The Surgical Management of Severe Scoliosis in Immature Patient with a Very Rare Disease Costello Syndrome—Clinical Example and Brief Literature Review
Abstract
1. Introduction
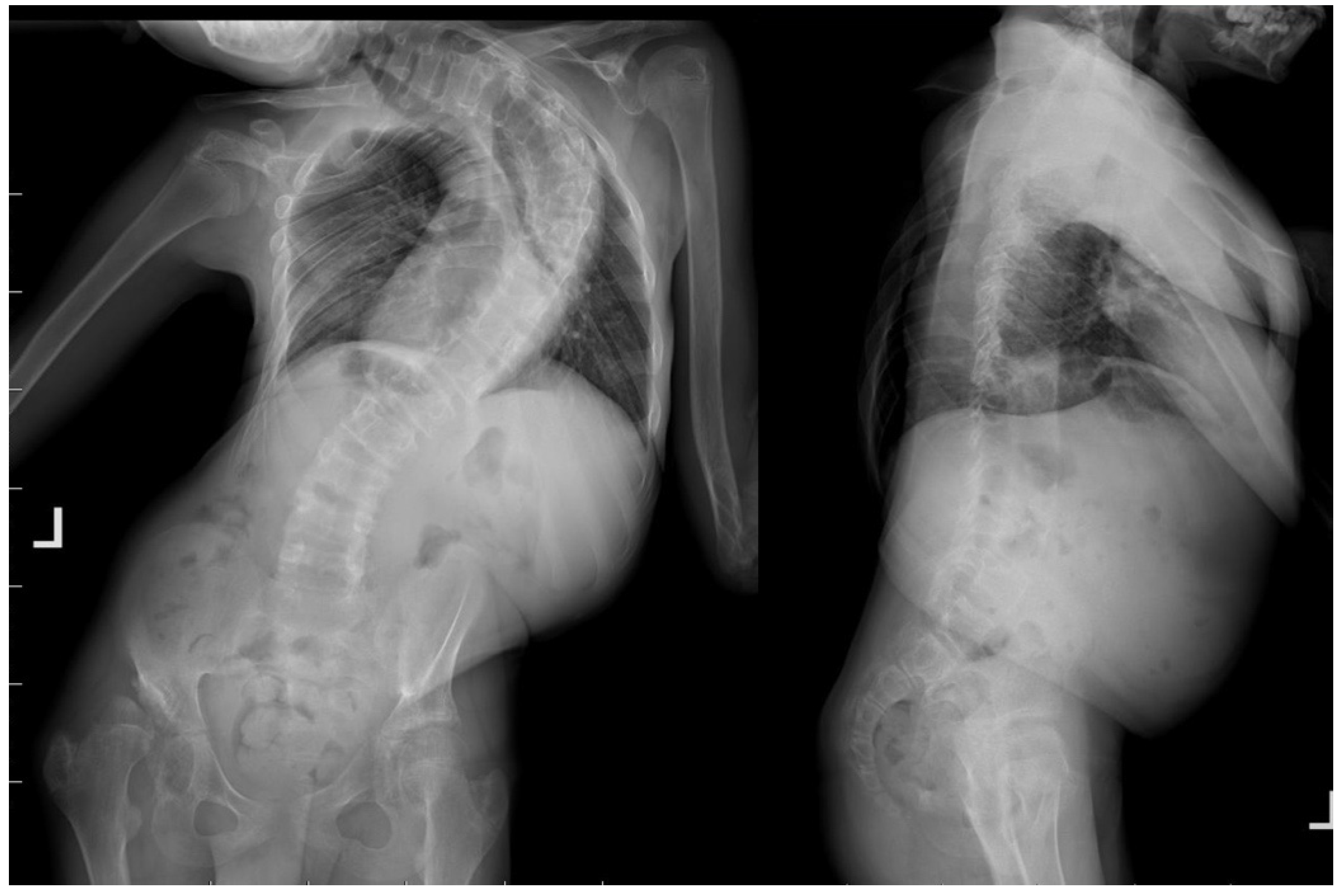
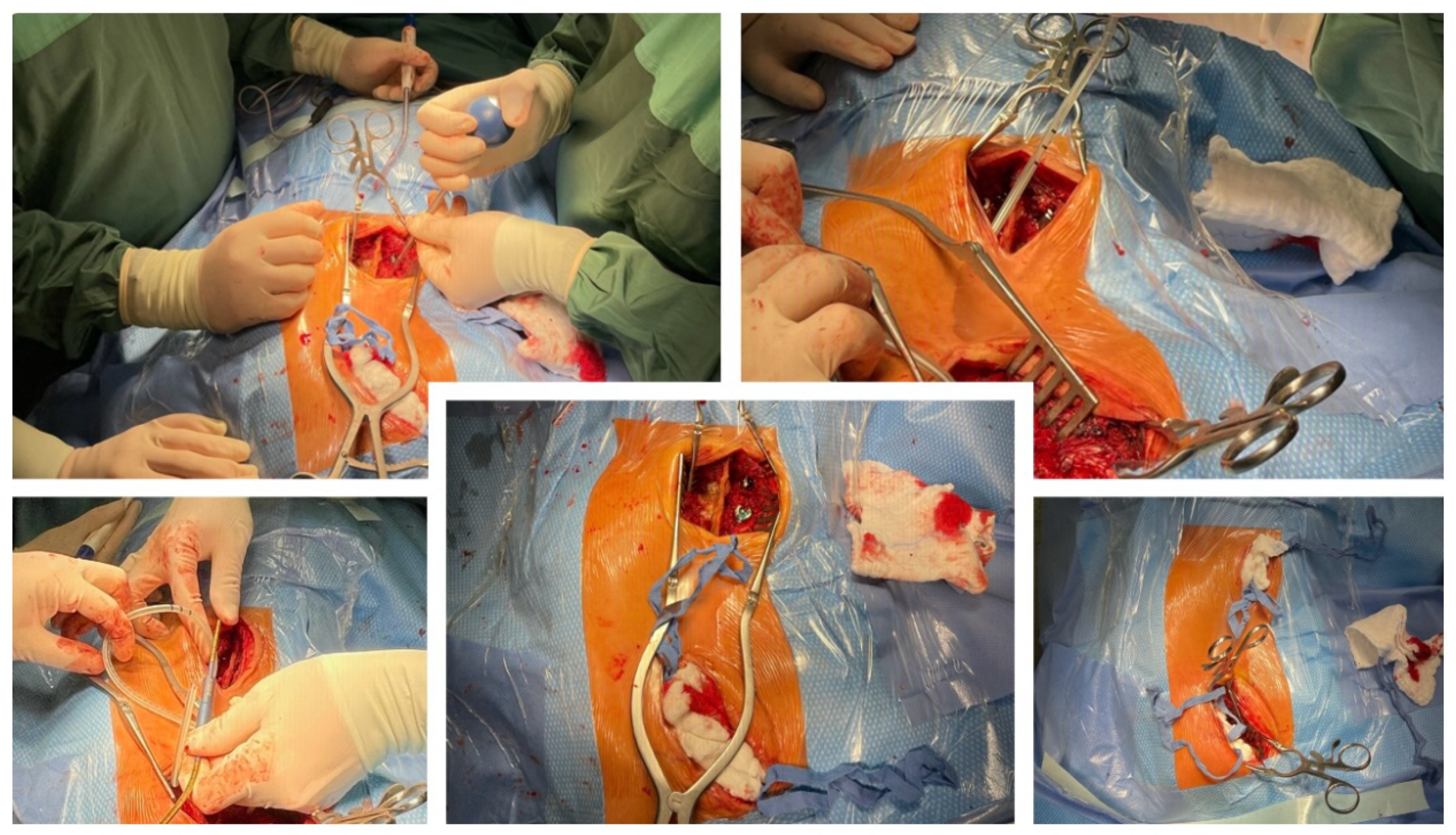
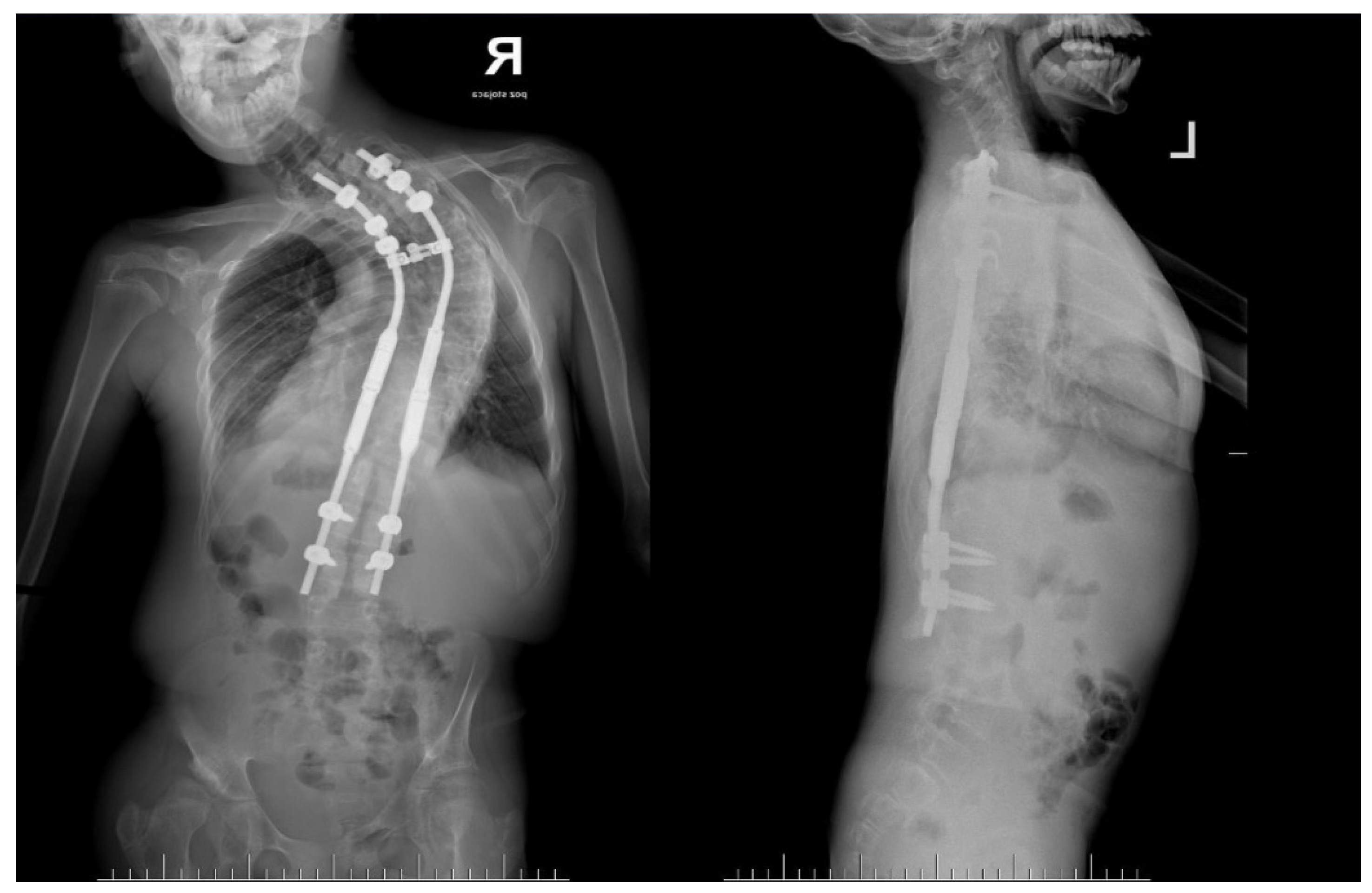
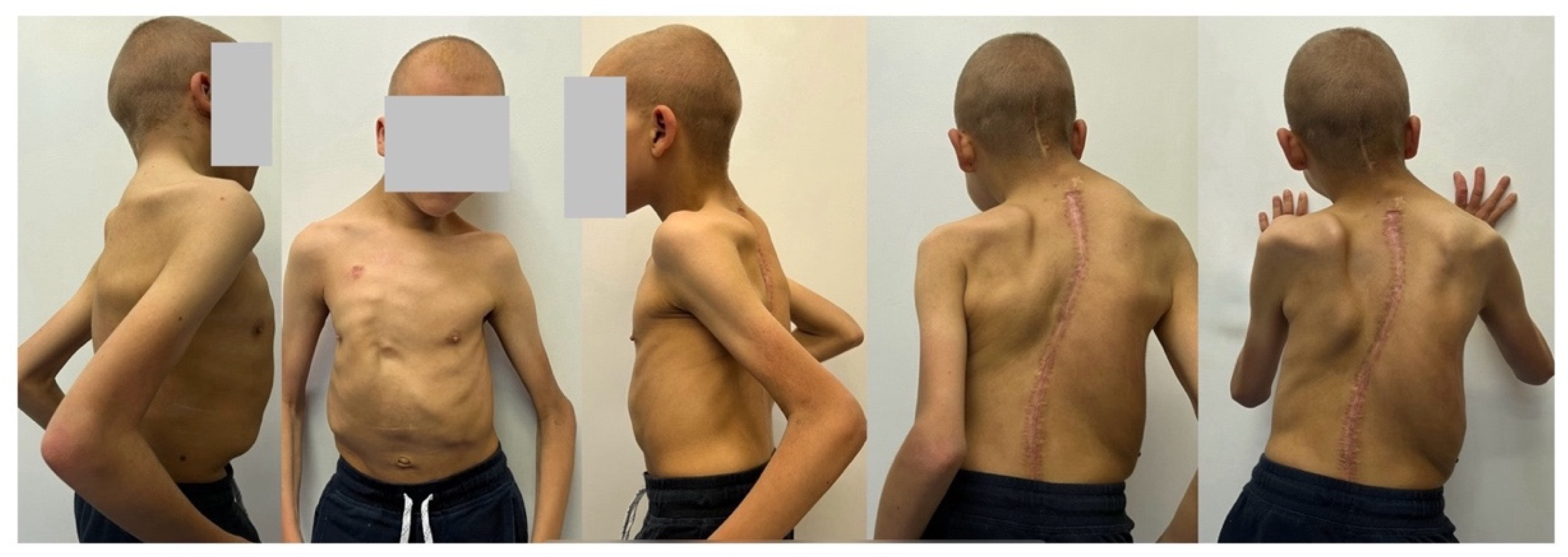
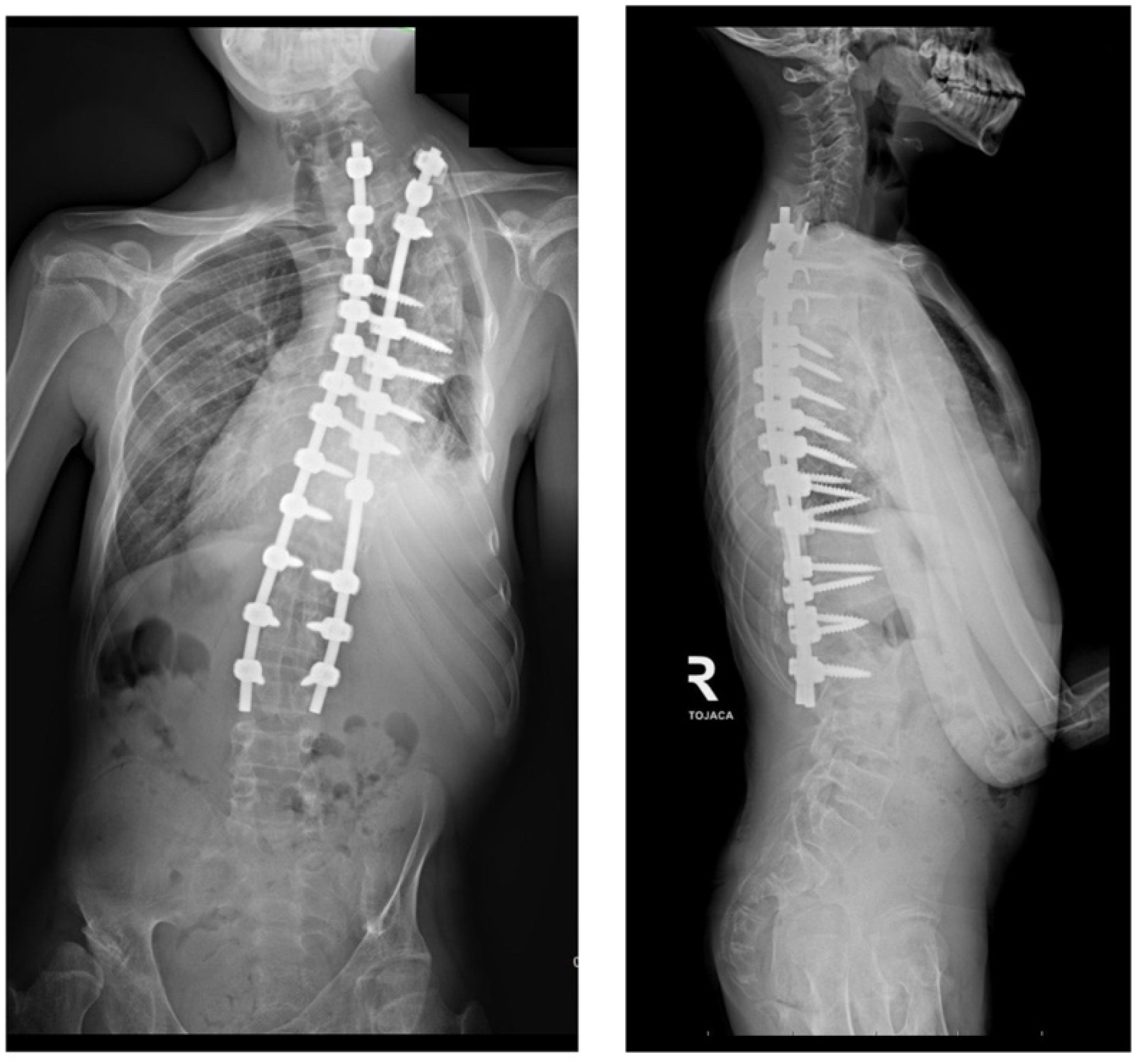
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leoni, C.; Viscogliosi, G.; Tartaglia, M.; Aoki, Y.; Zampino, G. Multidisciplinary Management of Costello Syndrome: Current Perspectives. J. Multidiscip. Healthc. 2022, 15, 1277–1296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rauen, K.A. The RASopathies. Annu. Rev. Genomics Hum. Genet. 2013, 14, 355–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leoni, C.; Blandino, R.; Delogu, A.B.; De Rosa, G.; Onesimo, R.; Verusio, V.; Marino, M.V.; Lanza, G.A.; Rigante, D.; Tartaglia, M.; et al. Genotype-cardiac phenotype correlations in a large single-center cohort of patients affected by RASopathies: Clinical implications and literature review. Am. J. Med. Genet. A 2022, 188, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.M. A new syndrome: Mental subnormality and nasal papillomata. Aust. Paediatr. J. 1977, 13, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Aoki, Y.; Kuriyama, S.; Kawame, H.; Okamoto, N.; Kurosawa, K.; Ohashi, H.; Mizuno, S.; Ogata, T.; Kure, S.; et al. Costello and CFC syndrome study group in Japan. Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: Findings from a nationwide epidemiological survey. Am. J. Med. Genet. A 2012, 158A, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Morse, L.A.; Axelrad, M.; Chatfield, K.C.; Chidekel, A.; Dobyns, W.; Doyle, D.; Kerr, B.; Lin, A.E.; Schwartz, D.D.; et al. Costello syndrome: Clinical phenotype, genotype, and management guidelines. Am. J. Med. Genet. A 2019, 179, 1725–1744. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tidyman, W.E.; Rauen, K.A. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 2009, 19, 230–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aoki, Y.; Niihori, T.; Kawame, H.; Kurosawa, K.; Ohashi, H.; Tanaka, Y.; Filocamo, M.; Kato, K.; Suzuki, Y.; Kure, S.; et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat. Genet. 2005, 37, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, S.; Thacker, M.M.; Hopkins, E.; Conway, L.; Gripp, K.W. Orthopedic manifestations and implications for individuals with Costello syndrome. Am. J. Med. Genet. A 2013, 161A, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.A.; Viscogliosi, G.; Leoni, C. Bone health in RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Shikany, A.R.; Baker, L.; Stabley, D.L.; Robbins, K.; Doyle, D.; Gripp, K.W.; Weaver, K.N. Medically actionable comorbidities in adults with Costello syndrome. Am. J. Med. Genet. A 2020, 182, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Chennappan, S.; Andrasch, Y.; Fidan, M.; Engler, M.; Ahmad, M.; Tuckermann, J.P.; Zenker, M.; Cirstea, I.C. Increased