Deciphering the Interaction between Coniella granati and Pomegranate Fruit Employing Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pomegranates, Pathogen Inoculation, and Physiological Indices
2.2. Transcriptome Sequencing, Mapping, and Bioinformatics Analysis
2.3. Validation of DEGs by RT-qPCR
3. Results
3.1. Coniella granati Inoculation on Pomegranate Fruit and Symptoms
3.2. Physiological Changes on Pomegranate Fruit in Response to Coniella granati Infection
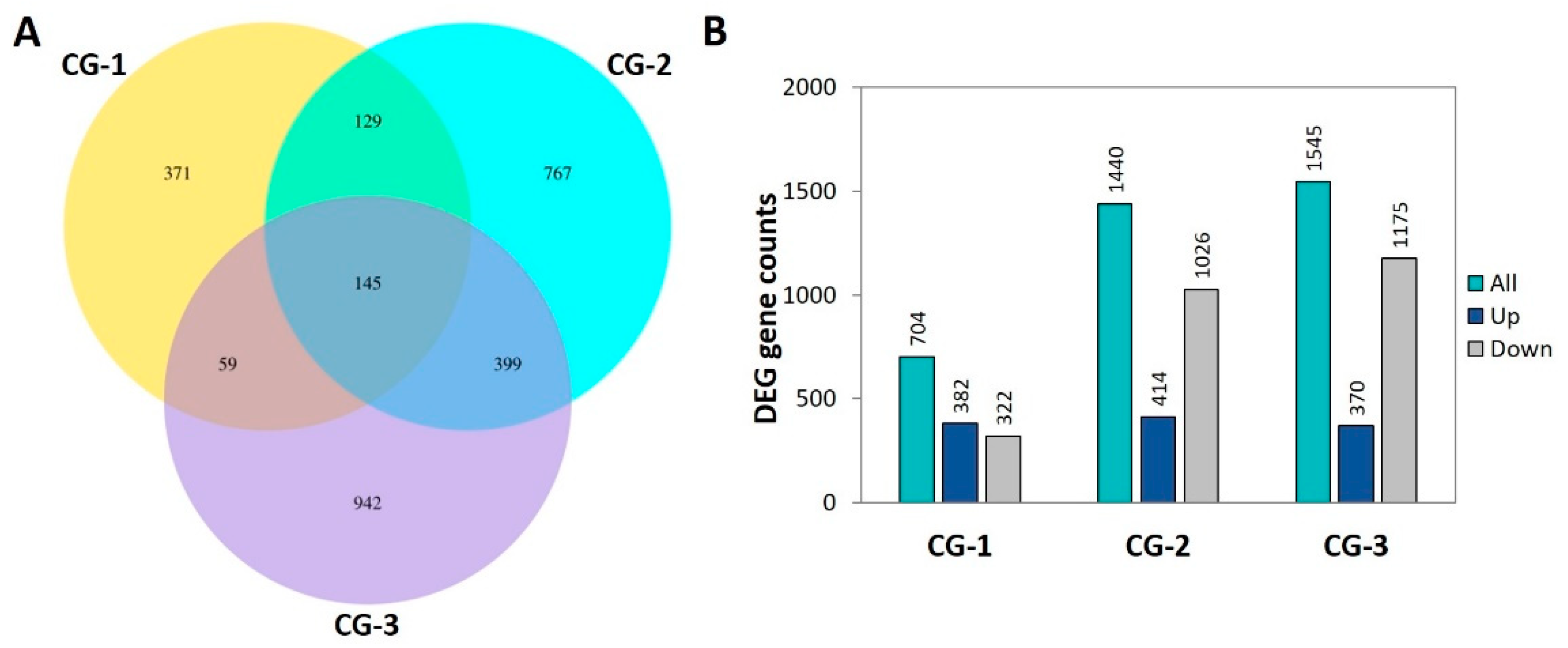
3.3. RNA-seq Analysis
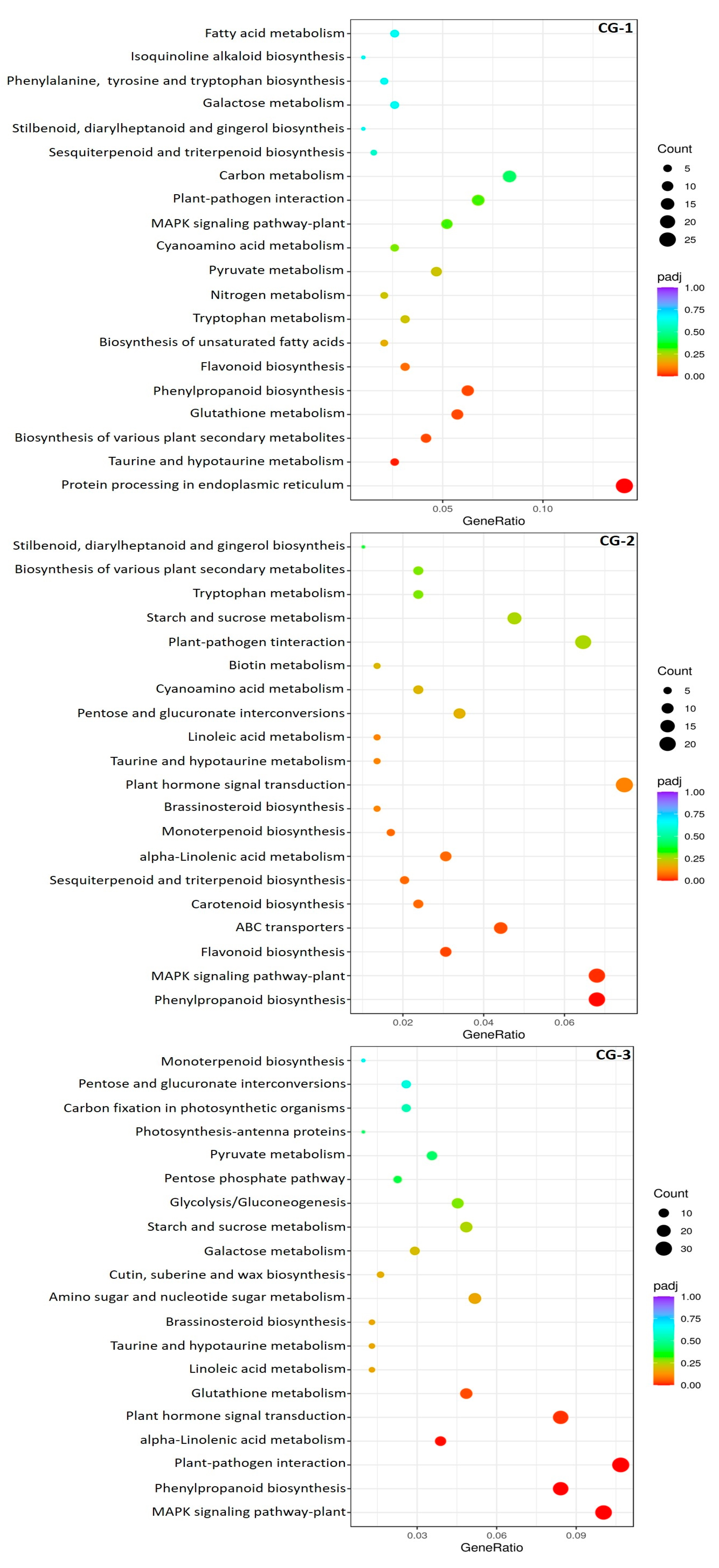
3.4. Functional Annotations and Classifications of DEGs
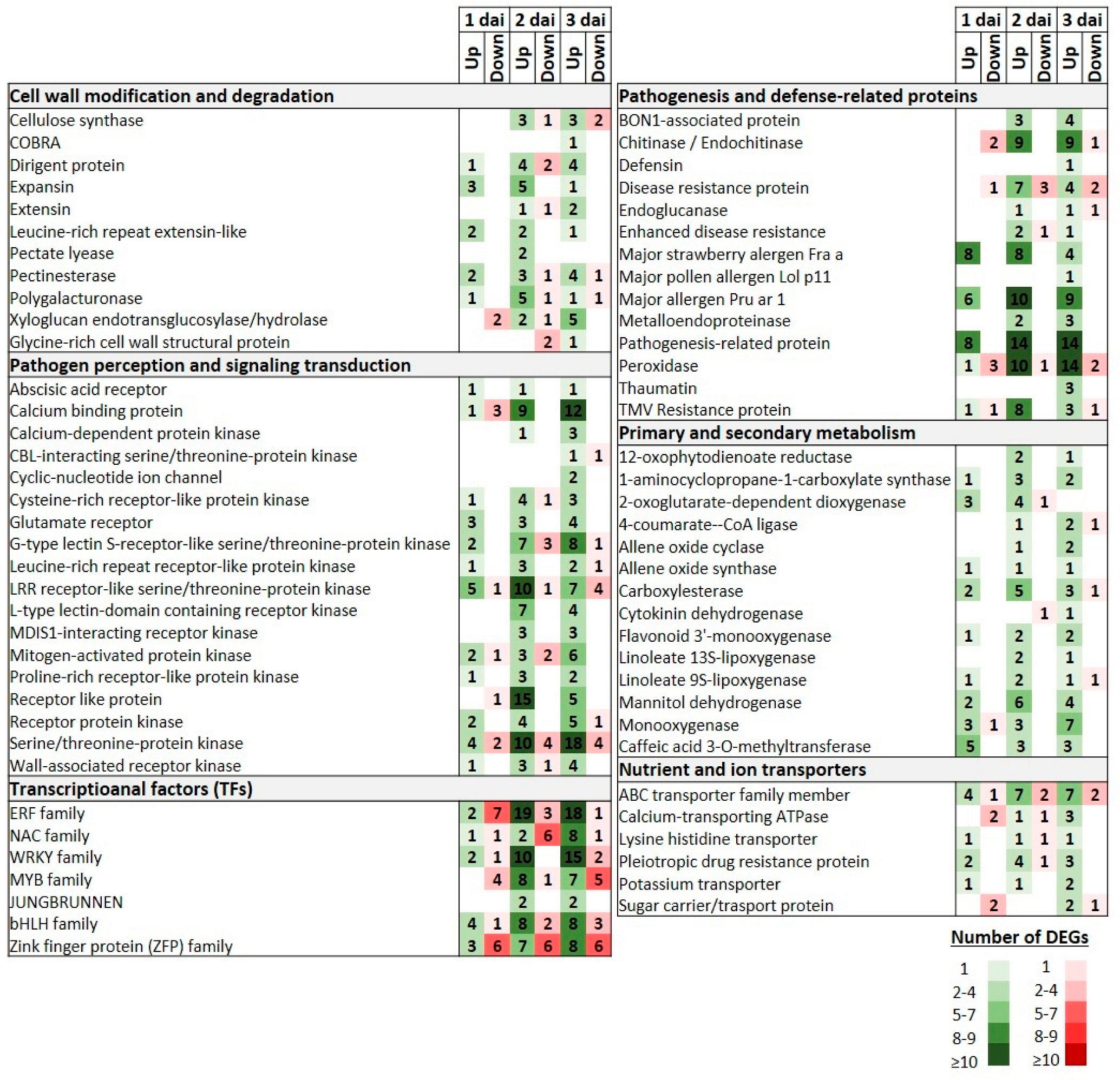
3.5. DEGs Transcriptional Profiles
3.6. Validation of DEGs by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mincuzzi, A.; Ippolito, A.; Brighenti, V.; Marchetti, L.; Benvenuti, S.; Ligorio, A.; Pellati, F.; Sanzani, S.M. The Effect of Polyphenols on Pomegranate Fruit Susceptibility to Pilidiella granati Provides Insights into Disease Tolerance Mechanisms. Molecules 2020, 25, 515. [Google Scholar] [CrossRef]
- Mincuzzi, A.; Sanzani, S.M.; Palou, L.; Ragni, M.; Ippolito, A. Postharvest Rot of Pomegranate Fruit in Southern Italy: Characterization of the Main Pathogens. J. Fungi 2022, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Belay, Z.A.; Caleb, O.J.; Vorster, A.; van Heerden, C.; Opara, U.L. Transcriptomic changes associated with husk scald incidence on pomegranate fruit peel during cold storage. Food Res. Int. 2020, 135, 109285. [Google Scholar] [CrossRef]
- Palou, L.; Taberner, V.; Guardado, A.; del Rio, M.A.; Monstesinos-Herrero, C. Incidence and etiology of postharvest fungal diseases of pomegranate (Punica granatum cv. Mollar de Elche) in Spain. Phytopathol. Mediterr. 2013, 52, 478–489. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Kaur, A.; Sarao, N.K.; Sharma, D. Pathogenesis-Related Proteins and Their Transgenic Expression for Developing Disease-Resistant Crops: Strategies Progress and Challenges. In Plant Breeding—New Perspectives; IntechOpen: London, UK, 2022; pp. 6–8. [Google Scholar] [CrossRef]
