Characteristics, Relationships, and Anatomical Basis of Leaf Hydraulic Traits and Economic Traits in Temperate Desert Shrub Species
Abstract
1. Introduction
2. Materials and Methods
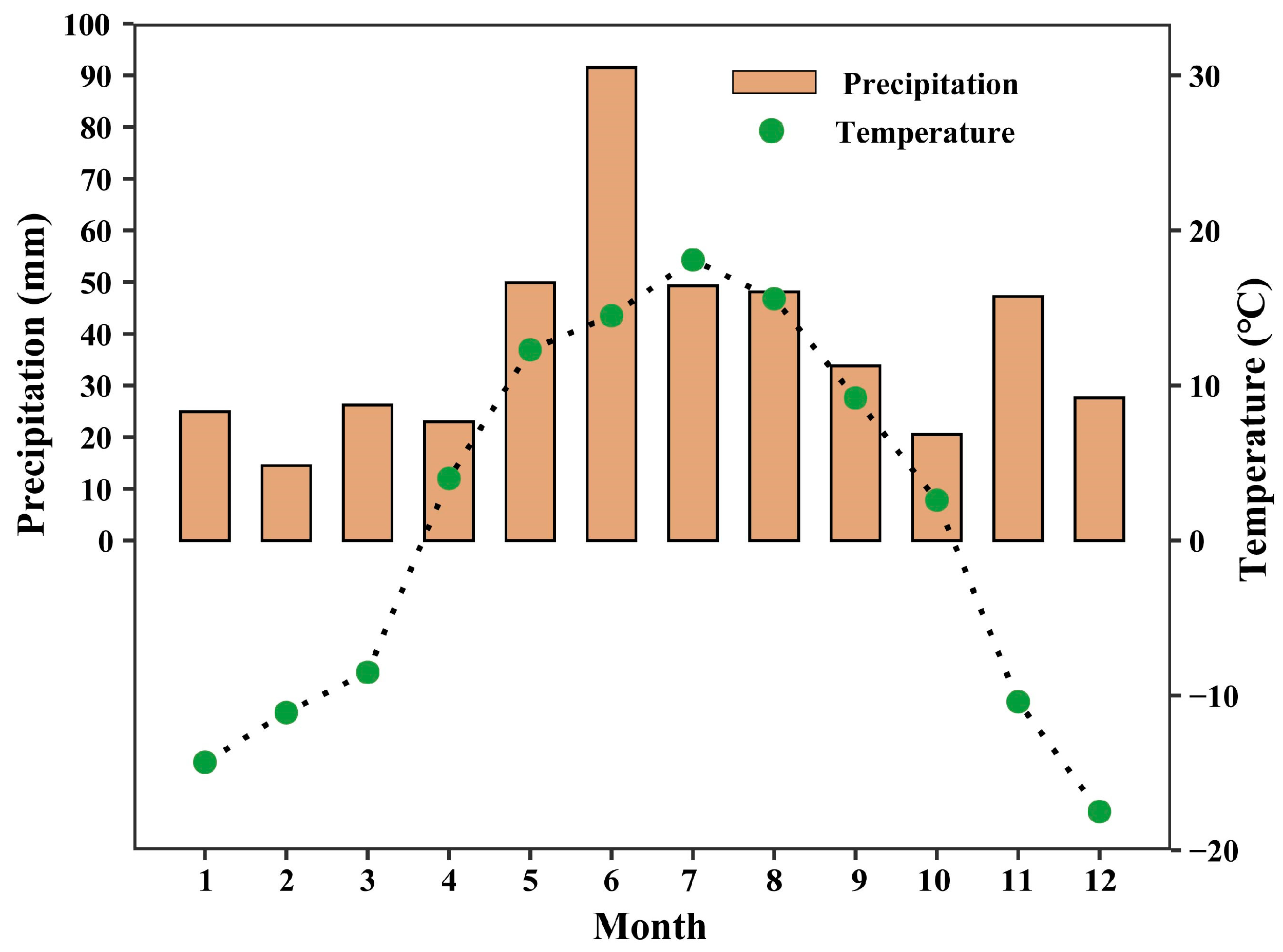
2.1. Study Sites and Species
2.2. Method
2.2.1. Determination of Anatomical Traits
2.2.2. Determination of Economic Traits
2.2.3. Determination of Hydraulic Traits
2.3. Data Analysis
3. Results
3.1. Characteristics of Leaf Hydraulic Traits
3.2. Characteristics of Leaf Economic Traits
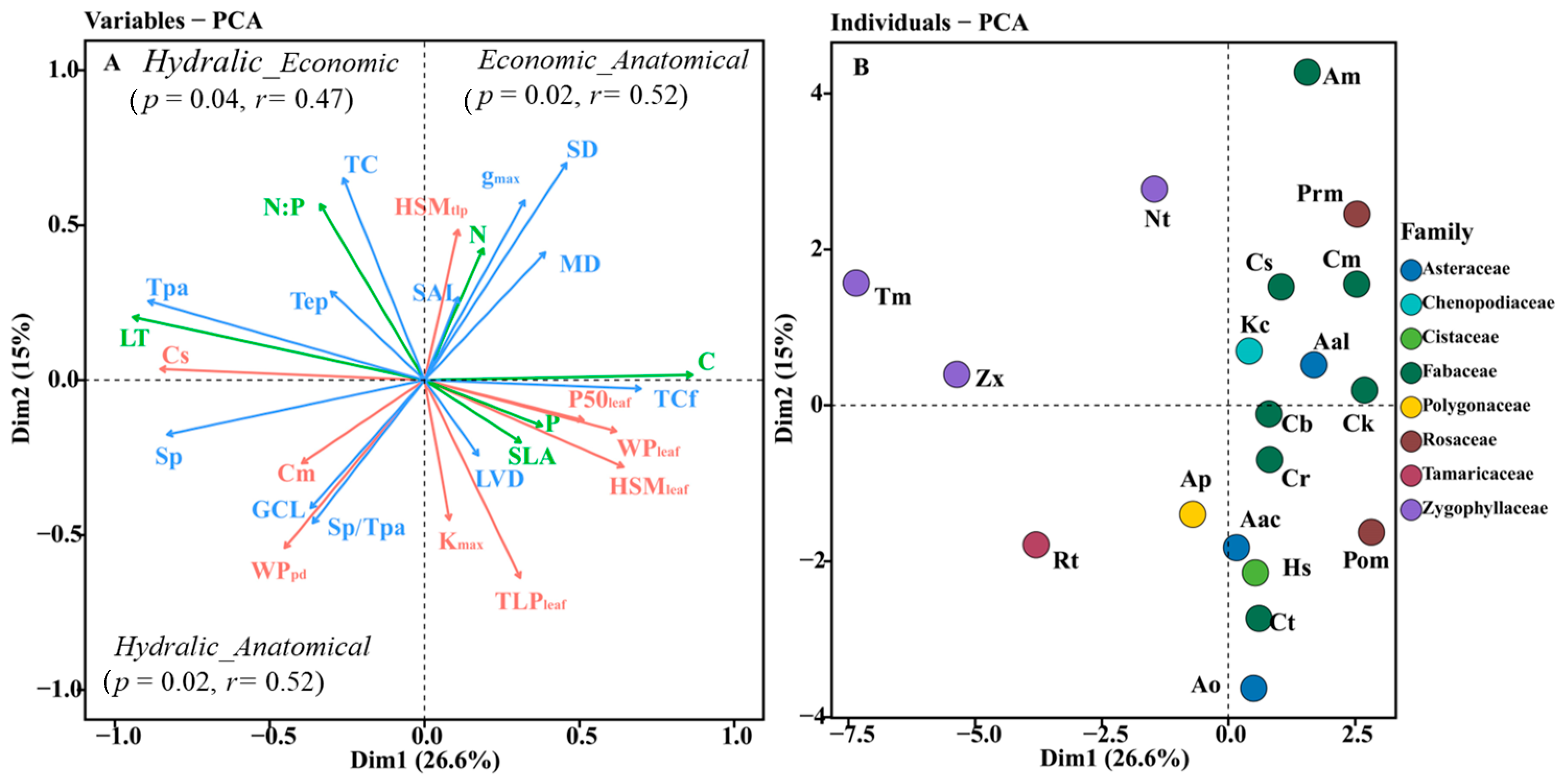
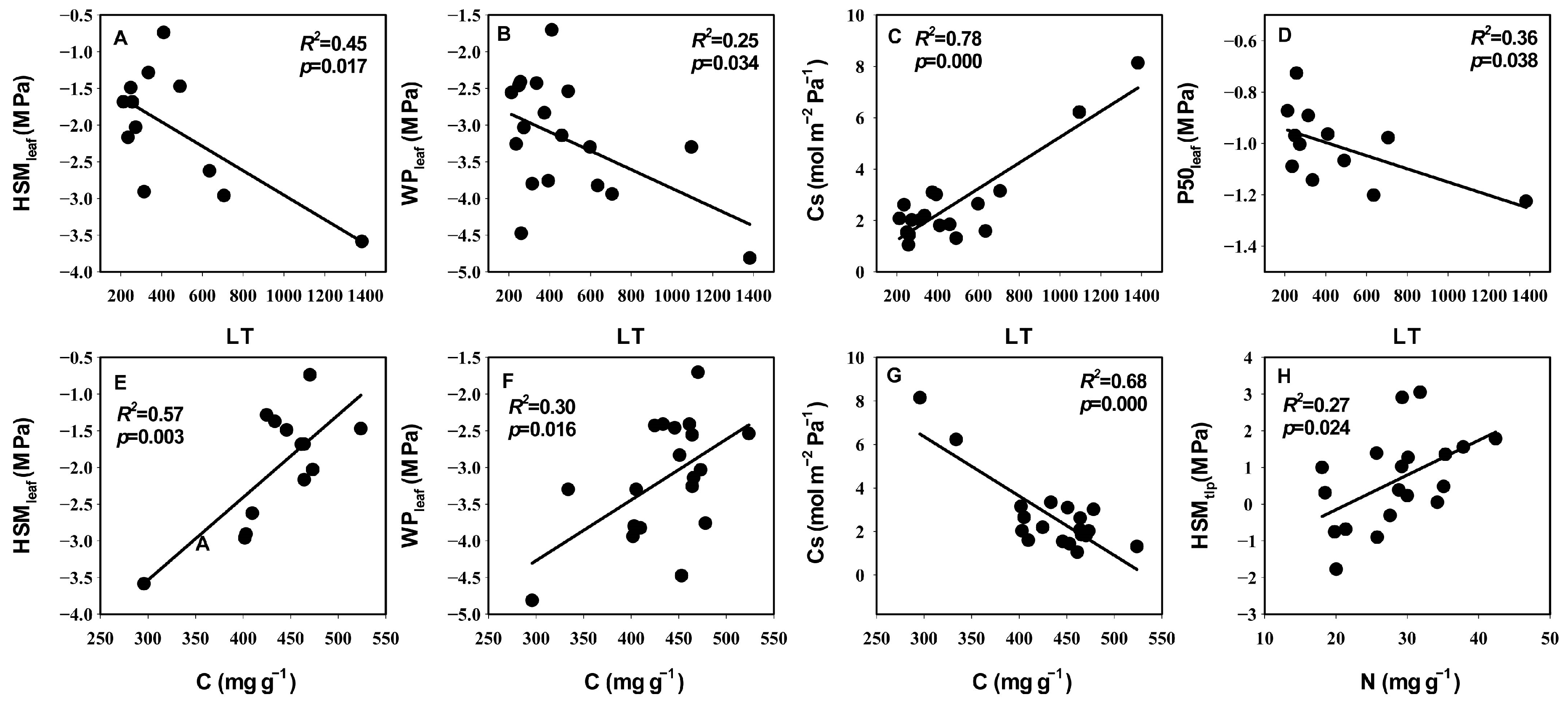
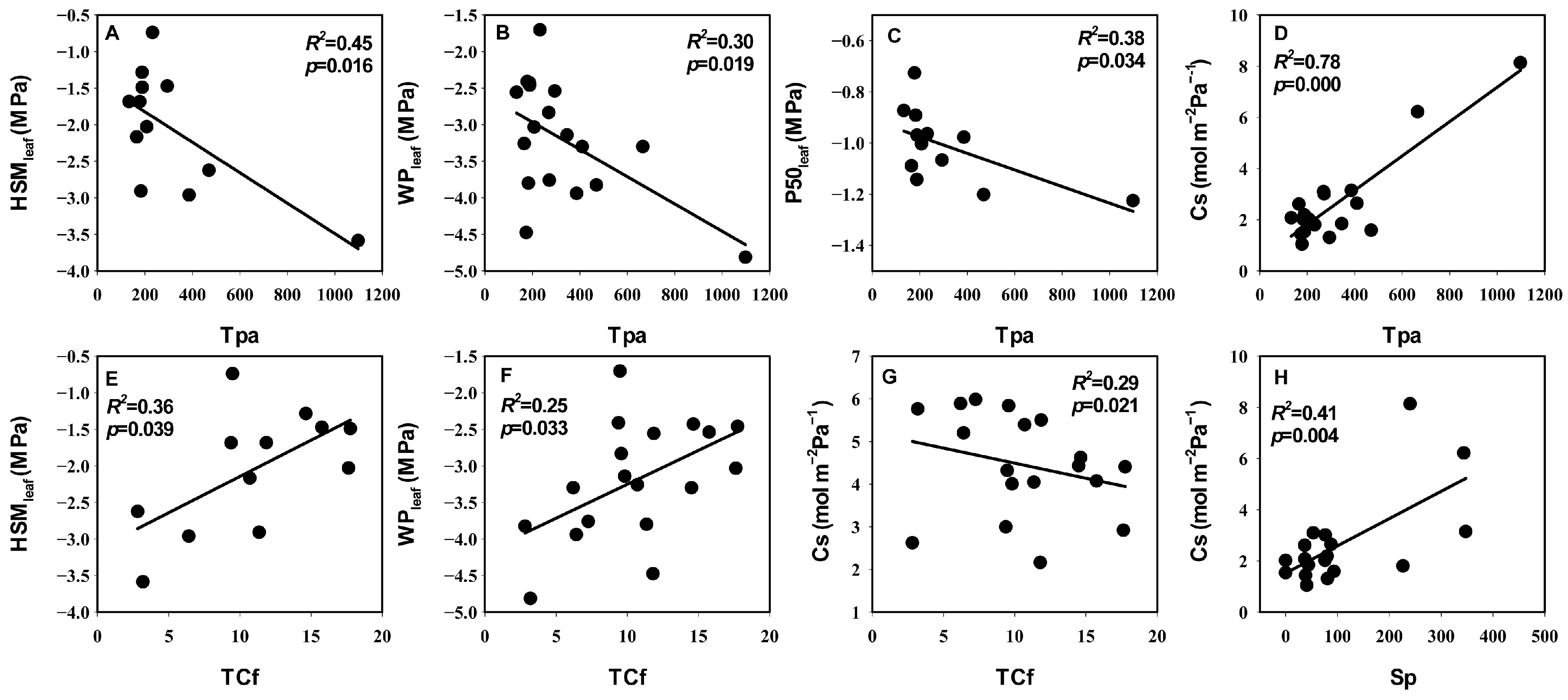
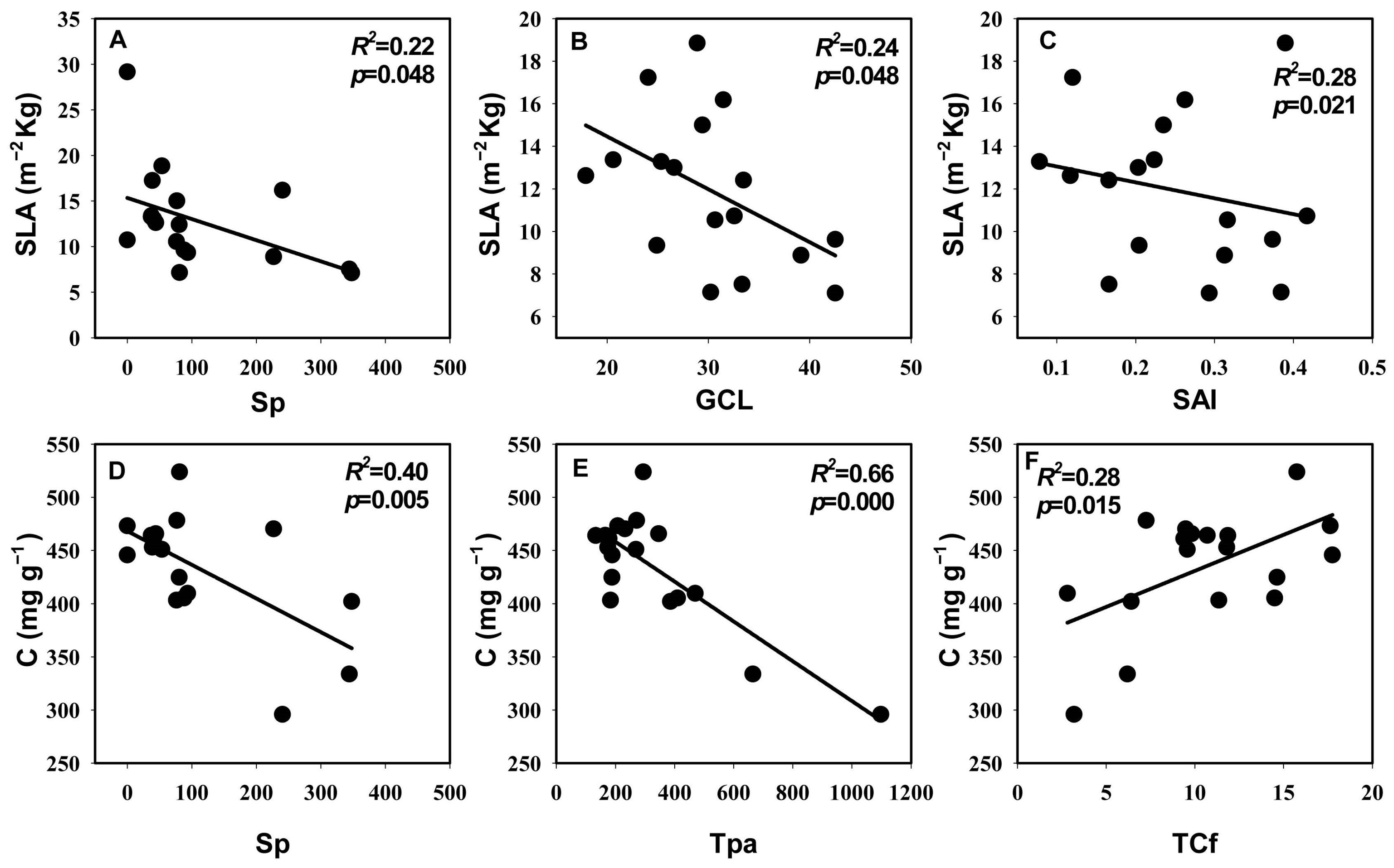
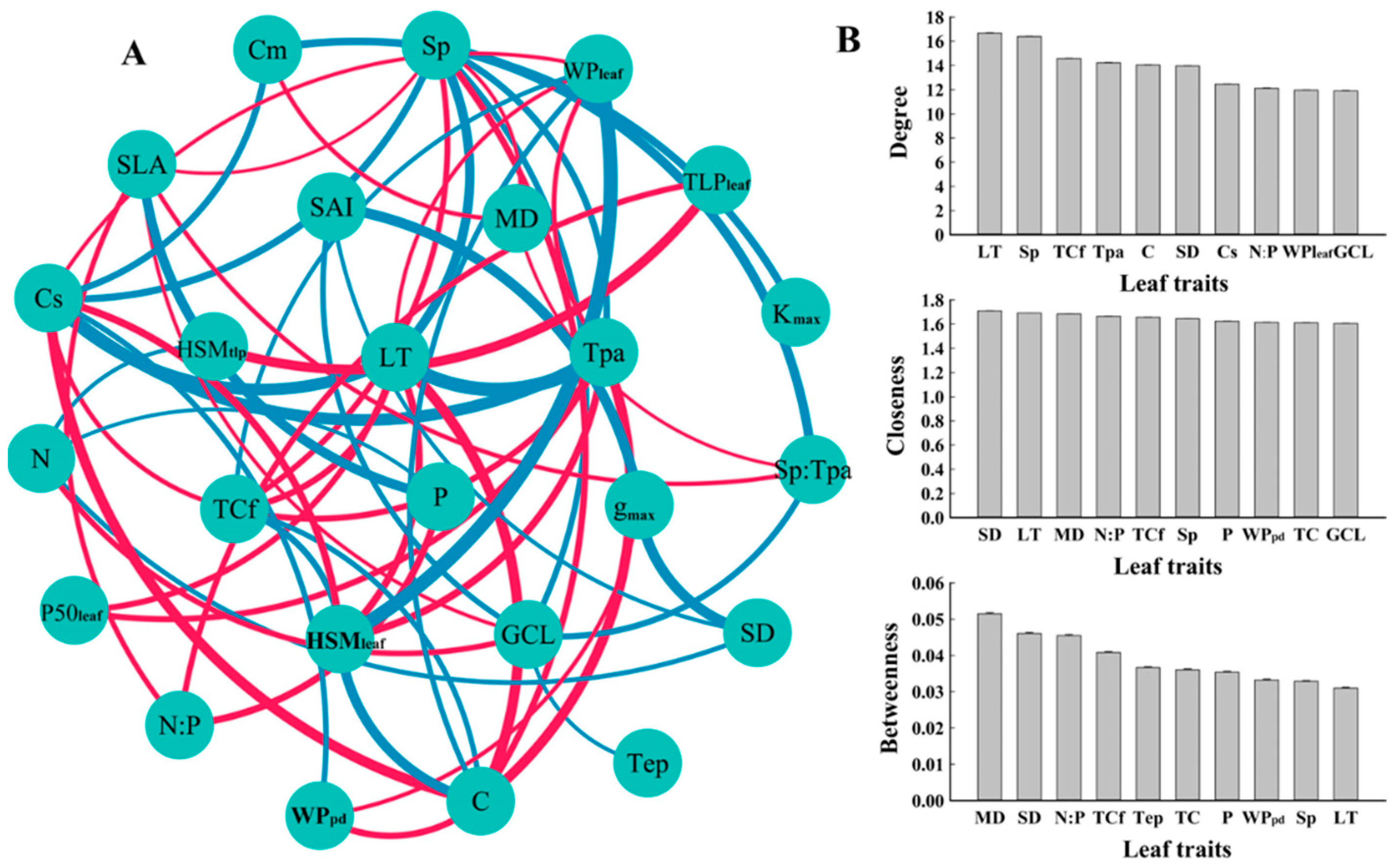
3.3. Relationships between Leaf Hydraulic Traits, Economic Traits, and Anatomical Traits
4. Discussion
4.1. No Trade-Off between Hydraulic Efficiency and Hydraulic Safety
4.2. Leaf Economic Strategies
4.3. The Coupled Relationship between Leaf Hydraulic Traits and Economic Traits and Their Association with Anatomical Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noy-Meir, I. Desert Ecosystems: Environment and Producers. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Whitford, W.G. Ecology of Desert Systems. J. Mammal. 2002, 84, 1122–1124. [Google Scholar]
- Huo, J.; Shi, Y.; Chen, J.; Zhang, H.; Feng, L.; Zhao, Y.; Zhang, Z. Hydraulic trade-off and coordination strategies mediated by leaf functional traits of desert shrubs. Front. Plant Sci. 2022, 13, 938758. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, B.; Zhang, M.; Jie, M.; Guo, S.; Wang, Y. Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities. Plants 2023, 12, 4065. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Vendramini, F.; Díaz, S.; Gurvich, D.E.; Wilson, P.J.; Thompson, K.; Hodgson, J.G. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf Hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, Z.; Niinemets, Ü.; Guo, D. Three Key Sub-leaf Modules and the Diversity of Leaf Designs. Front. Plant Sci. 2017, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, U.A.; You, W.H.; Yan, E.R. Correlations between leaf economics, hydraulic, and shade-tolerance traits among co-occurring individual trees. Acta Oecologica 2021, 110, 103673. [Google Scholar] [CrossRef]
