The Effect of Stress-Reducing Interventions on Heart Rate Variability in Cardiovascular Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Process
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Synthesis
2.6. Meta-Analysis
3. Results
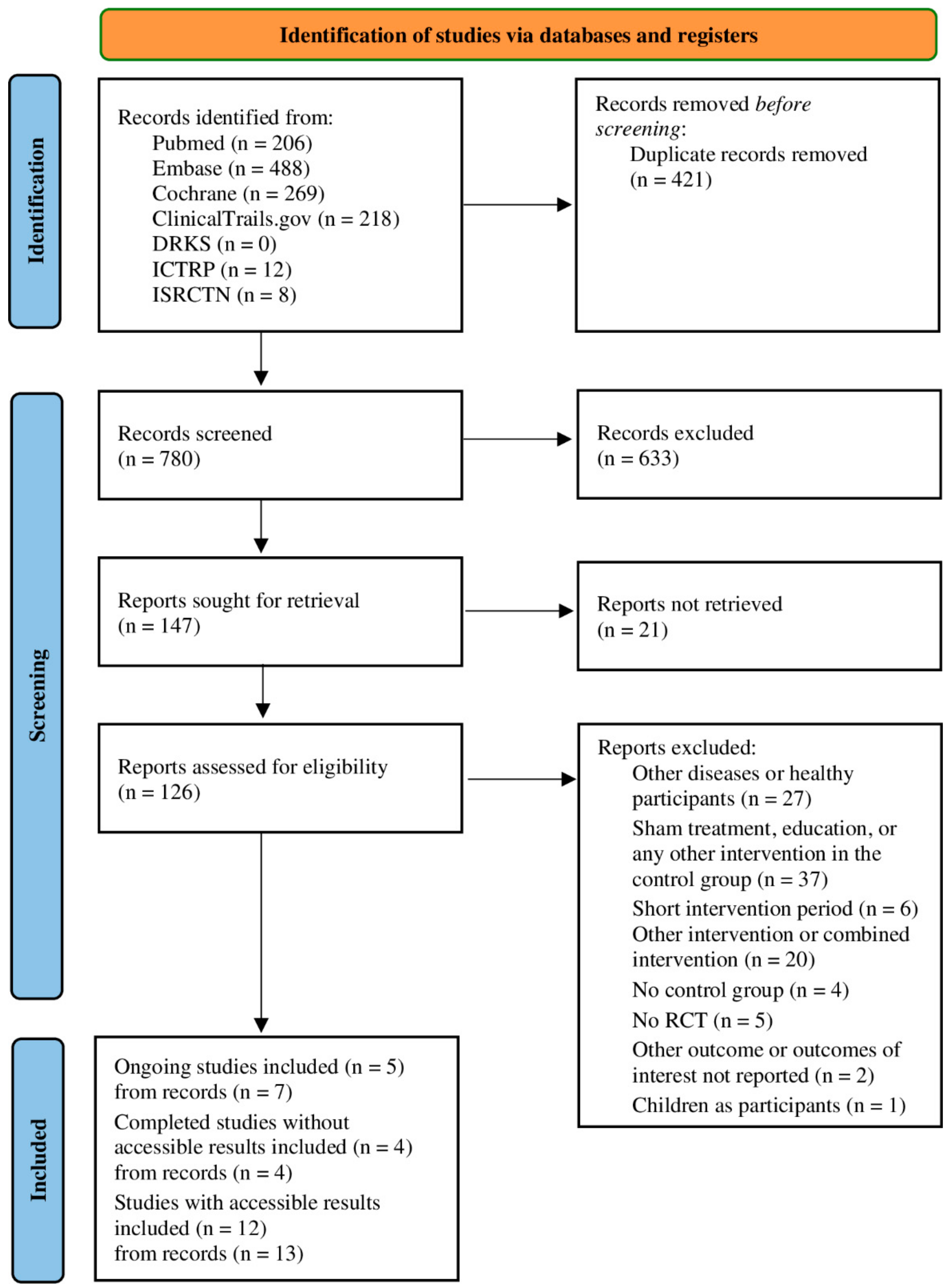
3.1. Study Selection and Characteristics
3.2. Study Selection and Characteristics
3.3. Meta-Analysis
3.4. Outcome Parameters
3.4.1. C-Reactive Protein (CRP)
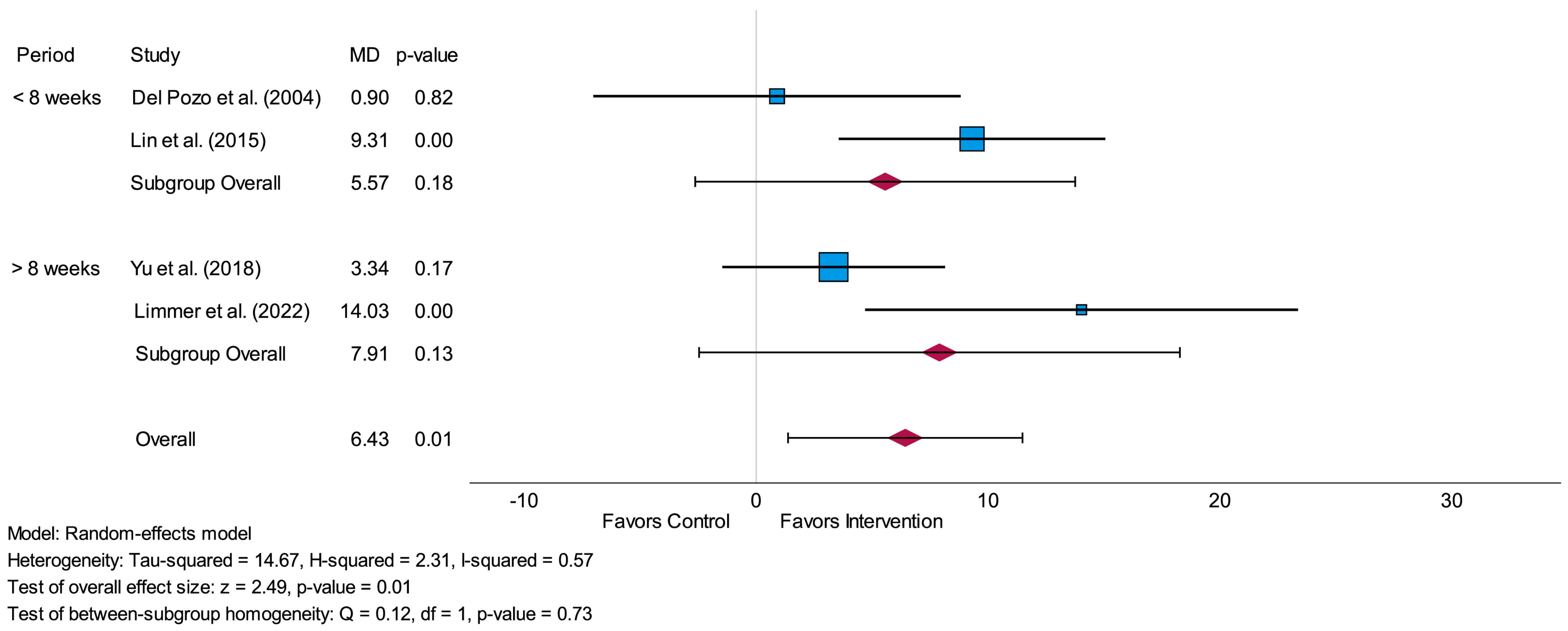
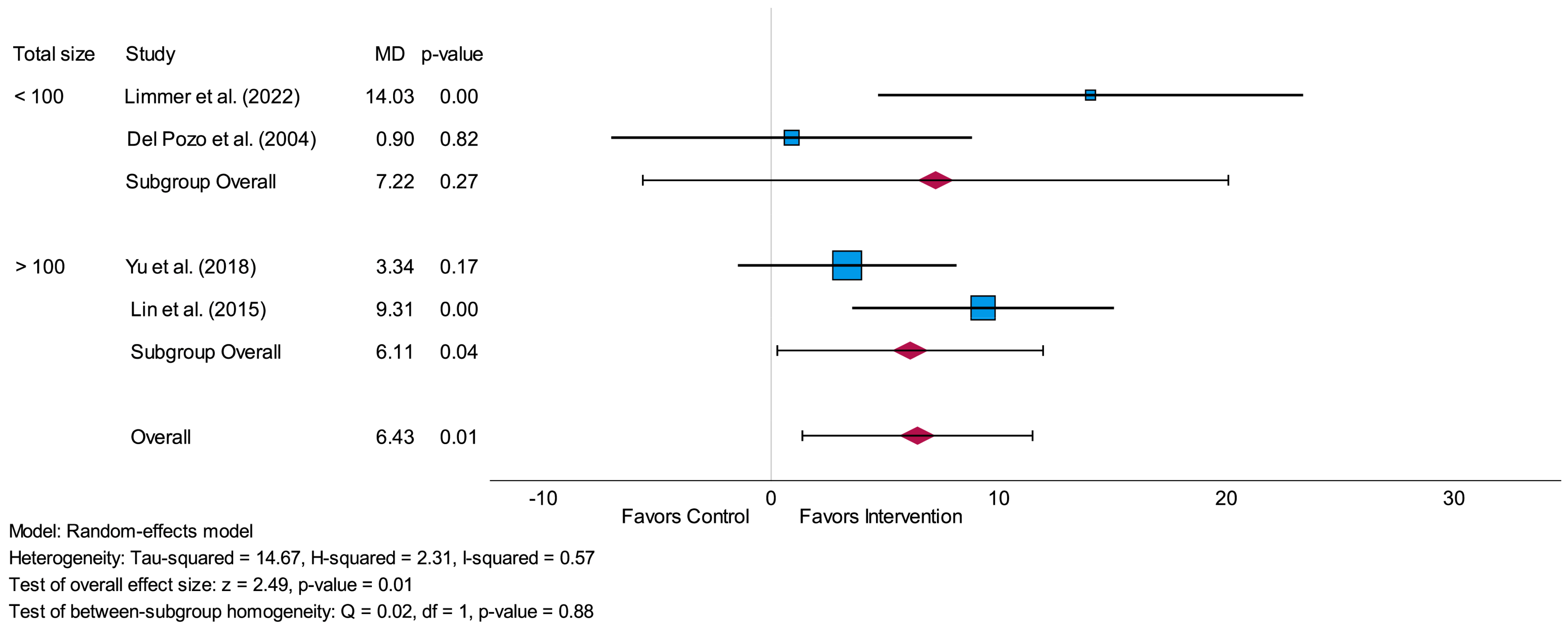
3.4.2. Standard Deviation of Normal-to-Normal Intervals (SDNN)
3.4.3. Total Power (TP)
3.4.4. Low-Frequency Power (LF)
3.4.5. High-Frequency Power (HF) and HF in Normalized Units (nHF)
3.5. Stress Management Interventions
3.5.1. HRV-Biofeedback
3.5.2. Tai Chi
3.5.3. Yoga
3.6. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | coronary artery disease |
| CHF | congestive heart failure |
| CRP | c-reactive protein |
| CVD | cardiovascular disease |
| ECG | electrocardiogram |
| HF | high-frequency power |
| HRV | heart rate variability |
| hsCRP | high-sensitivity c-reactive protein |
| LF | low-frequency power |
| MD | mean difference |
| MeSH | Medical Subject Headings |
| MI | myocardial infarction |
| nHF | HF in normalized units |
| nLF | LF in normalized units |
| PRISMA | Preferred Reporting of Items for Systematic Meta-analysis |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PSNS | parasympathetic nervous system |
| RCT | randomized controlled trial |
| SD | standard deviation |
| SDNN | standard deviation of Normal-to-Normal intervals |
| SMD | standard mean difference |
| SNS | sympathetic nervous system |
| TP | total power |
References
- Cygankiewicz, I.; Zareba, W. Heart rate variability. Handb. Clin. Neurol. 2013, 117, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef] [PubMed]
