Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial
Abstract
1. Introduction
2. Methods
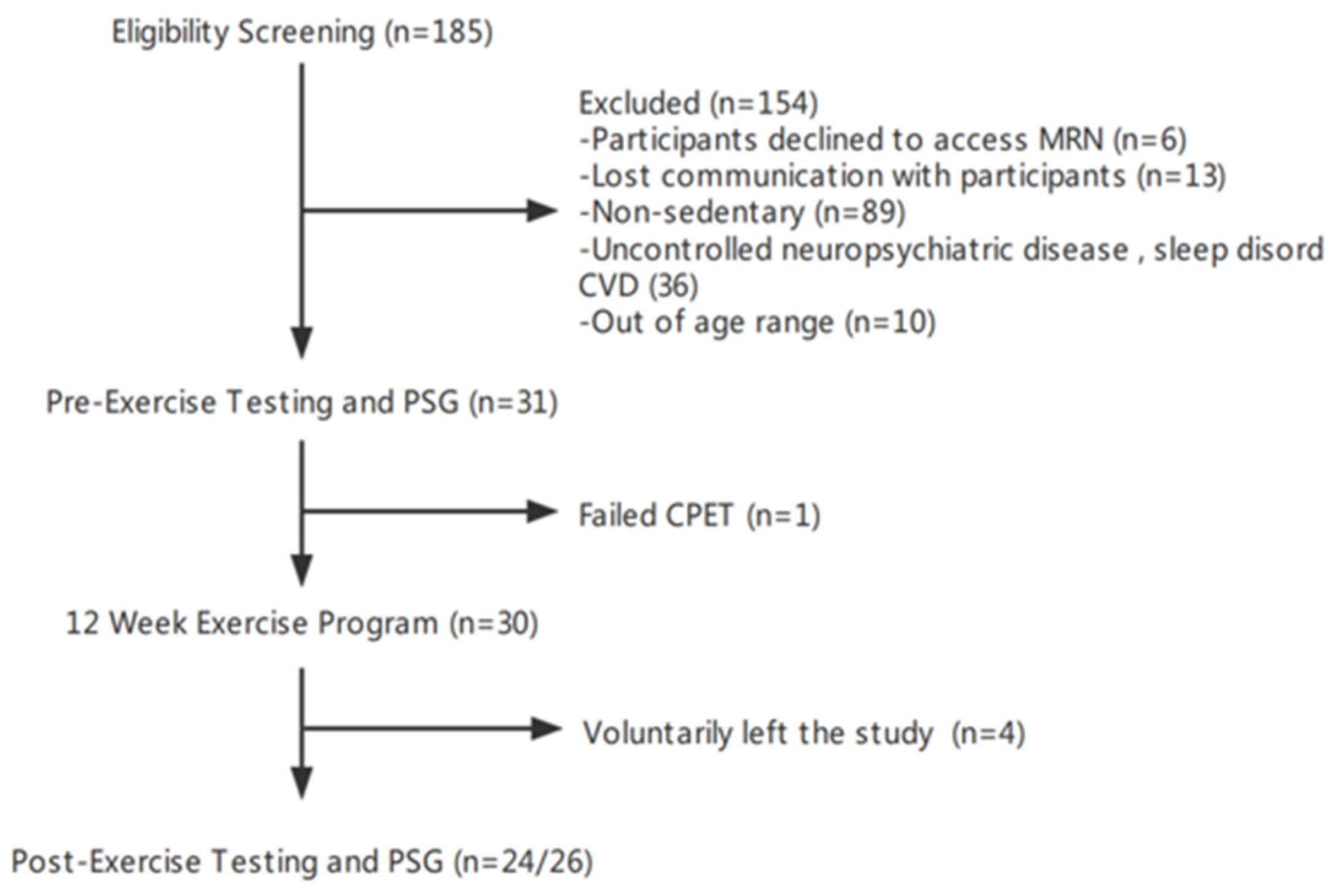
2.1. Study Design and Participants
2.2. Cardiopulmonary Exercise Testing
2.3. Cognitive Testing
2.4. Plasma Biomarkers Level Assessment
2.5. Diagnostic Polysomnography (PSG)
2.6. 12-Week Exercise Regimen
2.7. Home Data Collection
2.8. Exercising Time and Sleeping Heart Rate
2.9. EEG Preprocessing and Artifact Removal
2.10. Brain Age Computation and Spindle Analysis
2.11. Sleep Metrics Analysis
- Sleep efficiency = TST/TIB × 100%;
- Awakening index = (# of transitions sleep to wake)/TIB;
- WASO = total wake time after sleep and before final awakening;
2.12. Statistical Analysis
3. Results
3.1. Participant Characteristics
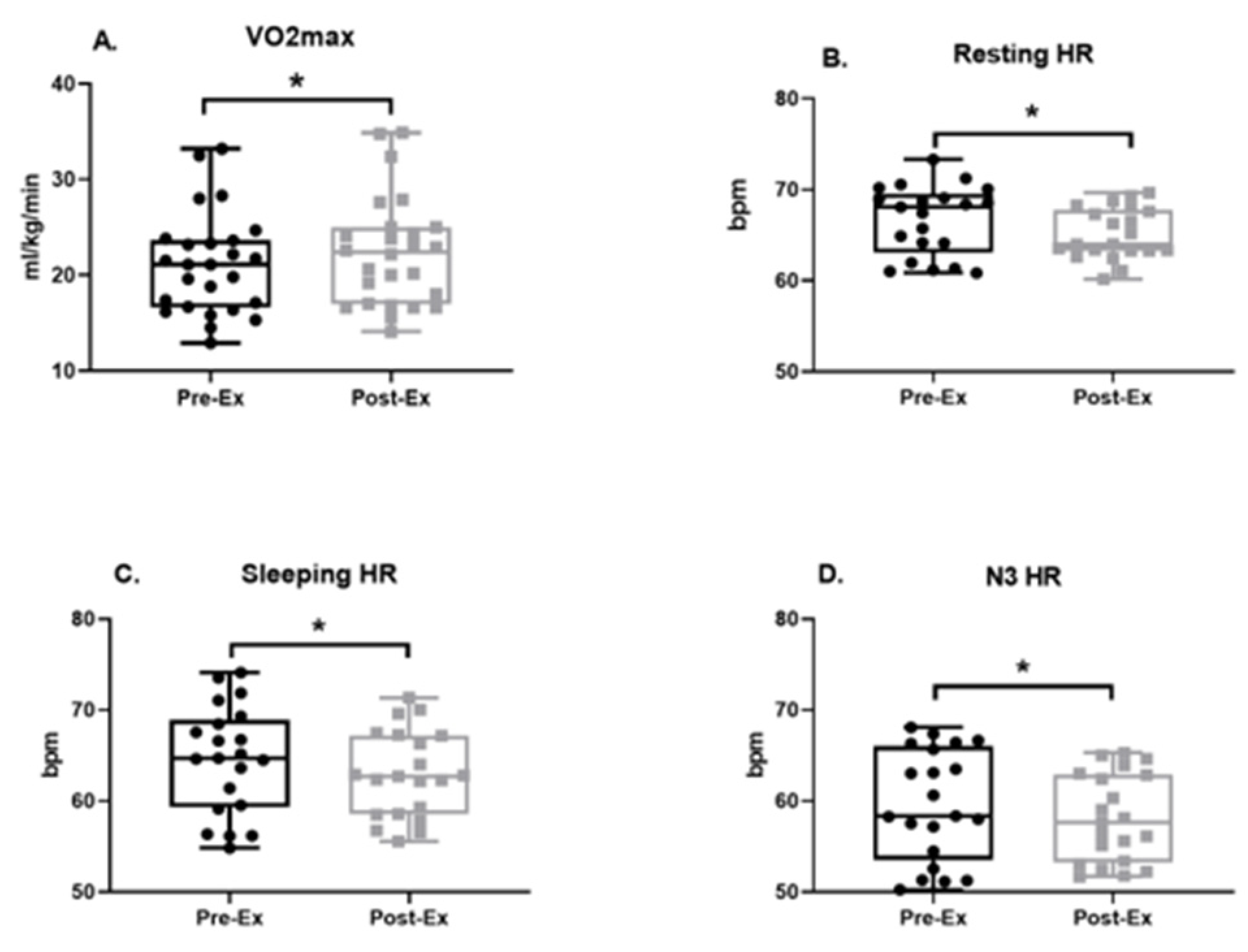
3.2. Physical Fitness
3.3. Cognition Performance Score
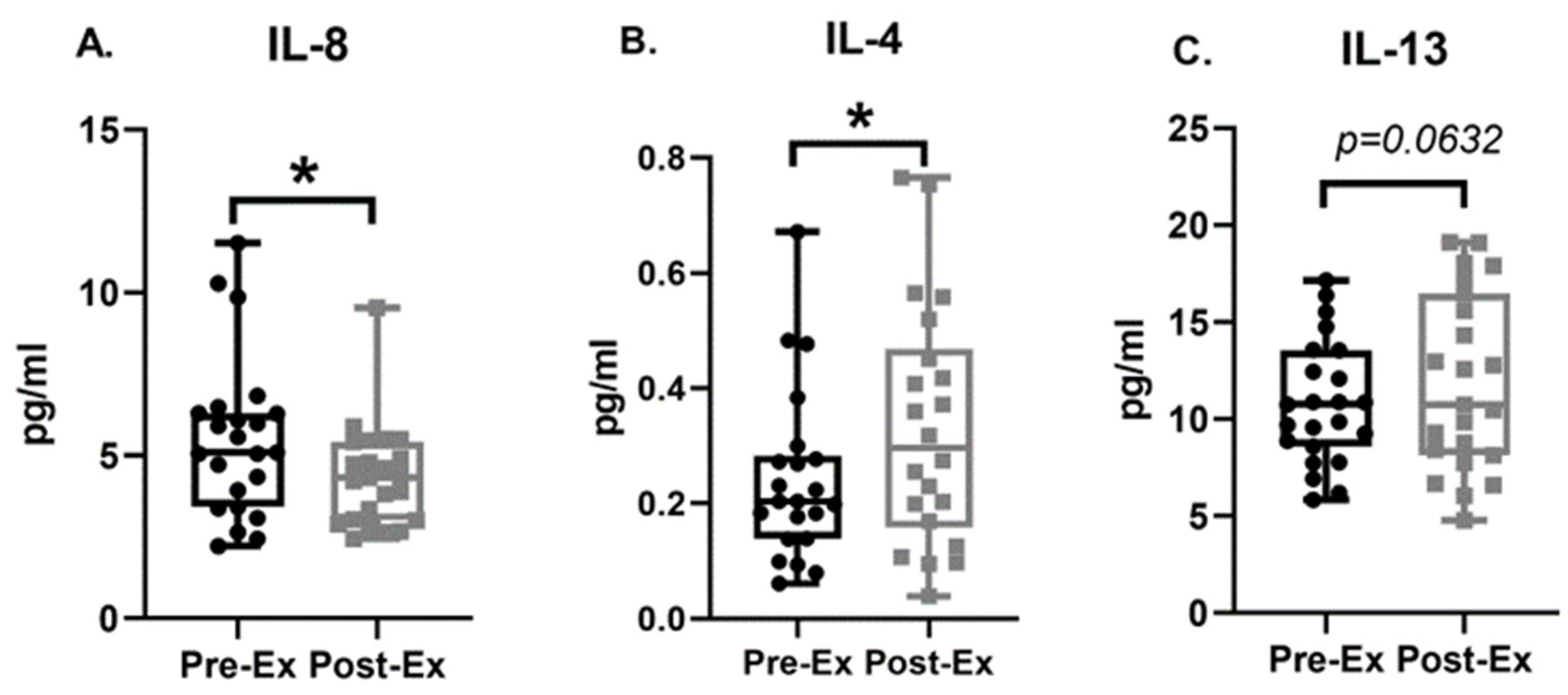
3.4. Plasma Biomarkers
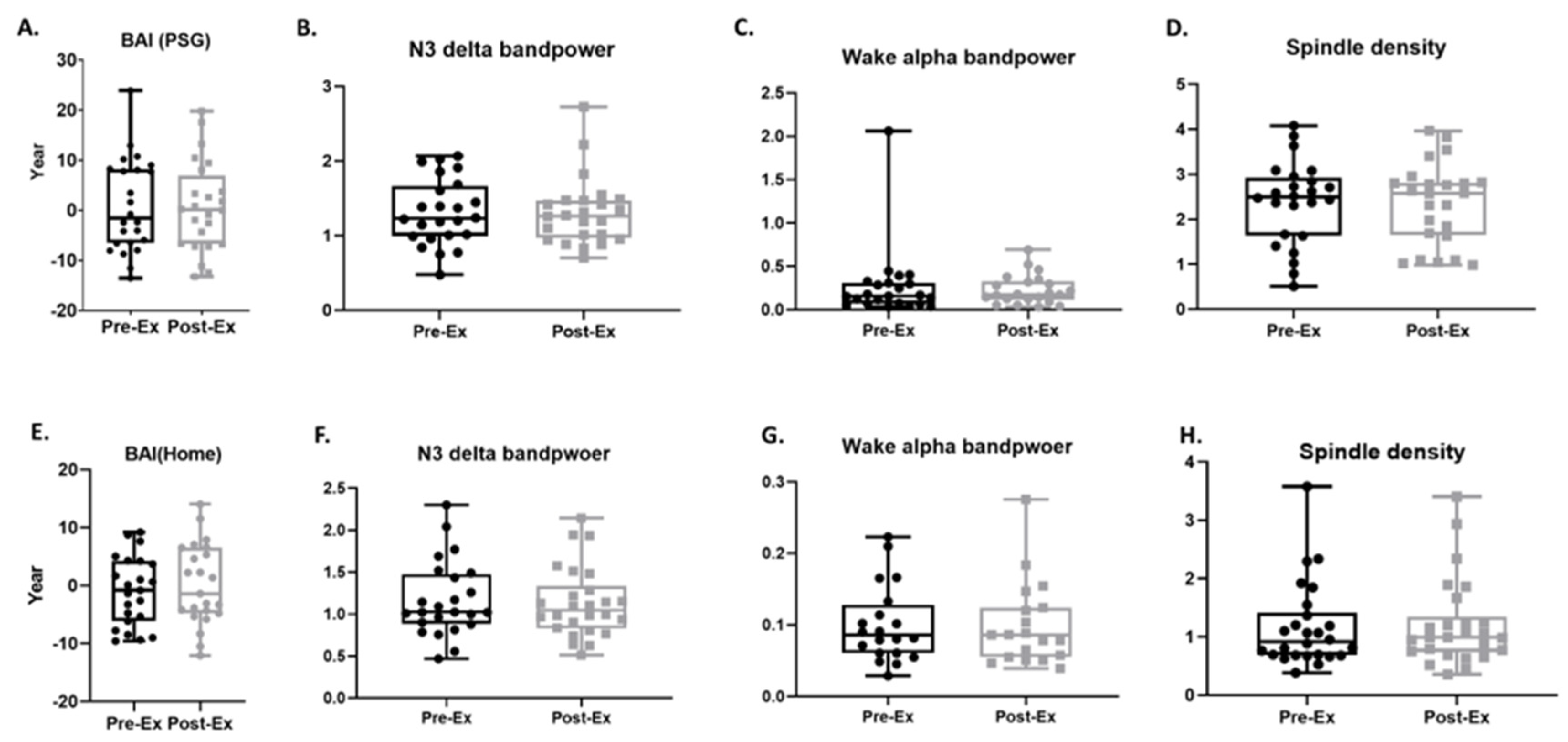
3.5. BAI and Sleep Micro-Architecture
3.6. Sleep Macro-Architecture
3.7. Associations of the Study Outcomes
4. Discussion
4.1. Improvements in Cognitive Performance
4.2. Sleep and Sleep EEG-Based Brain Age Index
4.3. Improvements in Aerobic Fitness
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Lo, J.C.; Loh, K.K.; Zheng, H.; Sim, S.K.; Chee, M.W. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep 2014, 37, 821. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Kumar, R.; Ogren, J.A.; Macey, P.M. Sleep-disordered breathing: Effects on brain structure and function. Respir. Physiol. Neurobiol. 2013, 188, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A.I. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar] [PubMed]
- Motomura, Y.; Kitamura, S.; Oba, K.; Terasawa, Y.; Enomoto, M.; Katayose, Y.; Hida, A.; Moriguchi, Y.; Higuchi, S.; Mishima, K. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS ONE 2013, 8, e56578. [Google Scholar] [CrossRef]
- Goldstein, A.N.; Walker, M.P. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef]