- Fu, X.; Meinzer, F.C.; Woodruff, D.R.; Liu, Y.; Smith, D.D.; McCulloh, K.A.; Howard, A.R. Coordination and trade-offs between leaf and stem hydraulic traits and stomatal regulation along a spectrum of isohydry to anisohydry. Plant Cell Environ. 2019, 42, 2245–2258. [Google Scholar] [CrossRef] [PubMed]
- González-Rebeles, G.; Terrazas, T.; Méndez-Alonzo, R.; Paz, H.; Brodribb, T.J.; Tinoco-Ojanguren, C. Leaf water relations reflect canopy phenology rather than leaf life span in Sonoran Desert trees. Tree Physiol. 2021, 41, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: Berlin, Germany, 2002. [Google Scholar]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Marias, D.E.; Smith, D.D.; McCulloh, K.A.; Howard, A.R.; Magedman, A.L. Mapping ‘hydroscapes’ along the iso- to anisohydric continuum of stomatal regulation of plant water status. Ecol. Lett. 2016, 19, 1343–1352. [Google Scholar] [CrossRef]
- Hartmann, H.; Link, R.M.; Schuldt, B. A whole-plant perspective of isohydry: Stem-level support for leaf-level plant water regulation. Tree Physiol. 2021, 41, 901–905. [Google Scholar] [CrossRef]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, Ü.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; Johnson, D.M.; Buckley, T.N.; Brodribb, T.J. The Anatomical Determinants of Leaf Hydraulic Function. Funct. Ecol. Xylem Anat. 2015, 255–271. [Google Scholar]
- Zwieniecki, M.A.; Brodribb, T.J.; Holbrook, N.M. Hydraulic design of leaves: Insights from rehydration kinetics. Plant Cell Environ. 2007, 30, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hao, G.; Hammond, W.M.; Yu, K.; Liu, X.; Ye, Q.; Zhou, Z.; Wang, C. Aridity-dependent sequence of water potentials for stomatal closure and hydraulic dysfunctions in woody plants. Glob. Change Biol. 2023, 29, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef]
- Li, S.; Hamani, A.K.M.; Zhang, Y.; Liang, Y.; Gao, Y.; Duan, A. Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 2021, 21, 536. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Jia, G. The anatomical structure of woody plants in arid habitats is closely related to nonstructural carbohydrates storage. For. Ecosyst. 2022, 9, 100073. [Google Scholar] [CrossRef]
- Mediavilla, S.; Martín, I.; Escudero, A. Vein and stomatal traits in leaves of three co-occurring Quercus species differing in leaf life span. Eur. J. For. Res. 2020, 139, 829–840. [Google Scholar] [CrossRef]