- Kivimaki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, A.I.; Hamer, M.; Gaze, D.; Collinson, P.; Rumley, A.; Lowe, G.; Steptoe, A. The association between fibrinogen reactivity to mental stress and high-sensitivity cardiac troponin T in healthy adults. Psychoneuroendocrinology 2015, 59, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Huang, Y.; Shen, Y.; Sun, L. Automated Stress Recognition Using Supervised Learning Classifiers by Interactive Virtual Reality Scenes. IEEE Trans. Neural. Syst. Rehabil. Eng. 2022, 30, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Tipton, M.J.; Harper, A.; Paton, J.F.R.; Costello, J.T. The human ventilatory response to stress: Rate or depth? J. Physiol. 2017, 595, 5729–5752. [Google Scholar] [CrossRef] [PubMed]
- Ayada, C.; Toru, U.; Korkut, Y. The relationship of stress and blood pressure effectors. Hippokratia 2015, 19, 99–108. [Google Scholar] [PubMed]
- Yang, J.; Kershaw, K.N. Feasibility of using ecological momentary assessment and continuous heart rate monitoring to measure stress reactivity in natural settings. PLoS ONE 2022, 17, e0264200. [Google Scholar] [CrossRef] [PubMed]
- Samson, C.; Koh, A. Stress Monitoring and Recent Advancements in Wearable Biosensors. Front. Bioeng. Biotechnol. 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 2021, 15, 46–59. [Google Scholar] [PubMed]
- Pacharra, M.; Schaper, M.; Kleinbeck, S.; Blaszkewicz, M.; Golka, K.; van Thriel, C. Neurobehavioral effects of exposure to propionic acid revisited-Does psychosocial stress interfere with distractive effects in volunteers? Neurotoxicology 2016, 55, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health. Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Craighead, L.; Watkins, L.L.; Ingle, K.; Tyson, C.C.; Lin, P.H.; Kraus, W.E.; et al. Effects of Lifestyle Modification on Psychosocial Function in Patients with Resistant Hypertension: Secondary Outcomes from the Triumph randomized clinical trial. J. Cardiopulm. Rehabil. Prev. 2023, 44, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.; Stefano, G.B. The neurobiology of stress management. Neuro Endocrinol. Lett. 2010, 31, 19–39. [Google Scholar] [PubMed]
