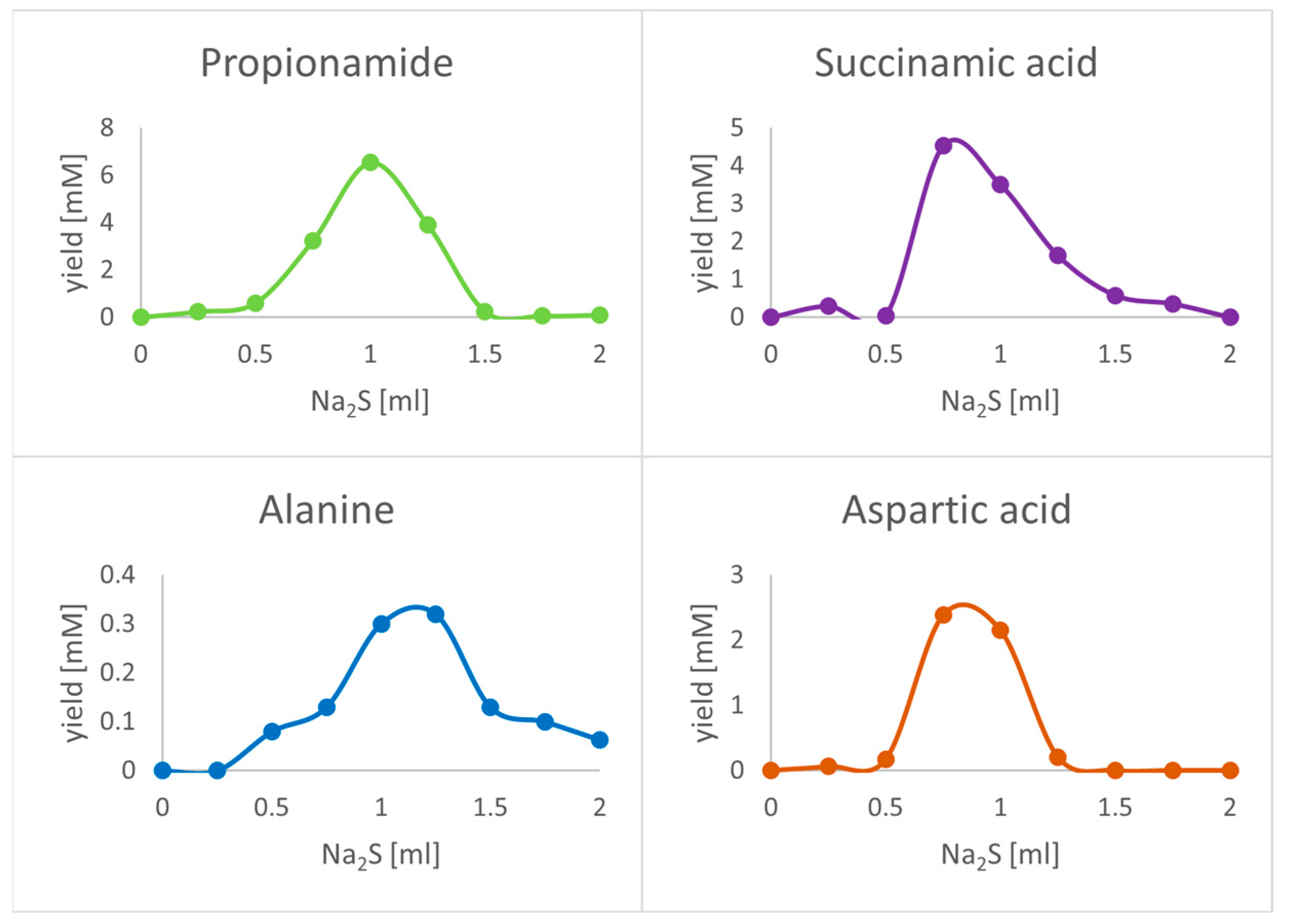
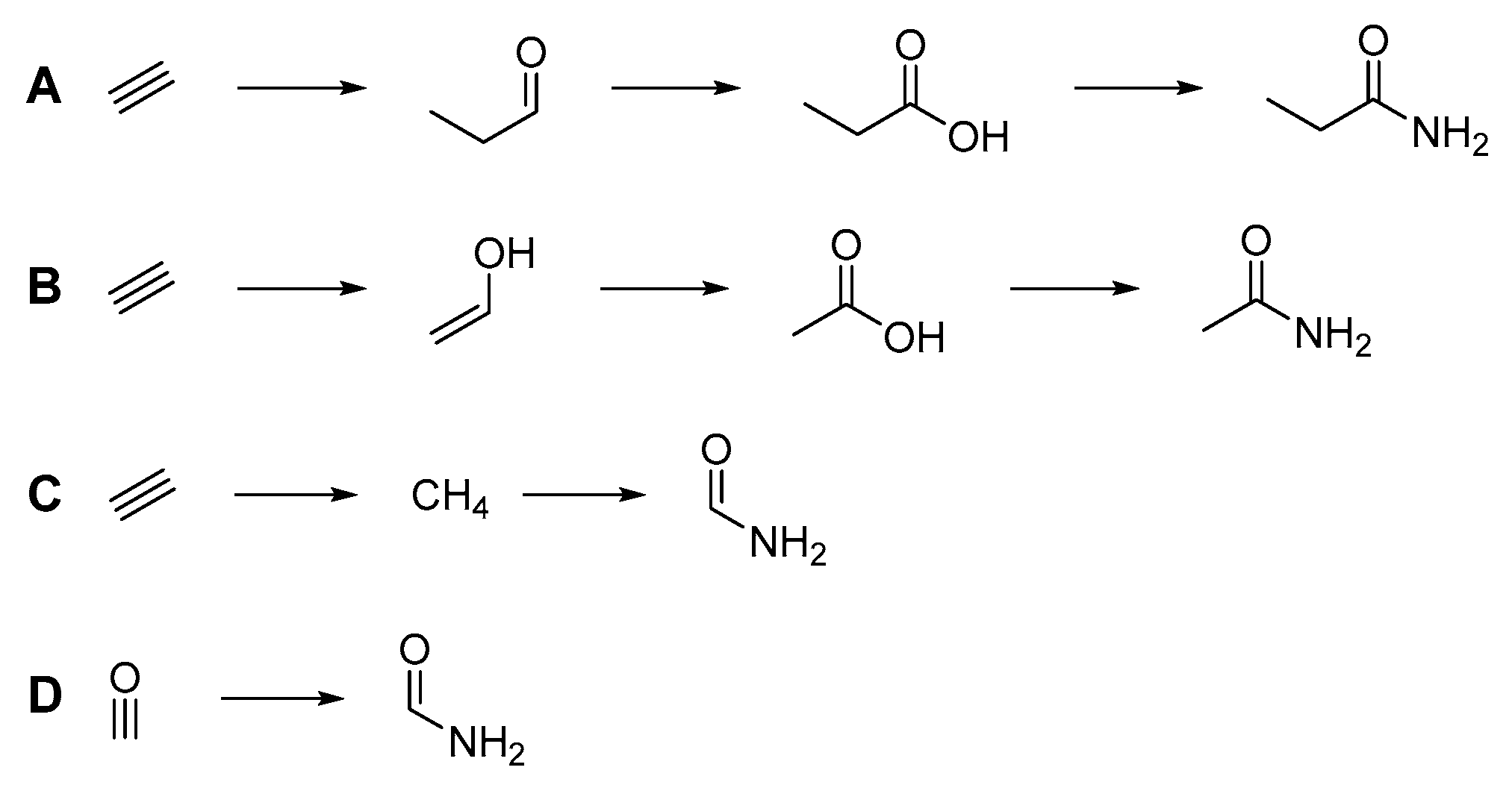

| Compound | # C | # N | 13C2H2 | 15NH4Cl | 13CO |
|---|---|---|---|---|---|
| Amino acids | |||||
| Glycine | 2 | 1 | 2 | 1 | - |
| Alanine | 3 | 1 | 3 | 1 | - |
| β-Alanine | 3 | 1 | 2 | 1 | 1 |
| Aspartic acid | 4 | 1 | 2 | 1 | 2 |
| β-Homoserine | 4 | 1 | 2 | 1 | 2 |
| Amides | |||||
| Formamide | 1 | 1 | 1 | 1 | - |
| Urea | 1 | 2 | - | 2 | 1 |
| Acetamide | 2 | 1 | 2 | 1 | - |
| Acrylamide | 3 | 1 | 2 | 1 | 1 |
| Propionamide | 3 | 1 | 2 | 1 | 1 |
| β-Alanine amide | 3 | 1 | 2 | 2 | 1 |
| Succinamic acid | 4 | 1 | 2 | 1 | 2 |
| Fumaramic acid | 4 | 1 | 2 | 1 | 2 |
| Pentenoic amides | 5 | 1 | 4 | 1 | 1 |
| Pentanoic amide | 5 | 1 | 4 | 1 | 1 |
| 2-Aminobenzamide | 7 | 2 | 6 | 2 | 1 |
| 2,4-Heptadienoic amide | 7 | 1 | 6 | 1 | 1 |
| Benzamide | 7 | 1 | 6 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitz, C.; Geisberger, T.; West, A.R.; Fertl, J.; Eisenreich, W.; Huber, C. From Zero to Hero: The Cyanide-Free Formation of Amino Acids and Amides from Acetylene, Ammonia and Carbon Monoxide in Aqueous Environments in a Simulated Hadean Scenario. Life 2024, 14, 719. https://doi.org/10.3390/life14060719
Seitz C, Geisberger T, West AR, Fertl J, Eisenreich W, Huber C. From Zero to Hero: The Cyanide-Free Formation of Amino Acids and Amides from Acetylene, Ammonia and Carbon Monoxide in Aqueous Environments in a Simulated Hadean Scenario. Life. 2024; 14(6):719. https://doi.org/10.3390/life14060719
Chicago/Turabian StyleSeitz, Christian, Thomas Geisberger, Alexander Richard West, Jessica Fertl, Wolfgang Eisenreich, and Claudia Huber. 2024. "From Zero to Hero: The Cyanide-Free Formation of Amino Acids and Amides from Acetylene, Ammonia and Carbon Monoxide in Aqueous Environments in a Simulated Hadean Scenario" Life 14, no. 6: 719. https://doi.org/10.3390/life14060719
APA StyleSeitz, C., Geisberger, T., West, A. R., Fertl, J., Eisenreich, W., & Huber, C. (2024). From Zero to Hero: The Cyanide-Free Formation of Amino Acids and Amides from Acetylene, Ammonia and Carbon Monoxide in Aqueous Environments in a Simulated Hadean Scenario. Life, 14(6), 719. https://doi.org/10.3390/life14060719