- Jain, P.; Huber, A.E.; Rockwell, F.E.; Sen, S.; Holbrook, N.M.; Stroock, A.D. New approaches to dissect leaf hydraulics reveal large gradients in living tissues of tomato leaves. New Phytol. 2024, 242, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Field, T.S. Stem hydraulic supply is linked to leaf photosynthetic capacity: Evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ. 2000, 23, 1381–1388. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, Z.; Zhang, Y.-J.; Zhu, S.-D.; Cao, K.-F. Canopy water status and photosynthesis of tropical trees are associated with trunk sapwood hydraulic properties. Plant Physiol. Biochem. 2019, 139, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.-J.; Siddiq, Z.; Zhang, J.-L.; Zhang, S.-B.; Jansen, S.; Cao, K.-F. Hydraulic traits and photosynthesis are coordinated with trunk sapwood capacitance in tropical tree species. Tree Physiol. 2023, 43, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Graciano, C.; Sardans, J.; Zeng, F.; Hughes, A.C.; Ahmed, Z.; Ullah, A.; Ali, S.; Gao, Y.; Peñuelas, J. Plant root mechanisms and their effects on carbon and nutrient accumulation in desert ecosystems under changes in land use and climate. New Phytol. 2024, 242, 916–934. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.A.; Niu, Z.M. Effect of Soil Environment on Species Diversity of Desert Plant Communities. Plants 2023, 12, 3465. [Google Scholar] [CrossRef]
- Ivanova, N. Global Overview of the Application of the Braun-Blanquet Approach in Research. Forests 2024, 15, 937. [Google Scholar] [CrossRef]
- Westhoff, V.E.; van der Maarel, E. The Braun–Blanquet approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; Springer: Dordrecht, The Netherlands, 1978; pp. 287–399. [Google Scholar]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [PubMed]
- Tan, F.S.; Li, X.; Cao, W.X.; Zhu, S.D.; Duan, N.; Li, Q.H. A whole-plant perspective of hydraulic strategy in temperate desert shrub species. Tree Physiol. 2024; submitted. [Google Scholar]
- Brodribb, T.J.; Holbrook, N.M. Stomatal protection against hydraulic failure: A comparison of coexisting ferns and angiosperms. New Phytol. 2004, 162, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 2010, 188, 1113–1123. [Google Scholar] [PubMed]