- Wang, F.; Lee, E.K.; Wu, T.; Benson, H.; Fricchione, G.; Wang, W.; Yeung, A.S. The effects of tai chi on depression, anxiety, and psychological well-being: A systematic review and meta-analysis. Int. J. Behav. Med. 2014, 21, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Worthen, M.; Cash, E. Stress Management. In StatPearls [Internet]; Stat Pearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zhang, B.; Shi, H.; Cao, S.; Xie, L.; Ren, P.; Wang, J.; Shi, B. Revealing the magic of acupuncture based on biological mechanisms: A literature review. Biosci. Trends 2022, 16, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Shirotsuki, K.; Sugaya, N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc. Med. 2021, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Massage therapy research review. Complement. Ther. Clin. Pract. 2014, 20, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Lin, I.M.; Fan, S.Y.; Chien, C.L.; Lin, T.H. One-Year Cardiovascular Prognosis of the Randomized, Controlled, Short-Term Heart Rate Variability Biofeedback Among Patients with Coronary Artery Disease. Int. J. Behav. Med. 2018, 25, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.; Laser, M.; Schutz, A. Mobile Heart Rate Variability Biofeedback as a Complementary Intervention After Myocardial Infarction: A Randomized Controlled Study. Int. J. Behav. Med. 2022, 29, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.M.; Gevirtz, R.N.; Scher, B.; Guarneri, E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am. Heart J. 2004, 147, E11. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.M.; Fan, S.Y.; Lu, H.C.; Lin, T.H.; Chu, C.S.; Kuo, H.F.; Lee, C.S.; Lu, Y.H. Randomized controlled trial of heart rate variability biofeedback in cardiac autonomic and hostility among patients with coronary artery disease. Behav. Res. Ther. 2015, 70, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.; Birgander, L.S.; Jansson, J.H.; Lindahl, B.; Burell, G.; Asplund, K.; Mattsson, C. Cognitive-behavioural stress management does not improve biological