- Bizuneh, G.K. The chemical diversity and biological activities of phytoalexins. Tradit. Med. 2021, 21, 31–43. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Iseppi, R.; Pinzi, L.; Mincuzzi, A.; Ippolito, A.; Messi, P.; Sanzani, S.M.; Rastelli, G.; Pellati, F. Antifungal Activity and DNA Topoisomerase Inhibition of Hydrolysable Tannins from Punica granatum L. Int. J. Mol. Sci. 2021, 22, 4175. [Google Scholar] [CrossRef]
- Nibedita, C.; Jolly, B. Transcriptomics: A successful approach to unravel the molecular mechanism of plant-pathogen interaction in post-genomic era. Res. J. Biotechnol. 2017, 8, 90–100. [Google Scholar]
- Tyagi, P.; Singh, D.; Mathur, S.; Singh, A.; Ranjan, R. Upcoming progress of transcriptomics studies on plants: An overview. Front. Plant. Sci. 2022, 13, 1030890. [Google Scholar] [CrossRef]
- Zambounis, A.; Ganopoulos, I.; Valasiadis, D.; Karapetsi, L.; Madesis, P. RNA sequencing-based transcriptome analysis of kiwifruit infected by Botrytis cinerea. Physiol. Mol. Plant Pathol. 2020, 111, 101514. [Google Scholar] [CrossRef]
- Doddaraju, P.; Kumar, P.; Dashyal, M.S.; Girigowda, M. Identification of suitable reference genes for expression studies in pomegranate under different biotic and abiotic stress conditions. Mol. Biol. Rep. 2021, 48, 3935–3943. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, X.; Hu, L.; Ni, H.; Langjia, R.; Yuan, F.; Zhang, M.; Zhang, S. Unraveling the pomegranate genome: Comprehensive analysis of R2R3-MYB transcription factors. Horticulturae 2023, 9, 779. [Google Scholar] [CrossRef]
- Chen, L.; Ge, D.; Ren, Y.; Wang, Y.; Yan, M.; Zhao, X.; Yuan, Z. Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L. Agronomy 2023, 13, 332. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, S.X.; Li, X.S.; Li, C.Y. Transcriptome studies with the third-generation sequencing technology. Life Sci. Instrum. 2018, 16, 114–121. [Google Scholar]
- Naidoo, S.; Visser, E.A.; Zwart, L.; du Toit, Y.; Bhadauria, V.; Shuey, L.S. Dual RNA-seq to elucidate the plant–pathogen duel. Curr. Issues Mol. Biol. 2018, 27, 127–142. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Boutsika, A.; Papageorgiou, A.G.; Dalianis, A.; Michaliou, M.; Chatzidimopoulos, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Zambounis, A. Global Transcriptome Analysis of the Peach (Prunus persica) in the Interaction System of Fruit–Chitosan–Monilinia fructicola. Plants 2024, 13, 567. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Papageorgiou, A.; Boutsika, A.; Chatzidimopoulos, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Zambounis, A. Insights into the Interaction between the Biocontrol Agent Bacillus amyloliquefaciens QST 713, the Pathogen Monilinia fructicola and Peach Fruit. Agronomy 2024, 14, 771. [Google Scholar] [CrossRef]