- Nardini, A.; Pedà, G.; Rocca, N.L. Trade-offs between leaf hydraulic capacity and drought vulnerability: Morpho-anatomical bases, carbon costs and ecological consequences. New Phytol. 2012, 196, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.L. A Study on the Leaf Economic Traits and Hydraulic Traits of Woody Plants on the Loess Plateau. Ph.D. Thesis, Northwest University, Xi’an, China, 2019. (In Chinese with English abstract). [Google Scholar]
- Tognetti, R.; Raschi, A.; Jones, M. Seasonal changes in tissue elasticity and water transport efficiency in three co-occurring Mediterranean shrubs under natural long-term CO2 enrichment. Funct. Plant Biol. 2002, 29, 1097–1106. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Davies, S.J.; Bunyavejchewin, S.; Noor, N.S.M. The role of desiccation tolerance in determining tree species distributions along the Malay-Thai Peninsula. Funct. Ecol. 2008, 22, 221–231. [Google Scholar] [CrossRef]
- Fang, X.W.; Turner, N.C.; Li, F.M.; Li, W.J.; Guo, X.S. Caragana korshinskii seedlings maintain positive photosynthesis during short-term, severe drought stress. Photosynthetica 2011, 49, 603–609. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T.; Holbrook, N.M. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005, 167, 403–413. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A. Hydraulic conductance and stomatal sensitivity to changes of leaf water status in six deciduous tree species. Biol. Plant. 2001, 44, 65–73. [Google Scholar] [CrossRef]
- Hao, G.-Y.; Hoffmann, W.A.; Scholz, F.G.; Bucci, S.J.; Meinzer, F.C.; Franco, A.C.; Cao, K.-F.; Goldstein, G. Stem and leaf hydraulics of congeneric tree species from adjacent tropical savanna and forest ecosystems. Oecologia 2008, 155, 405–415. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Wei, H.; Luo, T.; Wu, B. Optimal balance of water use efficiency and leaf construction cost with a link to the drought threshold of the desert steppe ecotone in northern China. Ann. Bot. 2016, 118, 541–553. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 2003, 161, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Richards, L.; Westoby, M. The importance of leaf cuticle for carbon economy and mechanical strength. New Phytol. 2012, 196, 441–447. [Google Scholar] [CrossRef]
- John, G.P.; Scoffoni, C.; Buckley, T.N.; Villar, R.; Poorter, H.; Sack, L. The anatomical and compositional basis of leaf mass per area. Ecol. Lett. 2017, 20, 412–425. [Google Scholar] [CrossRef]