- Bah, T.M.; Goodman, J.; Iliff, J.J. Sleep as a therapeutic target in the aging brain. Neurotherapeutics 2019, 16, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Piani, A.; Gigli, G.; Cancelli, I.; Rinaldi, A.; Baroselli, A.; Serafini, A.; Zanchettin, B.; Valente, M. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med. 2010, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Nebes, R.D.; Buysse, D.J.; Halligan, E.M.; Houck, P.R.; Monk, T.H. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J. Gerontol. Ser. B 2009, 64, 180–187. [Google Scholar] [CrossRef]
- Schmutte, T.; Harris, S.; Levin, R.; Zweig, R.; Katz, M.; Lipton, R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: Results from the Bronx aging study. Behav. Sleep Med. 2007, 5, 39–56. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef]
- Benz, R.L.; Pressman, M.R.; Hovick, E.T.; Peterson, D.D. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am. J. Kidney Dis. 2000, 35, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Dew, M.A.; Hoch, C.C.; Buysse, D.J.; Monk, T.H.; Begley, A.E.; Houck, P.R.; Hall, M.; Kupfer, D.J.; Reynolds III, C.F. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom. Med. 2003, 65, 63–73. [Google Scholar] [CrossRef]
- Reid, K.J.; Baron, K.G.; Lu, B.; Naylor, E.; Wolfe, L.; Zee, P.C. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010, 11, 934–940. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Pruitt, L.A.; Woo, S.; Castro, C.M.; Ahn, D.K.; Vitiello, M.V.; Woodward, S.H.; Bliwise, D.L. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Pitkälä, K.H. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement. Geriatr. Cogn. Disord. 2014, 38, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Baril, A.-A.; Beiser, A.S.; Mysliwiec, V.; Sanchez, E.; DeCarli, C.S.; Redline, S.; Gottlieb, D.J.; Maillard, P.; Romero, J.R.; Satizabal, C.L. Slow-wave sleep and MRI markers of brain aging in a community-based sample. Neurology 2021, 96, e1462–e1469. [Google Scholar] [CrossRef]
