Different Doses of Methamphetamine Are Needed to Produce Locomotor or Blood Pressure Sensitization in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
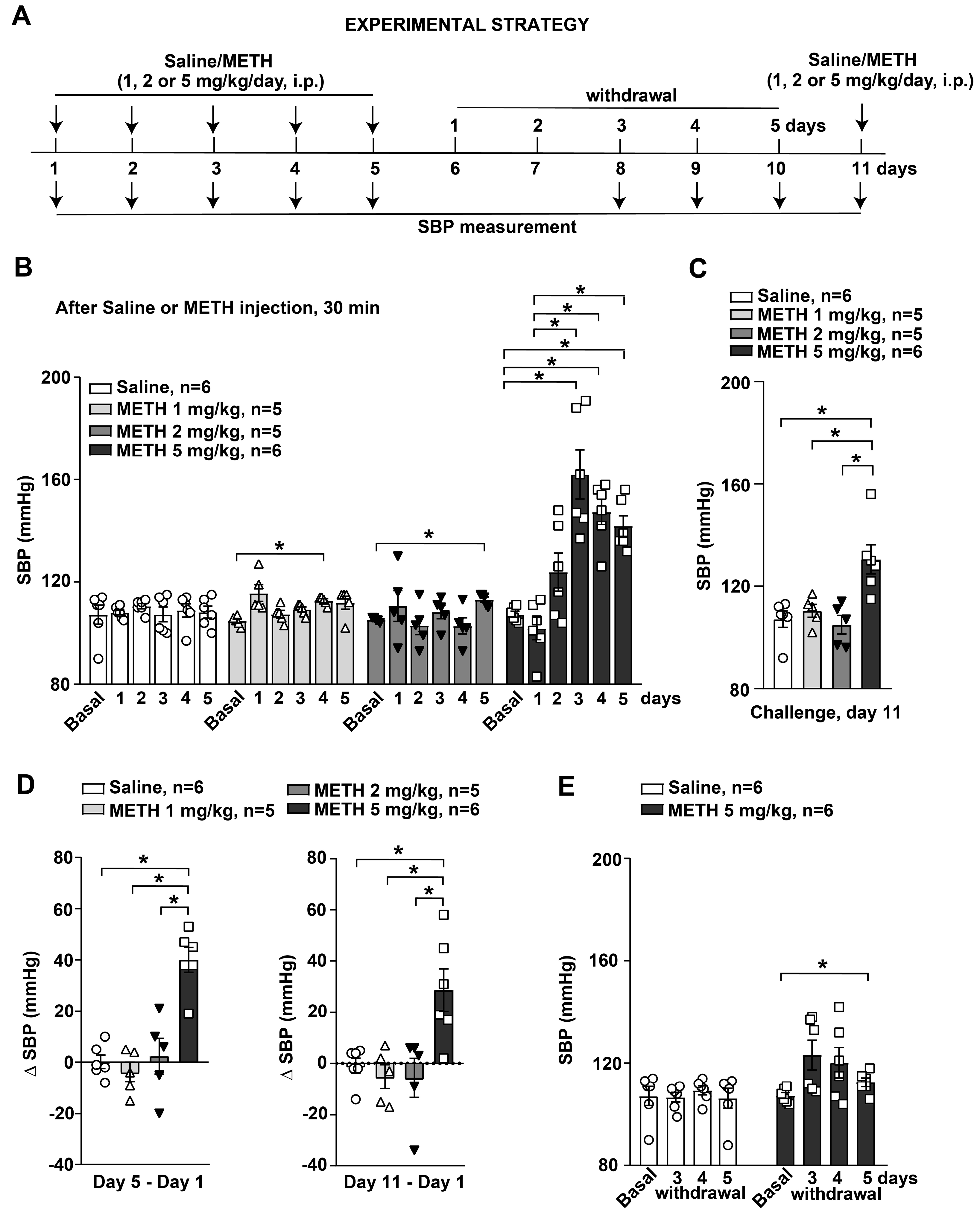
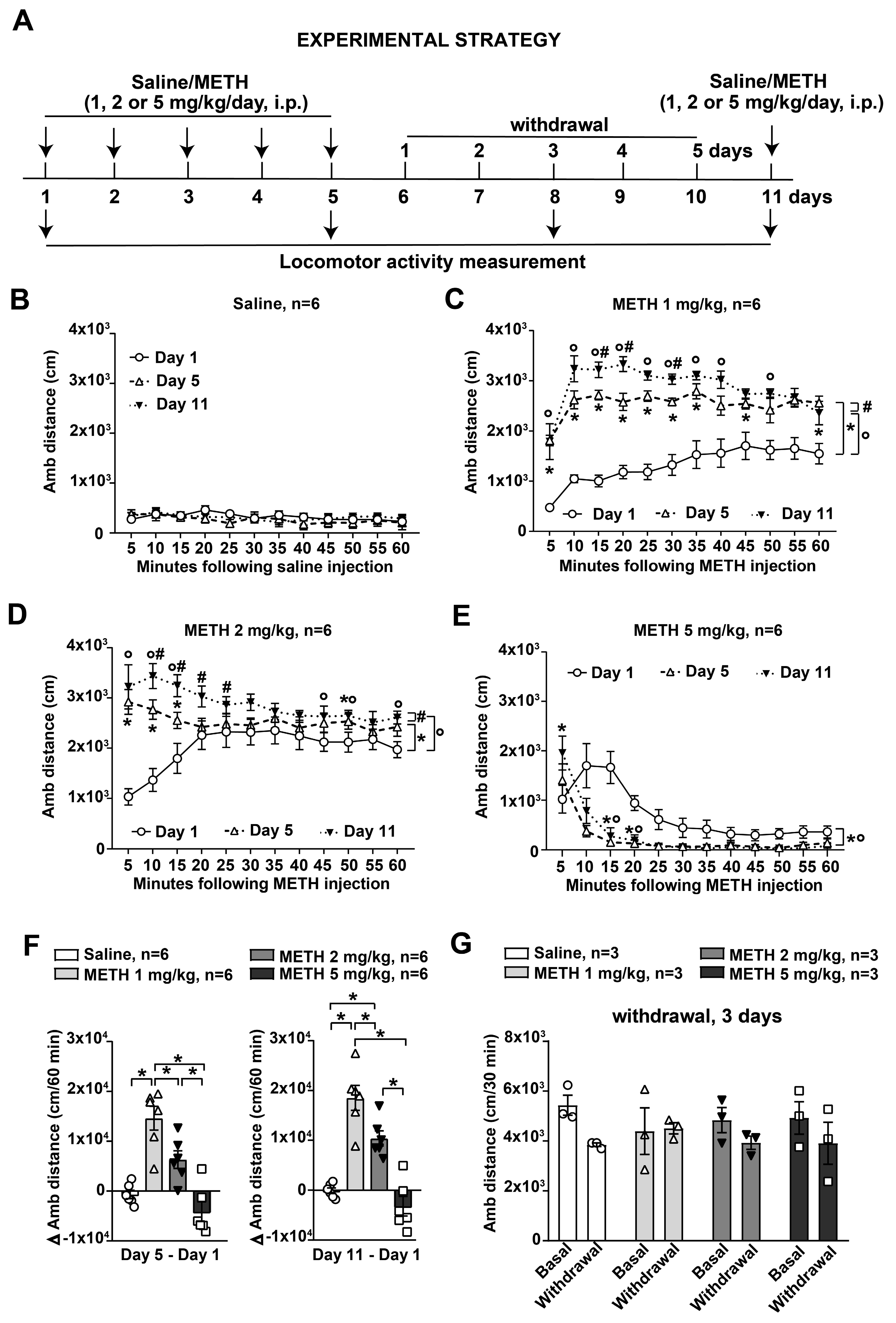
2.3. Experimental Strategy
2.4. Tail-Cuff Plethysmography
2.5. Open Field Test
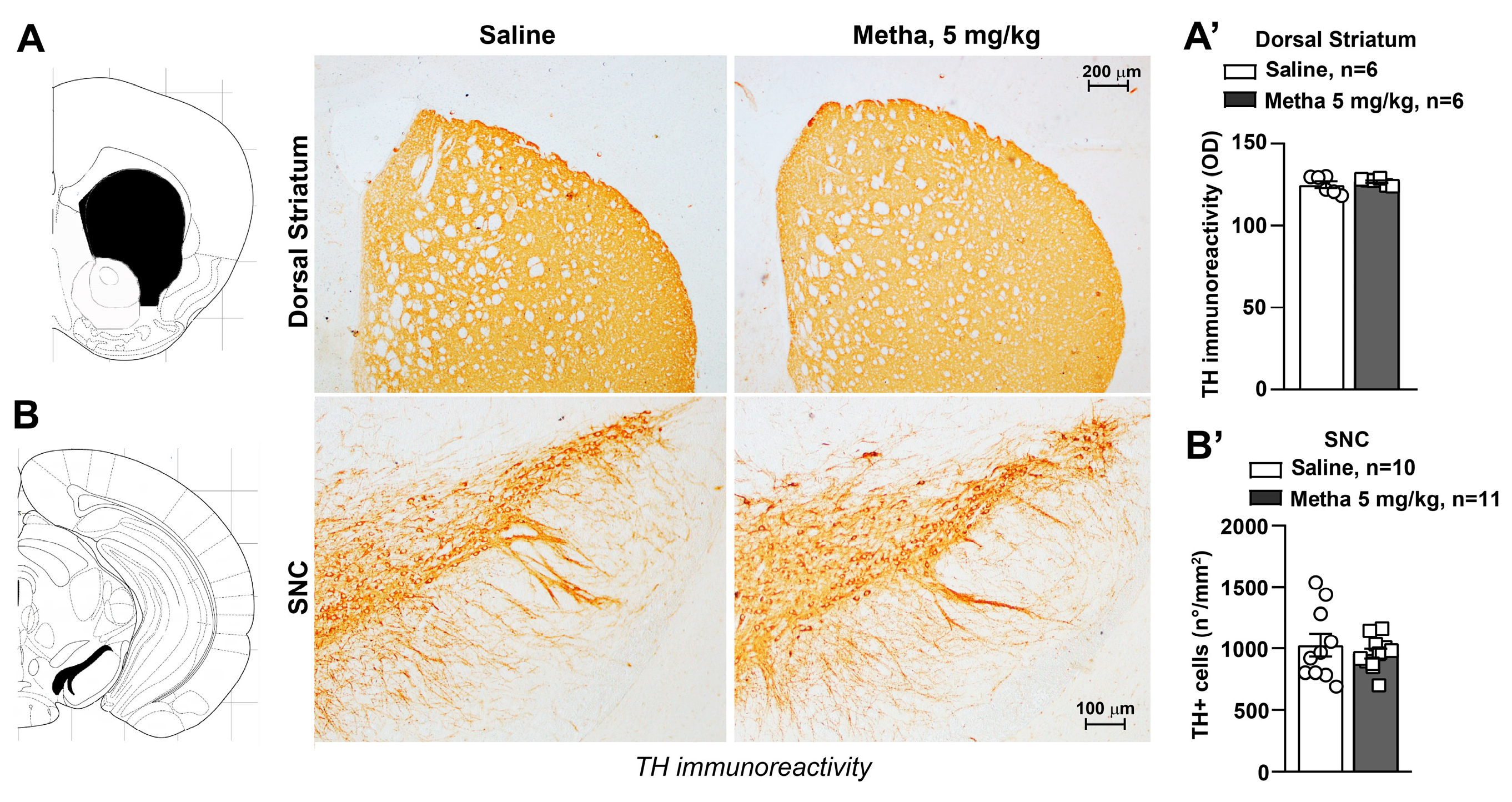
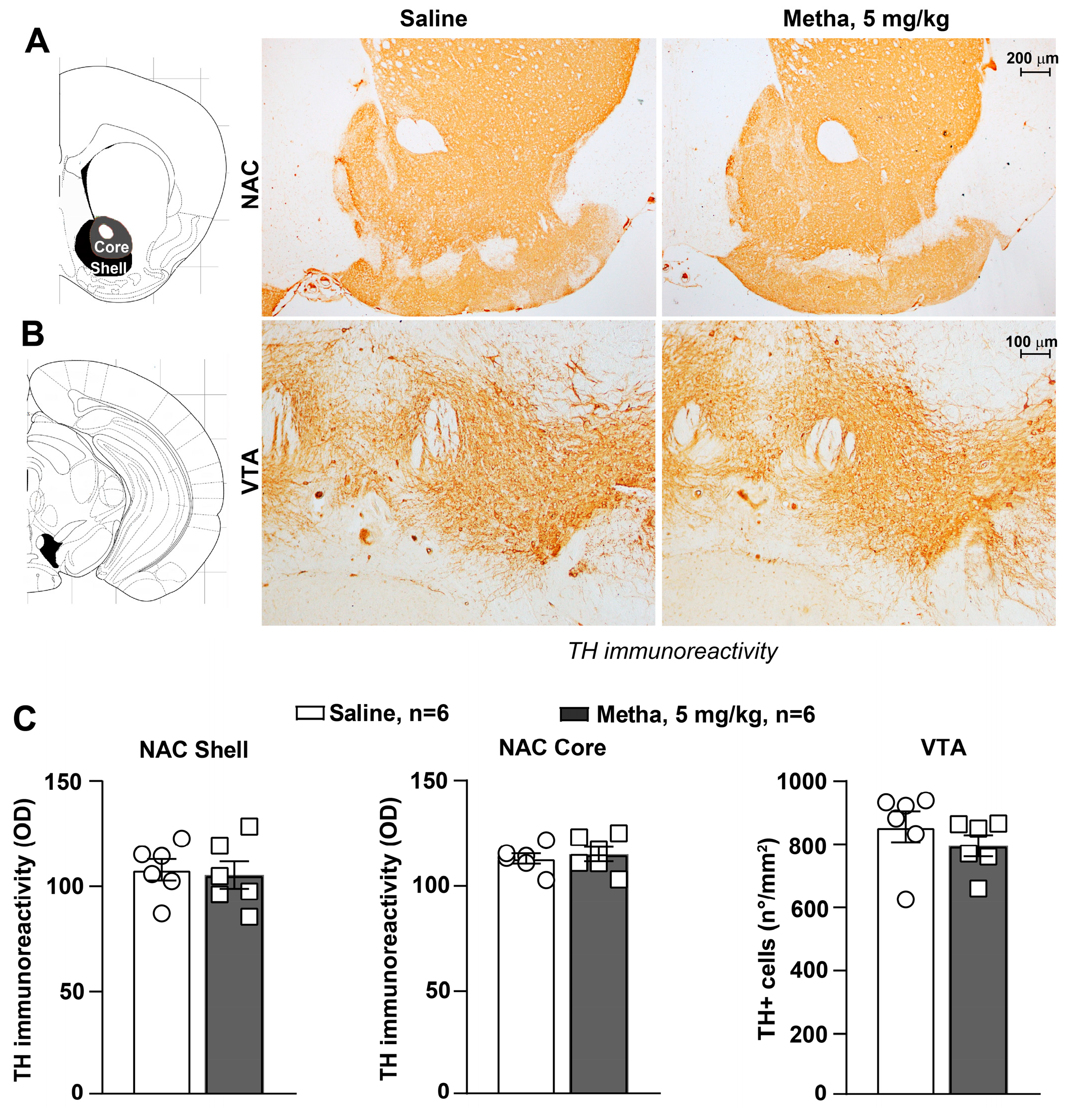
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Repeated Injection with the Highest Dose of METH Induced SBP Sensitization in Mice
3.2. Ambulatory Sensitization in Response to METH
3.3. METH Treatment Led to a Specific Regional Profile of c-fos-Related Neuronal Activation
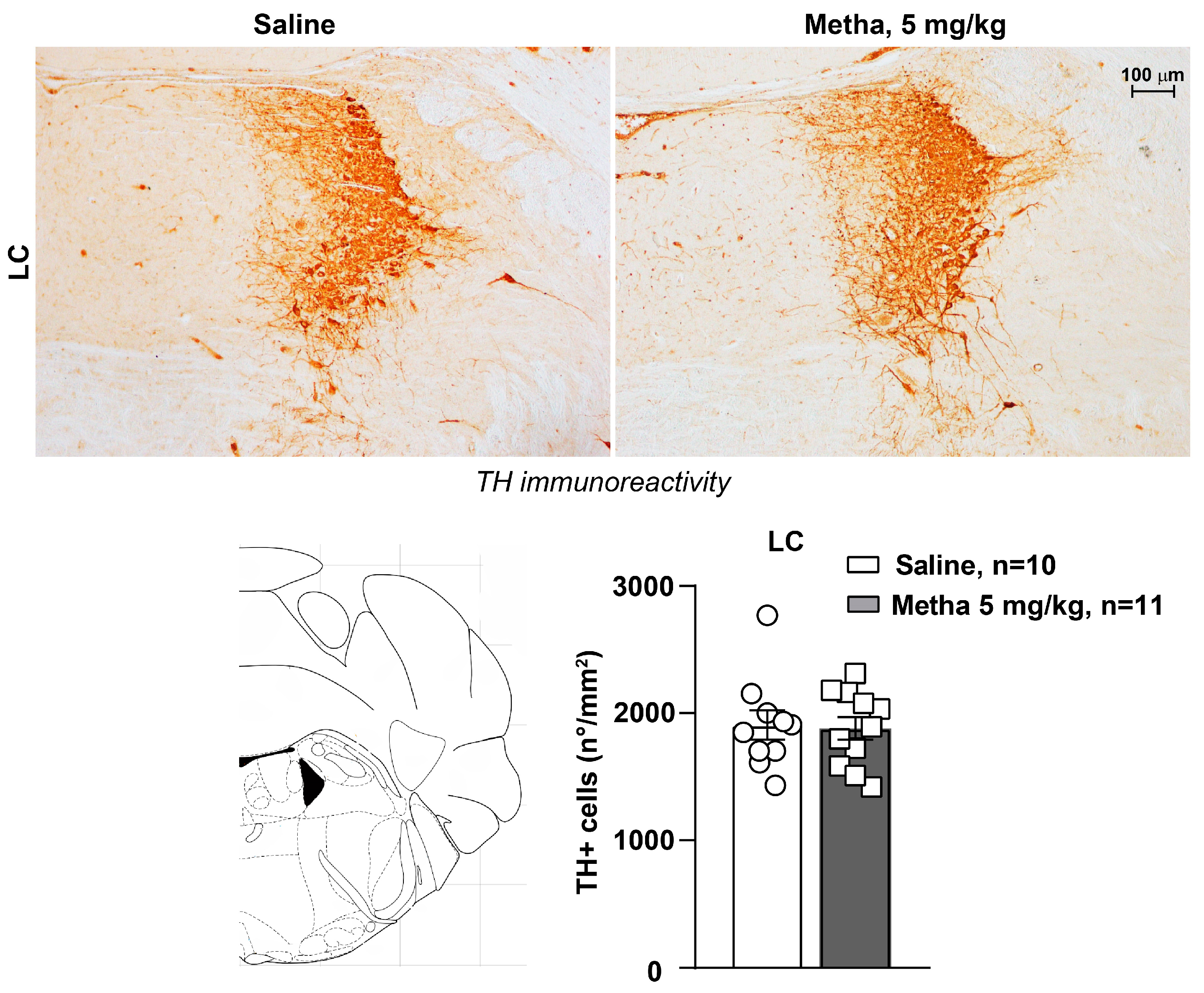
3.4. METH Treatment Did Not Induce Catecholamine Neuron Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellinwood, E.H.; Cohen, S. Amphetamine abuse. Science 1971, 171, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, R.; Khairnar, A.; Simola, N.; Granado, N.; García-Montes, J.R.; Porceddu, P.F.; Tizabi, Y.; Costa, G.; Morelli, M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog. Neurobiol. 2017, 155, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Busceti, C.L.; Celli, R.; Biagioni, F.; Fornai, F. Autophagy as a gateway for the effects of methamphetamine: From neurotransmitter release and synaptic plasticity to psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2021, 204, 102112. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.E.; Birdsall, E.; Seferian, K.S.; Crosby, M.A.; Keefe, K.A.; Gibb, J.W.; Hanson, G.R.; Fleckenstein, A.E. Methamphetamine-induced dopaminergic deficits and refractoriness to subsequent treatment. Eur. J. Pharmacol. 2009, 607, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Dews, P.B. Studies on behavior. IV. Stimulant actions of methamphetamine. J. Pharmacol. Exp. Ther. 1958, 122, 137–147. [Google Scholar]
- Seiden, L.S.; Sabol, K.E.; Ricaurte, G.A. Amphetamine: Effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 639–677. [Google Scholar] [CrossRef]
- Cadet, J.L.; Sheng, P.; Ali, S.; Rothman, R.; Carlson, E.; Epstein, C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J. Neurochem. 1994, 62, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Lenzi, P.; Gesi, M.; Ferrucci, M.; Lazzeri, G.; Capobianco, L.; de Blasi, A.; Battaglia, G.; Nicoletti, F.; Ruggieri, S.; et al. Similarities between methamphetamine toxicity and proteasome inhibition. Ann. N. Y. Acad. Sci. 2004, 1025, 162–170. [Google Scholar] [CrossRef]
- Ricaurte, G.A.; Schuster, C.R.; Seiden, L.S. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: A regional study. Brain Res. 1980, 193, 153–163. [Google Scholar] [CrossRef]
- Seiden, L.S. Methamphetamine: Toxicity to dopaminergic neurons. NIDA Res. Monogr. 1985, 62, 100–116. [Google Scholar]