- Kawai, K.; Okada, N. How are leaf mechanical properties and water-use traits coordinated by vein traits? A case study in Fagaceae. Funct. Ecol. 2016, 30, 527–536. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Poorter, H. Construction costs and payback time of biomass: A whole plant perspective. In A Whole Plant Perspective on Carbon-nitrogen Interactions; Utrecht University: Utrecht, The Netherlands, 1994; pp. 111–127. [Google Scholar]
- Zhang, L.; Luo, T.; Liu, X.; Wang, Y. Altitudinal variation in leaf construction cost and energy content of Bergenia purpurascens. Acta Oecologica 2012, 43, 72–79. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative Photosynthesis of Sun and Shade Plants. Annu. Rev. Plant Biol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Poorter, L.; Kwant, R.; Hernandez, R.; Medina, E.; Werger, M.J.A. Leaf optical properties in Venezuelan cloud forest trees. Tree Physiol. 2000, 20, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, M.; Trinogga, J.; Cebrián-Piqueras, M.A.; Trenkamp, A.; Fløjgaard, C.; Ejrnæs, R.; Bouma, T.J.; Minden, V.; Maier, M.; Mantilla-Contreras, J.; et al. Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 2019, 107, 829–842. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Sack, L.; Xu, L.; Li, M.; Zhang, J.; He, N. Leaf trait network architecture shifts with species-richness and climate across forests at continental scale. Ecol. Lett. 2022, 25, 1442–1457. [Google Scholar] [CrossRef]
- Schulte, P.J.; Hinckley, T.M. A comparison of pressure-volume curve data analysis techniques. J. Exp. Bot. 1985, 36, 590–602. [Google Scholar] [CrossRef]
| Species | Family | Code | Leaf Habit | Height (m) |
|---|---|---|---|---|
| Nitraria tangutorum Bobr | Zygophyllaceae | Nt | Deciduous | 1–2 |
| Tetraena mongolica Maxim | Zygophyllaceae | Tm | Deciduous | 0.4–0.9 |
| Zygophyllum xanthoxylum (Bunge) Maxim | Zygophyllaceae | Zx | Deciduous | 0.5–1 |
| Reaumuria trigyna Maxim | Tamaricaceae | Rt | Deciduous | 0.1–0.3 |
| Prunus mongolica (Maxim.) Ricker | Rosaceae | Prm | Deciduous | 1–2 |
| Potaninia mongolica Maxim | Rosaceae | Pom | Deciduous | 0.3–0.4 |
| Atraphaxis pungens (Bieb.) Jaub. et Spach | Polygonaceae | Ap | Deciduous | 0.8–1.5 |
| Ammopiptanthus mongolicus (Maxim. ex Kom.) Cheng f | Fabaceae | Am | Evergreen | 1.5–2 |
| Caragana korshinskii Kom | Fabaceae | Ck | Deciduous | 1–4 |