osteoclastogenesis contributes to bone loss in the Costello syndrome Hras G12V mouse model. Front. Cell Dev. Biol. 2022, 10, 1000575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Motosuneya, T.; Asazuma, T.; Tsuji, T.; Watanabe, H.; Nakayama, Y.; Nemoto, K. Severe scoliosis associated with Costello syndrome: A case report. J. Orthop. Surg. 2006, 14, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Rocos, B.; Taira, K.; Nemoto, N.; Oikawa, N.; Ohashi, H.; Machida, M.; Kinoshita, T.; Kamata, Y.; Nakanishi, K. Costello syndrome-associated orthopaedic manifestations focussed on kyphoscoliosis: A case series describing the natural course. J. Pediatr. Orthop. B 2023, 32, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Bisanti, C.; Viscogliosi, G.; Onesimo, R.; Massese, M.; Giorgio, V.; Corbo, F.; Acampora, A.; Cipolla, C.; Flex, E.; et al. Bone tissue homeostasis and risk of fractures in Costello syndrome: A 4-year follow-up study. Am. J. Med. Genet. A 2022, 188, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Grabala, P. Minimally Invasive Controlled Growing Rods for the Surgical Treatment of Early-Onset Scoliosis—A Surgical Technique Video. J. Pers. Med. 2024, 14, 548. [Google Scholar] [CrossRef]
- Chamberlin, K.; Galgano, M.; Grabala, P. Magnetically Controlled Growing Rods for Early-Onset Scoliosis: 2-Dimensional Operative Video. Oper. Neurosurg. 2023, 25, e279. [Google Scholar] [CrossRef] [PubMed]