- Melancon, M.O.; Lorrain, D.; Dionne, I.J. Sleep depth and continuity before and after chronic exercise in older men: Electrophysiological evidence. Physiol. Behav. 2015, 140, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Diaz, J.; Matsumoto, S.; Iwayama, K.; Nabekura, Y.; Ogata, H.; Kayaba, M.; Aoyagi, A.; Yajima, K.; Satoh, M.; et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci. Rep. 2021, 11, 4410. [Google Scholar] [CrossRef]
- Aritake-Okada, S.; Tanabe, K.; Mochizuki, Y.; Ochiai, R.; Hibi, M.; Kozuma, K.; Katsuragi, Y.; Ganeko, M.; Takeda, N.; Uchida, S. Diurnal repeated exercise promotes slow-wave activity and fast-sigma power during sleep with increase in body temperature: A human crossover trial. J. Appl. Physiol. 2019, 127, 168–177. [Google Scholar] [CrossRef]
- Dworak, M.; Wiater, A.; Alfer, D.; Stephan, E.; Hollmann, W.; Struder, H.K. Increased slow wave sleep and reduced stage 2 sleep in children depending on exercise intensity. Sleep Med. 2008, 9, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Sewitch, D.E.; Epstein, L.H.; Bulik, C.; McGowen, C.R.; Robertson, R.J. Exercise and subsequent sleep in male runners: Failure to support the slow wave sleep-mood-exercise hypothesis. Neuropsychobiology 1985, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Floyd, T.C.; Fein, G.; Cavness, C.; Lualhati, R.; Feinburg, I. Effects of exercise on sleep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, 2015, CD006489. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Huang, M.-Z.; Liao, L.-R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Medicine, A.C.O.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Sun, H.; Paixao, L.; Oliva, J.T.; Goparaju, B.; Carvalho, D.Z.; van Leeuwen, K.G.; Akeju, O.; Thomas, R.J.; Cash, S.S.; Bianchi, M.T. Brain age from the electroencephalogram of sleep. Neurobiol. Aging 2019, 74, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.M.; Sun, H.M.; Lam, A.D.; Westover, M.B. Validation of EEG-based brain age index as a biomarker for dementia: Biomarkers (non-neuroimaging)/novel biomarkers. Alzheimer’s Dement. 2020, 16, e044025. [Google Scholar] [CrossRef]