| Caragana brachypoda Pojark | Fabaceae | Cb | Deciduous | 0.2–0.3 |
| Caragana tibetica Kom | Fabaceae | Ct | Deciduous | 0.2–0.3 |
| Caragana microphylla Lam | Fabaceae | Cm | Deciduous | 1–3 |
| Caragana stenophylla Pojark | Fabaceae | Cs | Deciduous | 0.3–0.8 |
| Caragana roborovskyi Kom | Fabaceae | Cr | Deciduous | 0.3–1 |
| Helianthemum songaricum Schrenk | Cistaceae | Hs | Deciduous | 0.1–0.12 |
| Krascheninnikovia ceratoides (Linnaeus) Gueldenstaedt | Chenopodiaceae | Kc | Deciduous | 0.1–1 |
| Artemisia ordosica Krasch | Asteraceae | Ao | Deciduous | 0.5–1 |
| Asterothamnus alyssoides (Turcz.) Novopokr | Asteraceae | Aal | Deciduous | 0.1–0.2 |
| Ajania achilleoides (Turcz.) Poljakov ex Grubov | Asteraceae | Aac | Deciduous | 0.1–0.2 |
| Species | Longitude | Latitude | Association | Cover Classification | Soil Data | Soil Acid-Base Properties | Soil Sand Content (%) |
|---|---|---|---|---|---|---|---|
| Nitraria tangutorum Bobr | 107°12′3.86″ | 40°13′10.84″ | Tetraena mongolica + Zygophyllum xanthoxylum | 2 | Sandy brown soil | alkaline | 69.5 |
| Tetraena mongolica Maxim | 107°12′3.86″ | 40°13′10.84″ | Tetraena mongolica + Zygophyllum xanthoxylum | 2 | Sandy brown soil | alkaline | 69.5 |
| Zygophyllum xanthoxylum (Bunge) Maxim | 107°12′3.86″ | 40°13′10.84″ | Tetraena mongolica + Zygophyllum xanthoxylum | 2 | Sandy brown soil | alkaline | 69.5 |
| Reaumuria trigyna Maxim | 107°12′3.86″ | 40°13′10.84″ | Tetraena mongolica + Zygophyllum xanthoxylum | 2 | Sandy brown soil | alkaline | 69.5 |
| Prunus mongolica (Maxim.) Ricker | 106°41′57.00″ | 40°25′43.95″ | Prunus mongolica + Ammopiptanthus mongolicus | 2 | Sandy brown soil | alkaline | 52.3 |
| Potaninia mongolica Maxim | 107°30′10.15″ | 40°7′28.38″ | Tetraena mongolica + Zygophyllum xanthoxylum | 2 | Sandy brown soil | alkaline | 69.5 |
| Atraphaxis pungens (Bieb.) Jaub. et Spach | 107°27′13″ | 39°56′27.64″ | Caragana brachypoda + Atraphaxis pungens | 2 | Sandy brown soil | alkaline | 75.6 |
| Ammopiptanthus mongolicus (Maxim. ex Kom.) Cheng f | 106°41′57.00″ | 40°25′43.95″ | Prunus mongolica + Ammopiptanthus mongolicus | 2 | Sandy brown soil | alkaline | 52.3 |
| Caragana korshinskii Kom | 106°41′57.00″ | 40°25′43.95″ | Prunus mongolica + Ammopiptanthus mongolicus | 2 | Sandy brown soil | alkaline | 52.3 |