- Aci, M.M.; Tsalgatidou, P.C.; Boutsika, A.; Dalianis, A.; Michaliou, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Malacrino, A.; Schena, L.; et al. Comparative transcriptome profiling and co-expression network analysis uncover the key genes associated with pear petal defense responses against Monilinia laxa infection. Front. Plant Sci. 2024, 15, 1377937. [Google Scholar] [CrossRef]
- Mincuzzi, A.; Garganese, F.; Ippolito, A.; Sanzani, S.M. First report of Pilidiella granati causing postharvest fruit rot on pomegranate in southern Italy. J. Plant Pathol. 2016, 98, 377. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, E.I.; Karamichali, I.; Stefanidou, E.; Boutsika, A.; Tsitsigiannis, D.I.; Paplomatas, E.; Madesis, P.; Zambounis, A. Insights into the Transcriptional Reprogramming of Peach Leaves Inoculated with Taphrina deformans. Plants 2024, 13, 861. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.S.; Zhu, H.Y.; Bai, Y.B.; Hui, L.; Cheng, Z.M. RNA sequencing-based transcriptome analysis of mature strawberry fruit infected by necrotrophic fungal pathogen Botrytis cinerea. Physiol. Mol. Plant Pathol. 2018, 104, 77–85. [Google Scholar] [CrossRef]
- Haile, Z.M.; Guzman, N.-D.; Grace, E.; Moretto, M.; Sonego, P.; Engelen, K.; Zoli, L.; Moser, C.; Baraldi, E. Transcriptome profiles of strawberry (Fragaria vesca) fruit interacting with Botrytis cinerea at different ripening stages. Front. Plant Sci. 2019, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, G.; He, Z.; Yan, M.; Zou, M.; Jiang, J. Transcriptome analysis of Actinidia chinensis in response to Botryosphaeria dothidea infection. PLoS ONE 2020, 15, e0227303. [Google Scholar] [CrossRef]
- Liu, X.T.; Cao, X.Q.; Shi, S.C.; Zhao, N.; Li, D.D.; Fang, P.H.; Chen, X.; Qi, W.C.; Zhang, Z. Comparative RNA-Seq analysis reveals a critical role for brassinosteroids in rose (Rosa hybrida) petal defense against Botrytis cinerea infection. BMC Genet. 2018, 19, 62. [Google Scholar] [CrossRef]