cardiovascular risk indicators in women with ischaemic heart disease: A randomized-controlled trial. J. Intern. Med. 2006, 260, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Subhash, C.M.; Bhola, S.V.; Kushal, M.; Ashwini, M.; Jitendrapal, S.S. Effect of Yoga Lifestyle in Patients with Heart Failure: A Randomized Control Trial. Int. J. Yoga 2022, 15, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.S.; Pung, M.A.; Wilson, K.; Bangen, K.J.; Delano-Wood, L.; Hurwitz, B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J. Psychosom. Res. 2020, 128, 109883. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, P.; Cottraux, J.; Mollard, E.; Sai, N.; Brun, S.; Burri, H.; Restier, L.; Adeleine, P. Prevention of implantable defibrillator shocks by cognitive behavioral therapy: A pilot trial. Am. Heart J. 2006, 151, 191. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.H.; Pal, P.; Pal, G.K.; Balachander, J.; Jayasettiaseelon, E.; Sreekanth, Y.; Sridhar, M.G.; Gaur, G.S. Effect of yoga therapy on heart rate, blood pressure and cardiac autonomic function in heart failure. J. Clin. Diagn. Res. 2014, 8, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.H.; Pal, P.; Pal, G.K.; Sridhar, M.G.; Balachander, J.; Jayasettiaseelon, E.; Sreekanth, Y.; Gaur, G.S. Yoga training in heart failure (NYHA I-II) reduces oxidative stress and inflammation. J. Exerc. Physiol. Online 2014, 17, 10. [Google Scholar]
- Blumenthal, J.A.; Sherwood, A.; Babyak, M.A.; Watkins, L.L.; Waugh, R.; Georgiades, A.; Bacon, S.L.; Hayano, J.; Coleman, R.E.; Hinderliter, A. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: A randomized controlled trial. JAMA 2005, 293, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Tsang, W.W. Short-term Tai Chi Training May Benefit Arterial Compliance but Not Heart Rate Variability Among Stroke Survivors: A Randomized Controlled Trial. J. Int. Soc. Chin. Health Pract. 2020, 1. Available online: http://www.ischp.org/ojs/index.php/jischp/article/view/4 (accessed on 29 May 2023).