| Caragana brachypoda Pojark | 107°27′13″ | 39°56′27.64″ | Caragana brachypoda + Atraphaxis pungens | 2 | Sandy brown soil | alkaline | 75.6 |
| Caragana tibetica Kom | 107°27′13″ | 39°56′27.64″ | Caragana brachypoda + Atraphaxis pungens | 2 | Sandy brown soil | alkaline | 75.6 |
| Caragana microphylla Lam | 107°27′13″ | 39°56′27.64″ | Caragana brachypoda + Atraphaxis pungens | 2 | Sandy brown soil | alkaline | 75.6 |
| Caragana stenophylla Pojark | 107°0′50.75″ | 39°20′29.58″ | Helianthemum songaricum + Ajania achilleoides | 2 | Sandy brown soil | alkaline | 51.7 |
| Caragana roborovskyi Kom | 107°0′50.67″ | 39°20′29.18″ | Helianthemum songaricum + Ajania achilleoides | 2 | Sandy brown soil | alkaline | 51.7 |
| Helianthemum songaricum Schrenk | 107°0′51.36″ | 39°20′29.11″ | Helianthemum songaricum + Ajania achilleoides | 2 | Sandy brown soil | alkaline | 51.7 |
| Krascheninnikovia ceratoides (Linnaeus) Gueldenstaedt | 107°27′56″ | 39°39′34.62″ | Artemisia ordosica + Krascheninnikovia ceratoides | 2 | Sandy brown soil | alkaline | 75.6 |
| Artemisia ordosica Krasch | 107°27′57″ | 39°39′34.62″ | Artemisia ordosica + Krascheninnikovia ceratoides | 3 | Sandy brown soil | alkaline | 75.6 |
| Asterothamnus alyssoides (Turcz.) Novopokr | 107°27′58″ | 39°39′34.62″ | Artemisia ordosica + Krascheninnikovia ceratoides | 2 | Sandy brown soil | alkaline | 75.6 |
| Ajania achilleoides (Turcz.) Poljakov ex Grubov | 107°0′50.78″ | 39°20′29.00″ | Helianthemum songaricum + Ajania achilleoides | 2 | Gravelly brown soil | alkaline | 51.7 |
| Leaf Traits | Abbrev | Unit | Mean | Max | Min | SE |
|---|---|---|---|---|---|---|
| leaf vein density | LVD | mm mm−2 | 5.08 | 9.29 | 1.36 | 0.45 |
| midrib diameter | MD | μm | 121.71 | 325.03 | 42.40 | 14.87 |
| stomatal density | SD | no. mm−2 | 285.94 | 525.87 | 121.60 | 8.52 |
| average guard cell length | GCL | μm | 30.22 | 42.53 | 17.90 | 0.49 |
| stomatal area index | SAI | mm2 mm−2 | 0.25 | 0.42 | 0.08 | 0.01 |
| maximum stomatal conductance | gmax | mol m−2s−1 | 2.40 | 3.91 | 0.89 | 0.06 |
| epidermis thickness | Tep | μm | 44.81 | 86.72 | 24.12 | 4.05 |
| spongy tissue thickness | Sp | μm | 105.87 | 347.53 | 0.00 | 24.74 |
| palisade tissue thickness | Tpa | μm | 325.56 | 1098.17 | 133.32 | 53.84 |
| leaf cuticle thickness | TC | μm | 18.58 | 38.38 | 7.42 | 1.54 |
| ratio of spongy tissue thickness to palisade tissue thickness | Sp/Tpa | % | 31.72 | 97.70 | 0.00 | 5.96 |