- Ricaurte, G.A.; McCann, U.D. Neurotoxic amphetamine analogues: Effects in monkeys and implications for humans. Ann. N. Y. Acad. Sci. 1992, 648, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Ritter, J.K.; Sonsalla, P.K.; Hanson, G.R.; Gibb, J.W. Role of dopamine in the neurotoxic effects of methamphetamine. J. Pharmacol. Exp. Ther. 1985, 233, 539–544. [Google Scholar]
- Tirelli, E.; Laviola, G.; Adriani, W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci. Biobehav. Rev. 2003, 27, 163–178. [Google Scholar] [CrossRef]
- Robinson, T.E.; Jurson, P.A.; Bennett, J.A.; Bentgen, K.M. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: A microdialysis study in freely moving rats. Brain Res. 1988, 462, 211–222. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Effects of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 1990, 5, 48–58. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Time-course of extracellular dopamine and behavioral sensitization to cocaine (I) dopamine axon terminals. J. Neurosci. 1993, 13, 266–275. [Google Scholar] [CrossRef]
- Paulson, P.E.; Robinson, T.E. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: A microdialysis study in behaving rats. Synapse 1995, 19, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Polesskaya, O.; Silva, J.; Sanfilippo, C.; Desrosiers, T.; Sun, A.; Shen, J.; Feng, C.; Polesskiy, A.; Deane, R.; Zlokovic, B.; et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011, 