- Lopes, C.P. Yôga e o Treinamento de Técnicas Respiratórias em Pacientes com Insuficiência Cardíaca com Fração de Ejeção Preservada: Ensaio Clínico Randomizado. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul. Porto Alegre, Rio Grande do Sul, Brazil, 2017. [Google Scholar]
- Lopes, C.P.; Danzmann, L.C.; Moraes, R.S.; Vieira, P.J.C.; Meurer, F.F.; Soares, D.S.; Chiappa, G.; Guimaraes, L.S.P.; Leitao, S.A.T.; Ribeiro, J.P.; et al. Yoga and breathing technique training in patients with heart failure and preserved ejection fraction: Study protocol for a randomized clinical trial. Trials 2018, 19, 405. [Google Scholar] [CrossRef] [PubMed]
- NCT05580718. Online Cognitive Behavioral Therapy Targeting Cardiac Anxiety (MI-CBT) [Trial Registry Record]. Available online: https://ClinicalTrials.gov/show/NCT05580718 (accessed on 29 May 2023).
- Berg, S.K.; Herning, M.; Schjødt, I.; Thorup, C.B.; Juul, C.; Svendsen, J.H.; Jorgensen, M.B.; Risom, S.S.; Christensen, S.W.; Thygesen, L.; et al. The heart & mind trial: Intervention with cognitive-behavioural therapy in patients with cardiac disease and anxiety: Randomised controlled trial protocol. BMJ Open 2021, 11, e057085. [Google Scholar] [CrossRef] [PubMed]
- NCT04582734. Screening and Intervention Reducing Anxiety in Patients with Cardiac Disease [Trial Registry Record]. Available online: https://ClinicalTrials.gov/show/NCT04582734 (accessed on 29 May 2023).
- NCT04505865. The Effect of Mindfulness on Vascular Inflammation in Stable Coronary Disease [Trial Registry Record]. Available online: https://ClinicalTrials.gov/show/NCT04505865 (accessed on 29 May 2023).
- NCT03826836. Mind Our Heart Study [Trial Registry Record]. Available online: https://clinicaltrials.gov/show/NCT03826836 (accessed on 29 May 2023).
- ChiCTR2100052417. Study on the Effect of Acupunture Treatment on Autonomic Nerve Dysfunction in Convalescent Period of Stroke Based on Heart Rate Variability Assessment Technique [Trial Registry Record]. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100052417 (accessed on 29 May 2023).
- Jia, S.; Lu, W.; Hang, M.; Zhang, C.; Ma, Z.; Xue, K.; Lu, Y.; Zhang, S.; Guo, Y.; Zhang, J.; et al. Study on the Effect of Acupunture Treatment on Autonomic Nerve Dysfunction in Convalescent Period of Stroke Based on Heart Rate Variability Assessment Technique. Medicine 2022, 101, e32355. [Google Scholar] [CrossRef] [PubMed]
- NCT01998555. Web-Based Psychological Intervention to Coronary Artery Heart Disease Patients [Trial Registry Record]. Available online: https://ClinicalTrials.gov/show/NCT01998555 (accessed on 29 May 2023).