- Reiter, K.; Nielson, K.A.; Smith, T.J.; Weiss, L.R.; Alfini, A.J.; Smith, J.C. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J. Int. Neuropsychol. Soc. 2015, 21, 757–767. [Google Scholar] [CrossRef]
- Kline, C.; Porcari, J.P.; Hintermeister, R.; Freedson, P.S.; Ward, A.; McCarron, R.F.; Rippe, J. Estimation of from a one-mile track walk, gender, age and body weight. Med. Sci. Sports Exerc. 1987, 19, 253–259. [Google Scholar] [CrossRef]
- Pober, D.M.; Freedson, P.S.; Kline, G.M.; McInnis, K.J.; Rippe, J.M. Development and validation of a one-mile treadmill walk test to predict peak oxygen uptake in healthy adults ages 40 to 79 years. Can. J. Appl. Physiol. 2002, 27, 575–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Slotkin, J.; Carlozzi, N.E.; Bauer, P.J.; Wallner-Allen, K.; Fox, N. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: Validation in an adult sample. J. Int. Neuropsychol. Soc. 2014, 20, 567–578. [Google Scholar] [CrossRef]
- Burguillos, M.A. Use of meso-scale discovery to examine cytokine content in microglia cell supernatant. Methods Mol. Biol. 2013, 1041, 93–100. [Google Scholar]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Zhang, C.; Carlyle, B.C.; Zhen, S.Y.; Trombetta, B.A.; Schultz, A.P.; Pruzin, J.J.; Fitzpatrick, C.D.; Yau, W.W.; Kirn, D.R.; et al. Plasma IL-12/IFN-gamma axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2021, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Quan, S.F.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Mao, M.-M.; Plante, D.T.; Pressman, M.R.; Troester, M.M. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Bou Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep 2020, 43, zsaa097. [Google Scholar] [CrossRef]
- Younes, M.; Soiferman, M.; Thompson, W.; Giannouli, E. Performance of a new portable wireless sleep monitor. J. Clin. Sleep Med. 2017, 13, 245–258. [Google Scholar] [CrossRef]