1373, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Fang, C.; Kuo, C.Y.; Wu, Y.W.; Lin, H.H. Activation of mGluR5 and NMDA receptor pathways in the rostral ventrolateral medulla as a central mechanism for methamphetamine-induced pressor effect in rats. Biomolecules 2020, 10, 149. [Google Scholar] [CrossRef]
- Stek, A.M.; Fisher, B.K.; Baker, R.S.; Lang, U.; Tseng, C.Y.; Clark, K.E. Maternal and fetal cardiovascular responses to methamphetamine in the pregnant sheep. Am. J. Obstet. Gynecol. 1993, 169, 888–897. [Google Scholar] [CrossRef]
- Schindler, C.W.; Zheng, J.W.; Tella, S.R.; Goldberg, S.R. Pharmacological mechanisms in the cardiovascular effects of methamphetamine in conscious squirrel monkeys. Pharmacol. Biochem. Behav. 1992, 42, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010, 48, 675–694. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Chen, J.H.; Lee, M.H.; Deng, J.F. Fatal and nonfatal methamphetamine intoxication in the intensive care unit. J. Toxicol. Clin. Toxicol. 1994, 32, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Darke, S.; Duflou, J.; McKetin, R. Methamphetamine-related fatalities in Australia: Demographics, circumstances, toxicology and major organ pathology. Addiction 2008, 103, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Sato, K.; Kashiwade, H.; Yamanami, S.; Zhou, H.; Yonemura, I.; Nakajima, H.; Hasekura, H. Sudden