- NCT03659409. Stroke of Mindfulness: Investigating Physiological and Psychological Well-Being [Trial Registry Record]. Available online: https://clinicaltrials.gov/show/NCT03659409 (accessed on 29 May 2023).
- ACTRN12612000358842. Evaluation of a 12 Week Yoga Intervention on Negative Affective States, Cardiovascular and Cognitive Function in Post-Cardiac Rehabilitation Patients [Trial Registry Record]. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12612000358842 (accessed on 29 May 2023).
- Yeung, A.; Kiat, H.; Denniss, A.R.; Cheema, B.S.; Bensoussan, A.; Machliss, B.; Colagiuri, B.; Chang, D. Randomised controlled trial of a 12 week yoga intervention on negative affective states, cardiovascular and cognitive function in post-cardiac rehabilitation patients. BMC Complement. Altern. Med. 2014, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- ChiCTR-IPR-17011893. The Treatment to the Autonomic Dysfunction in Acute Sroke by Auricular Acupuncture Mediated by ABVN: A Clinical Study [Trial Registry Record]. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPR-17011893 (accessed on 29 May 2023).
- Burr, R.L. Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep 2007, 30, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Heathers, J.A. Everything Hertz: Methodological issues in short-term frequency-domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Adlan, A.M.; Veldhuijzen van Zanten, J.; Lip, G.Y.H.; Paton, J.F.R.; Kitas, G.D.; Fisher, J.P. Acute hydrocortisone administration reduces cardiovagal baroreflex sensitivity and heart rate variability in young men. J. Physiol. 2018, 596, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Brunckhorst, C.B.; Holzmeister, J.; Scharf, C.; Binggeli, C.; Duru, F. Stress, depression and cardiac arrhythmias. Ther. Umsch. 2003, 60, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Sloan, R.P. Heart rate variability in depressive and anxiety disorders. Am Heart J 2000, 140, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Kartik, C.; Otsuka, K.; Pella, D.; Pella, J. Brain-heart connection and the risk of heart attack. Biomed. Pharmacother. 2002, 56 (Suppl. S2), 257s–265s. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Verdino, R.J.; Gottdiener, J.S.; O’Leary, S.T.; Bairey Merz, C.N.; Krantz, D.S. Changes in heart rate and heart rate variability before ambulatory ischemic events(1). J. Am. Coll. Cardiol. 2001, 38, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.E.; Press, S.A.; Helmert, E.; Hautzinger, M.; Khazan, I.; Vagedes, J. Comparing Effectiveness of HRV-Biofeedback and Mindfulness for Workplace Stress Reduction: A Randomized Controlled Trial. Appl. Psychophysiol. Biofeedback 2020, 45, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bogels, S.M.; de Bruin, E.I. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Sasaki, J.E.; Wei, G.X.; Huang, T.; Yeung, A.S.; Neto, O.B.; Chen, K.W.; Hui, S.S. Effects of Mind(-)Body Exercises (Tai Chi/Yoga) on Heart Rate Variability Parameters and Perceived Stress: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2018, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Weissman, N.J.; Zhu, J.; Bonsall, R.W.; Doyle, M.; Stretch, M.R.; Glaes, S.B.; Krantz, D.S.; Gottdiener, J.S.; Tracy, R.P. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am. J. Cardiol. 2008, 101, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Gathright, E.C.; Hughes, J.W.; Sun, S.; Storlazzi, L.E.; DeCosta, J.; Balletto, B.L.; Carey, M.P.; Scott-Sheldon, L.A.J.; Salmoirago-Blotcher, E. Effects of stress management interventions on heart rate variability in adults with cardiovascular disease: A systematic review and meta-analysis. J. Behav. Med. 2024, 47, 374–388. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Main Disease | Intervention | Intervention Period | Outcome of Interest | Sample Size | Mean Age | Female | |