- Grabala, P.; Helenius, I.J.; Chamberlin, K.; Galgano, M. Less-Invasive Approach to Early-Onset Scoliosis-Surgical Technique for Magnetically Controlled Growing Rod (MCGR) Based on Treatment of 2-Year-Old Child with Severe Scoliosis. Children 2023, 10, 555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grabala, P.; Chamberlin, K.; Grabala, M.; Galgano, M.A.; Helenius, I.J. No Benefits in Using Magnetically Controlled Growing Rod as Temporary Internal Distraction Device in Staged Surgical Procedure for Management of Severe and Neglected Scoliosis in Adolescents. J. Clin. Med. 2023, 12, 5352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocos, B.; Reda, L.; Lebel, D.E.; Dodds, M.K.; Zeller, R. The Use of Halo Gravity Traction in Severe, Stiff Scoliosis. J. Pediatr. Orthop. 2021, 41, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Koller, H.; Zenner, J.; Gajic, V.; Meier, O.; Ferraris, L.; Hitzl, W. The impact of halo-gravity traction on curve rigidity and pulmonary function in the treatment of severe and rigid scoliosis and kyphoscoliosis: A clinical study and narrative review of the literature. Eur. Spine J. 2012, 21, 514–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McIntosh, A.L.; Ramo, B.S.; Johnston, C.E. Halo Gravity Traction for Severe Pediatric Spinal Deformity: A Clinical Concepts Review. Spine Deform. 