death due presumably to internal use of methamphetamine. Forensic Sci. Int. 1993, 62, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Colfax, G.; Shoptaw, S. The methamphetamine epidemic: Implications for HIV prevention and treatment. Curr. HIV/AIDS Rep. 2005, 2, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.A., Jr.; Arsura, E.L.; Strategos, S. Methamphetamine-related stroke: Four cases. J. Emerg. Med. 1999, 17, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Pilski, A.; Graves, S.M. Repeated methamphetamine administration results in axon loss prior to somatic loss of substantia nigra pars compacta and locus coeruleus neurons in male but not female mice. Int. J. Mol. Sci. 2023, 24, 13039. [Google Scholar] [CrossRef]
- Singewald, N.; Kaehler, S.T.; Philippu, A. Noradrenaline release in the locus coeruleus of conscious rats is triggered by drugs, stress and blood pressure changes. Neuroreport 1999, 10, 1583–1587. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Stornetta, R.L.; Bochorishvili, G.; Depuy, S.D.; Burke, P.G.; Abbott, S.B. C1 neurons: The body’s EMTs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R187–R204. [Google Scholar] [CrossRef]
- Holloway, B.B.; Stornetta, R.L.; Bochorishvili, G.; Erisir, A.; Viar, K.E.; Guyenet, P.G. Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J. Neurosci. 2013, 33, 18792–18805. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, N.; Kitanaka, J.; Takemura, M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur. J. Pharmacol. 2003, 474, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Chen, Y.; Zhu, J.; Wang, L.; Cao, G.; Dang, Y.; Yan, C.; Wang, J.; Chen, T. Levo-tetrahydropalmatine attenuates the development and expression of methamphetamine-induced locomotor sensitization and the accompanying activation of ERK in the nucleus accumbens and caudate putamen in mice. Neuroscience 2014, 258, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Pang, K.; Liu, M.; Tan, X.; Hang, Z.; Mu, S.; Han, W.; Yue, Q.; Comai, S.; Sun, J. Activation of Kv7 channels normalizes hyperactivity of the VTA-NAcLat circuit and attenuates methamphetamine-induced conditioned place preference and sensitization in mice. Mol. Psychiatry 2023, 28, 5183–5194. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Raineri, M.; Cadet, J.L.; García-Rill, E.; Urbano, F.J.; Bisagno, V. Modafinil improves methamphetamine-induced object recognition deficits and restores prefrontal cortex ERK signaling in mice. Neuropharmacology 2014, 87, 188–197. [Google Scholar] [CrossRef] [PubMed]