- Kahn, R.A.; Randazzo, P.; Serafini, T.; Weiss, O.; Rulka, C.; Clark, J.; Amherdt, M.; Roller, P.; Orci, L.; Rothman, J.E. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J. Biol. Chem. 1992, 267, 13039–13046. [Google Scholar] [CrossRef]
- Biswal, S.; Sun, H.; Goparaju, B.; Westover, M.B.; Sun, J.; Bianchi, M.T. Expert-level sleep scoring with deep neural networks. J. Am. Med. Inform. Assoc. 2018, 25, 1643–1650. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Lindstrom, M.J.; Bates, D.M. Newton—Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J. Am. Stat. Assoc. 1988, 83, 1014–1022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Edwards, D.J. Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Res. Q. Exerc. Sport 1991, 62, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, T.R.; Pa, J.; Kutch, J.J.; Lane, C.J.; Duncan, D.; Yan, L.; Schroeder, E.T. 12 weeks of strength training improves fluid cognition in older adults: A nonrandomized pilot trial. PLoS ONE 2021, 16, e0255018. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef]
- Rathore, A.; Lom, B. The effects of chronic and acute physical activity on working memory performance in healthy participants: A systematic review with meta-analysis of randomized controlled trials. Syst. Rev. 2017, 6, 124. [Google Scholar] [CrossRef]
- Falleti, M.G.; Maruff, P.; Collie, A.; Darby, D.G. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J. Clin. Exp. Neuropsychol. 2006, 28, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef]
- Szabo, A.N.; McAuley, E.; Erickson, K.I.; Voss, M.; Prakash, R.S.; Mailey, E.L.; Wójcicki, T.R.; White, S.M.; Gothe, N.; Olson, E.A. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology 2011, 25, 545. [Google Scholar] [CrossRef] [PubMed]