2019, 7, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Grabala, P.; Helenius, I.J. Clinical and Radiological Outcomes of Less Invasive Temporary Internal Distraction Followed by Staged Pedicle Screw Instrumentation in Adolescents with Severe Idiopathic Scoliosis at 2-Year Minimum Follow-Up. World Neurosurg. 2020, 143, e464–e473. [Google Scholar] [CrossRef] [PubMed]
- Grabala, P.; Kowalski, P.; Grabala, M. The Influence of Increased Pedicle Screw Diameter and Thicker Rods on Surgical Results in Adolescents Undergoing Posterior Spinal Fusion for Idiopathic Scoliosis. J. Clin. Med. 2024, 13, 2174. [Google Scholar] [CrossRef]
- Teixeira da Silva, L.E.; de Barros, A.G.; de Azevedo, G.B. Management of severe and rigid idiopathic scoliosis. Eur. J. Orthop. Surg. Traumatol. 2015, 25 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehrpour, S.; Sorbi, R.; Rezaei, R.; Mazda, K. Posterior-only surgery with preoperative skeletal traction for management of severe scoliosis. Arch. Orthop. Trauma. Surg. 2017, 137, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; O’Brien, B.; Demmer, L.A.; Almeda, K.K.; Blanco, C.L.; Glasow, P.F.; Berul, C.I.; Hamilton, R.; Micheil Innes, A.; Lauzon, J.L.; et al. Prenatal features of Costello syndrome: Ultrasonographic findings and atrial tachycardia. Prenat. Diagn. 2009, 29, 682–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gripp, K.W.; Rauen, K.A. Costello Syndrome; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2006; pp. 1993–2023. [Google Scholar] [PubMed]