| ratio of cuticle thickness to leaf thickness | TCf | % | 10.56 | 17.75 | 2.82 | 1.01 |
| leaf thickness | LT | μm | 482.35 | 1382.63 | 212.66 | 72.00 |
| specific leaf area | SLA | m2 Kg−1 | 13.09 | 29.14 | 7.10 | 1.17 |
| Carbon content per unit mass | C | mg g−1 | 28.47 | 42.39 | 18.05 | 1.53 |
| Nitrogen content per unit mass | N | mg g−1 | 434.55 | 523.66 | 295.74 | 11.75 |
| Phosphorus content per unit mass | P | mg g−1 | 1.56 | 2.91 | 0.87 | 0.13 |
| the ratio of nitrogen content to phosphorus content per unit mass | N:P | — | 19.39 | 27.71 | 10.36 | 1.11 |
| leaf water potential at turgor loss point | TLPleaf | MPa | −3.81 | −1.98 | −6.21 | 0.26 |
| maximum conductance in leaves | Kmax | mmol Kg−1 MPa−1 s−1 | 451.86 | 716.84 | 157.31 | 48.38 |
| the water potential inducing 50% loss of maximum conductance in leaves | P50leaf | MPa | −1.01 | −0.73 | −1.23 | 0.04 |
| leaf predawn water potential | WPpd | MPa | −1.36 | −0.82 | −2.03 | 0.07 |
| leaf midday water potential | WPleaf | MPa | −3.16 | −1.70 | −4.81 | 0.18 |
| leaf hydraulic safety margin | HSMleaf | MPa | −2.00 | −0.74 | −3.59 | 0.22 |
| leaf hydraulic safety margins for wilting | HSMtlp | MPa | 0.65 | 3.05 | −1.78 | 0.28 |
| leaf water capacity per unit mass | Cm | mol Kg−1 Pa | 4.44 | 5.98 | 2.16 | 0.26 |
| leaf water capacity per unit area | Cs | mol m−2 Pa−1 | 2.69 | 8.14 | 1.05 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Cao, W.; Li, X.; Li, Q. Characteristics, Relationships, and Anatomical Basis of Leaf Hydraulic Traits and Economic Traits in Temperate Desert Shrub Species. Life 2024, 14, 834. https://doi.org/10.3390/life14070834
Tan F, Cao W, Li X, Li Q. Characteristics, Relationships, and Anatomical Basis of Leaf Hydraulic Traits and Economic Traits in Temperate Desert Shrub Species. Life. 2024; 14(7):834. https://doi.org/10.3390/life14070834
Chicago/Turabian StyleTan, Fengsen, Wenxu Cao, Xu Li, and Qinghe Li. 2024. "Characteristics, Relationships, and Anatomical Basis of Leaf Hydraulic Traits and Economic Traits in Temperate Desert Shrub Species" Life 14, no. 7: 834. https://doi.org/10.3390/life14070834
APA StyleTan, F., Cao, W., Li, X., & Li, Q. (2024). Characteristics, Relationships, and Anatomical Basis of Leaf Hydraulic Traits and Economic Traits in Temperate Desert Shrub Species. Life, 14(7), 834. https://doi.org/10.3390/life14070834