- Burzynska, A.Z.; Chaddock-Heyman, L.; Voss, M.W.; Wong, C.N.; Gothe, N.P.; Olson, E.A.; Knecht, A.; Lewis, A.; Monti, J.M.; Cooke, G.E. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS ONE 2014, 9, e107413. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.; Nielsen, A.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.; Lindegaard, B.; Petersen, A.; Taudorf, S. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; van Praag, H. Exercise and hippocampal memory systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, W.; Xiao, Q.; Beers, D.R.; Appel, S.H. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006, 99, 1176–1187. [Google Scholar] [CrossRef]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Westman, E.; Pelini, L.; Lindberg, O.; Muehlboeck, J.; Simmons, A.; Tarducci, R.; Floridi, P.; Chiarini, P.; Soininen, H. Differential associations of IL-4 with hippocampal subfields in Mild Cognitive Impairment and Alzheimer’s disease. Front. Aging Neurosci. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Ng, T.; Shwe, M.; Ho, H.; Foo, K.; Cham, M.; Lee, J.; Fan, G.; Tan, Y.; Yong, W. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 2015, 26, 1446–1451. [Google Scholar] [CrossRef]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef]
- Baune, B.T.; Ponath, G.; Golledge, J.; Varga, G.; Arolt, V.; Rothermundt, M.; Berger, K. Association between IL-8 cytokine and cognitive performance in an elderly general population—The MEMO-Study. Neurobiol. Aging 2008, 29, 937–944. [Google Scholar] [CrossRef]
- Dorneles, G.P.; Haddad, D.O.; Fagundes, V.O.; Vargas, B.K.; Kloecker, A.; Romao, P.R.; Peres, A. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight-obese individuals. Cytokine 2016, 77, 1–9. [Google Scholar] [CrossRef]
- Ito, K.; Hanazawa, T.; Tomita, K.; Barnes, P.; Adcock, I. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: Role of tyrosine nitration. Biochem. Biophys. Res. Commun. 2004, 315, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, A.; Olver, T.D.; Emter, C.A.; Fleenor, B.S. Chronic exercise training prevents coronary artery stiffening in aortic-banded miniswine: Role of perivascular adipose-derived advanced glycation end products. J. Appl. Physiol. 