- González, B.; González, C.; Bisagno, V.; Urbano, F.J. Effects of methamphetamine on locomotor activity and thalamic gene expression in leptin-deficient obese mice. Transl. Brain Rhythm. 2017, 2, 10.15761/TBR.1000112. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, H.; Yu, S.J.; Chen, Y.H.; Wang, Y. Alleviation of methamphetamine sensitization by partially lesioning dopaminergic terminals with 6-hydroxydopamine in nucleus accumbens. Cell Transplant. 2021, 30, 9636897211052300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.N.; Wu, S.J.; Huang, A.C.W. Environmental enrichment components required to reduce methamphetamine-induced behavioral sensitization in mice: Examination of behaviors and neural substrates. J. Clin. Med. 2022, 11, 3051. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Greenberg, M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4, 477–485. [Google Scholar] [CrossRef]
- Carrizzo, A.; Lenzi, P.; Procaccini, C.; Damato, A.; Biagioni, F.; Ambrosio, M.; Amodio, G.; Remondelli, P.; Del Giudice, C.; Izzo, R.; et al. Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway. Circulation 2015, 131, 1495–1505. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Cattaneo, F.; Carrizzo, A.; Paolillo, R.; Boccella, N.; Ambrosio, M.; Damato, A.; Pironti, G.; Franzone, A.; Russo, G.; et al. Akap1 regulates vascular function and endothelial cells behavior. Hypertension 2018, 71, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, F.; Ferese, R.; Limanaqi, F.; Madonna, M.; Lenzi, P.; Gambardella, S.; Fornai, F. Methamphetamine persistently increases