- Leoni, C.; Onesimo, R.; Giorgio, V.; Diamanti, A.; Giorgio, D.; Martini, L.; Rossodivita, A.; Tartaglia, M.; Zampino, G. Understanding Growth Failure in Costello Syndrome: Increased Resting Energy Expenditure. J. Pediatr. 2016, 170, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Giorgio, V.; Onesimo, R.; Kuczynska, E.; Zampino, G. Impact of Costello syndrome on growth patterns. Am. J. Med. Genet. A 2020, 182, 2797–2799. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.L.; Thrailkill, K.M.; Bunn, R.C. RASopathies: The musculoskeletal consequences and their etiology and pathogenesis. Bone 2021, 152, 116060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Axelrad, M.E.; Schwartz, D.D.; Fehlis, J.E.; Hopkins, E.; Stabley, D.L.; Sol-Church, K.; Gripp, K.W. Longitudinal course of cognitive, adaptive, and behavioral characteristics in Costello syndrome. Am. J. Med. Genet. A 2009, 149A, 2666–2672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, D.D.; Katzenstein, J.M.; Highley, E.J.; Stabley, D.L.; Sol-Church, K.; Gripp, K.W.; Axelrad, M.E. Age-related differences in prevalence of autism spectrum disorder symptoms in children and adolescents with Costello syndrome. Am. J. Med. Genet. A 2017, 173, 1294–1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Axelrad, M.E.; Glidden, R.; Nicholson, L.; Gripp, K.W. Adaptive skills, cognitive, and behavioral characteristics of Costello syndrome. Am. J. Med. Genet. A 2004, 128A, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.P.; Fallurin, R.; Watson, T.; Shankar, P.R.; Young, T.L.; Orel-Bixler, D.; Rauen, K.A. Ophthalmic manifestations in Costello syndrome caused by Ras pathway dysregulation during development. Ophthalmic Genet. 