|---|---|---|---|---|---|---|---|---|---|
| Claesson et al. [33] | 2006 | Ischemic heart disease | Cognitive behavioral stress management | 1 year | Fibrinogen, hsCRP | Intervention Control | 77 82 | 59.4 ± 9.3 62.2 ± 7.7 | 77 82 |
| Jain et al. [34] | 2022 | Congestive heart failure | Yoga | 3 months | C-reactive protein | Intervention Control | 30 30 | 51.9 ± 6.9 52.3 ± 6.6 | 6 9 |
| Redwine et al. [35] | 2020 | Congestive heart failure | Tai chi | 16 weeks | C-reactive protein | Intervention Control | 24 * 23 * | 63.0 ± 9.0 67.0 ± 7.0 | 2 3 |
| Yu et al. [29] | 2018 | Coronary artery disease | HRV-biofeedback | 18 weeks | Breathing rate, heart rate variability | Intervention Control | 75 59 | 61.2 ± 7. 4 60. 3 ± 6.9 | 9 6 |
| Limmer et al. [30] | 2022 | Myocardial infarction | HRV-biofeedback | 12 weeks | Breathing rate, heart rate variability | Intervention Control | 23 * 23 * | 57.4 ± 8.8 63.6 ± 9.9 | 2 5 |
| Chevalier et al. [36] | 2004 | Ventricular tachyarrhythmias | Cognitive behavioral therapy | 3 months | Heart rate variability | Intervention Control | 35 * 35 * | 58.5 ± 10.0 57.9 ± 11.0 | 5 1 |
| Del Pozo et al. [31] | 2004 | Coronary artery disease | HRV-biofeedback | 6 weeks | Heart rate variability | Intervention Control | 31 32 | 66.8 ± 8.4 68.0 ± 9.0 | 11 10 |
| Krishna et al. [37,38] | 2014 | Congestive heart failure | Yoga | 12 weeks | Heart rate variability, hsCRP | Intervention Control | 44 48 | 49.3 ± 5.7 50.1 ± 4.5 | 12 16 |
| Blumenthal et al. [39] | 2005 | Ischemic heart disease | Cognitive social learning model of behavior | 16 weeks | Heart rate variability | Intervention Control | 44 * 42 * | 63.0 ± 11.5 63.0 ± 9.0 | 15 10 |
| Chan and Tsang [40] | 2020 | Stroke | Tai chi | 12 weeks | Heart rate variability | Intervention Control | 19 * 19 * | 64.2 ± 8.2 61.8 ± 7.3 | 9 11 |
| Lin et al. [32] | 2015 | Coronary artery disease | HRV-biofeedback | 6 weeks | Heart rate variability | Intervention Control | 77 * 77 * | 61.0 ± 8.4 60.6 ± 8.0 | 6 11 |
| Lopes et al. [41,42] | 2017 | Congestive heart failure | Yoga | 8 weeks | Heart rate variability | Intervention Control | 11 10 | 67.0 ± 6.0 62.0 ± 6.0 | 10 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Malahi, O.; Mohajeri, D.; Bäuerle, A.; Mincu, R.; Rothenaicher, K.; Ullrich, G.; Rammos, C.; Teufel, M.; Rassaf, T.; Lortz, J. The Effect of Stress-Reducing Interventions on Heart Rate Variability in Cardiovascular Disease: A Systematic Review and Meta-Analysis. Life 2024, 14, 749. https://doi.org/10.3390/life14060749
El-Malahi O, Mohajeri D, Bäuerle A, Mincu R, Rothenaicher K, Ullrich G, Rammos C, Teufel M, Rassaf T, Lortz J. The Effect of Stress-Reducing Interventions on Heart Rate Variability in Cardiovascular Disease: A Systematic Review and Meta-Analysis. Life. 2024; 14(6):749. https://doi.org/10.3390/life14060749
Chicago/Turabian StyleEl-Malahi, Ouahiba, Darya Mohajeri, Alexander Bäuerle, Raluca Mincu, Korbinian Rothenaicher, Greta Ullrich, Christos Rammos, Martin Teufel, Tienush Rassaf, and Julia Lortz. 2024. "The Effect of Stress-Reducing Interventions on Heart Rate Variability in Cardiovascular Disease: A Systematic Review and Meta-Analysis" Life 14, no. 6: 749. https://doi.org/10.3390/life14060749
APA StyleEl-Malahi, O., Mohajeri, D., Bäuerle, A., Mincu, R., Rothenaicher, K., Ullrich, G., Rammos, C., Teufel, M., Rassaf, T., & Lortz, J. (2024). The Effect of Stress-Reducing Interventions on Heart Rate Variability in Cardiovascular Disease: A Systematic Review and Meta-Analysis. Life, 14(6), 749. https://doi.org/10.3390/life14060749