alpha-synuclein and suppresses gene promoter methylation within striatal neurons. Brain Res. 2019, 1719, 157–175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busceti, C.L.; Bucci, D.; De Lucia, M.; Ferrucci, M.; Scioli, M.; Carrizzo, A.; Nicoletti, F.; Vecchione, C.; Fornai, F. Different Doses of Methamphetamine Are Needed to Produce Locomotor or Blood Pressure Sensitization in Mice. Life 2024, 14, 723. https://doi.org/10.3390/life14060723
Busceti CL, Bucci D, De Lucia M, Ferrucci M, Scioli M, Carrizzo A, Nicoletti F, Vecchione C, Fornai F. Different Doses of Methamphetamine Are Needed to Produce Locomotor or Blood Pressure Sensitization in Mice. Life. 2024; 14(6):723. https://doi.org/10.3390/life14060723
Chicago/Turabian StyleBusceti, Carla Letizia, Domenico Bucci, Massimiliano De Lucia, Michela Ferrucci, Mariarosaria Scioli, Albino Carrizzo, Ferdinando Nicoletti, Carmine Vecchione, and Francesco Fornai. 2024. "Different Doses of Methamphetamine Are Needed to Produce Locomotor or Blood Pressure Sensitization in Mice" Life 14, no. 6: 723. https://doi.org/10.3390/life14060723
APA StyleBusceti, C. L., Bucci, D., De Lucia, M., Ferrucci, M., Scioli, M., Carrizzo, A., Nicoletti, F., Vecchione, C., & Fornai, F. (2024). Different Doses of Methamphetamine Are Needed to Produce Locomotor or Blood Pressure Sensitization in Mice. Life, 14(6), 723. https://doi.org/10.3390/life14060723