2022, 43, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Alexander, M.E.; Colan, S.D.; Kerr, B.; Rauen, K.A.; Noonan, J.; Baffa, J.; Hopkins, E.; Sol-Church, K.; Limongelli, G.; et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: A Ras/MAPK pathway syndrome. Am. J. Med. Genet. A 2011, 155A, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ospina, N.; Kuo, C.; Ananth, A.L.; Myers, A.; Brennan, M.L.; Stevenson, D.A.; Bernstein, J.A.; Hudgins, L. Respiratory system involvement in Costello syndrome. Am. J. Med. Genet. A 2016, 170, 1849–1857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hebron, K.E.; Hernandez, E.R.; Yohe, M.E. The RASopathies: From pathogenetics to therapeutics. Dis. Model. Mech. 2022, 15, dmm049107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gelb, B.D.; Yohe, M.E.; Wolf, C.; Andelfinger, G. New prospectives on treatment opportunities in RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 541–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lioncino, M.; Monda, E.; Verrillo, F.; Moscarella, E.; Calcagni, G.; Drago, F.; Marino, B.; Digilio, M.C.; Putotto, C.; Calabrò, P.; et al. Hypertrophic Cardiomyopathy in RASopathies: Diagnosis, Clinical Characteristics, Prognostic Implications, and Management. Heart Fail. Clin. 2022, 18, 