- Lu, L.; Ji, L.; Ma, Q.; Yang, M.; Li, S.; Tang, Q.; Qiao, L.; Li, F.; Guo, Q.; Wang, C. Depression of Fungal Polygalacturonase Activity in Solanum Lycopersicum Contributes to Antagonistic Yeast-Mediated Fruit Immunity to Botrytis. J. Agric. Food Chem. 2019, 67, 3293–3304. [Google Scholar] [CrossRef]
- Yang, L.; Huang, W.; Xiong, F.; Xian, Z.; Su, D.; Ren, M.; Li, Z. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef]
- De Cremer, K.; Mathys, J.; Vos, C.; Froenicke, L.; Michelmore, R.W.; Cammue, B.P.A.; De Coninck, B. RNA seq-based transcrip-tome analysis of Lactuca sativa, infected by the fungal necrotrophy Botrytis cinerea. Plant Cell Environ. 2013, 36, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Van Damme, E.J. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 397. [Google Scholar] [CrossRef] [PubMed]
- Delteil, A.; Gobbato, E.; Cayrol, B.; Estevan, J.; Michel-Romiti, C.; Dievart, A.; Kroj, T.; Morel, J.-B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Z.; Wang, G.X.; Zhou, J.M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M.; Khalid, N.; Islam, W.; Sanaullah, T.; Anwar, M.; Khan, S.; Ye, W.; Lou, Y. Zinc finger protein transcription factors: Integrated line of action for plant antimicrobial activity. Microb. Pathog. 2019, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Mertens, J.; Goossens, A. Role and functioning of bHLH transcription factors in jasmonate signaling. J. Exp. Bot. 2017, 68, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Q.; Zeng, X.; Chen, X.; Liu, S.; Han, Y. Deciphering the molecular signatures associated with resistance to Botrytis cinerea in strawberry flower by comparative and dynamic transcriptome analysis. Front. Plant Sci. 2022, 13, 888939. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, U.J.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Wang, C.T.; Yu, T.F.; Wang, D.M.; Li, M.; Zhao, D.; Li, X.-T.; Fu, J.-D.; Xu, Z.-S.; Song, X.-Y. Overexpression of ZmWRKY65 transcription factor from maize confers stress resistances in transgenic Arabidopsis. Sci. Rep. 2021, 11, 4024. [Google Scholar] [CrossRef]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, F.; Liu, D.; Imani, J.; Langen, G.; Kogel, K.-H. Matrix Metalloproteinases Operate Redundantly in Arabidopsis Immunity against Necrotrophic and Biotrophic Fungal Pathogens. PLoS ONE 2017, 12, e0183577. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Song, Q.; Wang, L.; Liu, S.; Hong, Y.; Huang, L.; Song, F. Tomato Sl3-MMP, a member of the Matrix metalloproteinase family, is required for disease resistance against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000. BMC Plant Biol. 2015, 15, 143. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Hua, J. The C2 domain protein BAP1 negatively regulates defense responses in Arabidopsis. Plant J. 2006, 48, 238–248. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Wang, K.; Lu, C.; Luo, M.; Shan, T.; Zhang, Z. A wheat caffeic acid 3-O-methyltransferase TaCOMT-3D positively contributes to both resistance to sharp eyespot disease and stem mechanical strength. Sci. Rep. 2018, 8, 6543. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Prasad, V.; Zehra, A.; Gupta, V.K.; Upadhyay, R.S. Mannitol metabolism during pathogenic fungal-host interactions under stressed conditions. Front. Microbiol. 2015, 6, 1019. [Google Scholar] [CrossRef]
- Vélëz, H.; Glassbrook, N.J.; Daub, M.E. Mannitol biosynthesis is required for plant pathogenicity by Alternaria alternata. FEMS Microbiol. Lett. 2008, 285, 122–129. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Lu, S.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Fang, Z.; Hou, X. Genome-wide identification and analysis of cytokinin dehydrogenase/oxidase (CKX) family genes in Brassica oleracea L. reveals their involvement in response to Plasmodiophora brassicae infections. Hortic. Plant J. 2022, 8, 68–80. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhao, Y.; Lan, T.; Yu, K.; Zhang, L.; Qin, Z.; Hu, Z.; Yao, Y.; Ni, Z.; et al. Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR 3 and jasmonate signalling pathway. Plant Biotechnol. J. 2020, 18, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Niu, J.; zi Peng, D.; Cheng, Q.; Luan, M.B.; Zhang, Z.Q. Clone and function verification of the OPR gene in Brassica napus related to linoleic acid synthesis. BMC Plant Biol. 2022, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Zhang, J.; Ge, L.; Lei, S.; Han, J.; Zhang, X.; Li, X.; Sun, X. A putative 12-oxophytodienoate reductase gene CsOPR3 from Camellia sinensis, is involved in wound and herbivore infestation responses. Gene 2017, 615, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Li, K.; Yang, D.; Liu, N.; Zhang, L.; Zhao, L.; Zhang, X.; Liu, Y.; Gao, L.; et al. Roles of the 2-Oxoglutarate-Dependent Dioxygenase Superfamily in the Flavonoid Pathway: A Review of the Functional Diversity of F3H, FNS I, FLS, and LDOX/ANS. Molecules 2021, 26, 6745. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Ramaroson, M.-L.; Koutouan, C.; Helesbeux, J.-J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.G.; Shirasu, K.; Menke, F.; Jones, A. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef]
- Khare, D.; Choi, H.; Huh, S.U.; Bassin, B.; Kim, J.; Martinoia, E.; Sohn, K.H.; Paek, K.-H.; Lee, Y. Arabidopsis ABCG34 Contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proc. Natl. Acad. Sci. USA 2017, 114, E5712–E5720. [Google Scholar] [CrossRef]

| Days after Inoculation (dai) | Total Flavonoids (mg Rutin/g FW) | Total Phenolics (mg GAE/g FW) | Lipid Peroxidation, TBRAS (nmole/g FW) | Hydrogen Peroxide (H2O2) (μmole/g FW) | |
|---|---|---|---|---|---|
| CG | 1 | 31.36 ± 1.20 d | 32.57 ± 0.60 b | 18.09 ± 0.13 cd | 1.18 ± 0.06 a |
| 2 | 29.24 ± 0.64 c | 31.48 ± 0.40 c | 18.10 ± 0.25 cd | 1.33 ± 0.04 ab | |
| 3 | 36.78 ± 0.50 e | 30.10 ± 0.51 c | 19.14 ± 0.39 d | 1.37 ± 0.06 ab | |
| CT | 1 | 21.50 ± 0.57 a | 23.27 ± 0.27 a | 14.06 ± 0.20 a | 0.97 ± 0.07 bc |
| 2 | 25.57 ± 0.36 b | 24.35 ± 0.53 a | 16.29 ± 0.76 b | 1.10 ± 0.06 cd | |
| 3 | 28.12 ± 0.48 c | 23.45 ± 0.38 a | 17.51 ± 0.48 c | 1.11 ± 0.06 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafouros, A.; Tsalgatidou, P.C.; Boutsika, A.; Delis, C.; Mincuzzi, A.; Ippolito, A.; Zambounis, A. Deciphering the Interaction between Coniella granati and Pomegranate Fruit Employing Transcriptomics. Life 2024, 14, 752. https://doi.org/10.3390/life14060752
Tsafouros A, Tsalgatidou PC, Boutsika A, Delis C, Mincuzzi A, Ippolito A, Zambounis A. Deciphering the Interaction between Coniella granati and Pomegranate Fruit Employing Transcriptomics. Life. 2024; 14(6):752. https://doi.org/10.3390/life14060752
Chicago/Turabian StyleTsafouros, Athanasios, Polina C. Tsalgatidou, Anastasia Boutsika, Costas Delis, Annamaria Mincuzzi, Antonio Ippolito, and Antonios Zambounis. 2024. "Deciphering the Interaction between Coniella granati and Pomegranate Fruit Employing Transcriptomics" Life 14, no. 6: 752. https://doi.org/10.3390/life14060752