19–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geddes, G.C.; Parent, J.J.; Lander, J.; Jeewa, A.; Ware, S.M.; Villa, C.; Chatfield, K.C.; Weaver, K.N. MEK Inhibition Improves Cardiomyopathy in Costello Syndrome. J. Am. Coll. Cardiol. 2023, 81, 1439–1441. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabala, P.; Kowalski, P.; Rudziński, M.J.; Polis, B.; Grabala, M. The Surgical Management of Severe Scoliosis in Immature Patient with a Very Rare Disease Costello Syndrome—Clinical Example and Brief Literature Review. Life 2024, 14, 740. https://doi.org/10.3390/life14060740
Grabala P, Kowalski P, Rudziński MJ, Polis B, Grabala M. The Surgical Management of Severe Scoliosis in Immature Patient with a Very Rare Disease Costello Syndrome—Clinical Example and Brief Literature Review. Life. 2024; 14(6):740. https://doi.org/10.3390/life14060740
Chicago/Turabian StyleGrabala, Pawel, Piotr Kowalski, Marek J. Rudziński, Bartosz Polis, and Michal Grabala. 2024. "The Surgical Management of Severe Scoliosis in Immature Patient with a Very Rare Disease Costello Syndrome—Clinical Example and Brief Literature Review" Life 14, no. 6: 740. https://doi.org/10.3390/life14060740
APA StyleGrabala, P., Kowalski, P., Rudziński, M. J., Polis, B., & Grabala, M. (2024). The Surgical Management of Severe Scoliosis in Immature Patient with a Very Rare Disease Costello Syndrome—Clinical Example and Brief Literature Review. Life, 14(6), 740. https://doi.org/10.3390/life14060740