2019, 127, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Evola, M.; Hall, A.; Wall, T.; Young, A.; Grammas, P. Oxidative stress impairs learning and memory in apoE knockout mice. Pharmacol. Biochem. Behav. 2010, 96, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, J.; Liu, X.-D.; Luo, M.-Y.; Xu, N.-J. Roles of physical exercise in neurodegeneration: Reversal of epigenetic clock. Transl. Neurodegener. 2021, 10, 30. [Google Scholar] [CrossRef]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef]
- Torsvall, L.; Åkerstedt, T.; Göran, L. Effects on sleep stages and EEG power density of different degrees of exercise in fit subjects. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 347–353. [Google Scholar] [CrossRef]
- Shapiro, C.M.; Bortz, R.; Mitchell, D.; Bartel, P.; Jooste, P. Slow-wave sleep: A recovery period after exercise. Science 1981, 214, 1253–1254. [Google Scholar] [CrossRef]
- Munz, M.T.; Prehn-Kristensen, A.; Thielking, F.; Molle, M.; Goder, R.; Baving, L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front. Cell. Neurosci. 2015, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Saebipour, M.R.; Joghataei, M.T.; Yoonessi, A.; Sadeghniiat-Haghighi, K.; Khalighinejad, N.; Khademi, S. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: A pilot study. J. Sleep Res. 2015, 24, 518–525. [Google Scholar] [CrossRef]
- Grimaldi, D.; Papalambros, N.A.; Zee, P.C.; Malkani, R.G. Neurostimulation techniques to enhance sleep and improve cognition in aging. Neurobiol. Dis. 2020, 141, 104865. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Chong, P.L.H.; Garic, D.; Shen, M.D.; Lundgaard, I.; Schwichtenberg, A.J. Sleep, cerebrospinal fluid, and the glymphatic system: A systematic review. Sleep Med. Rev. 2021, 61, 101572. [Google Scholar] [CrossRef]
- Thomas, R.J.; Mietus, J.E.; Peng, C.K.; Goldberger, A.L. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 2005, 28, 1151–1161. [Google Scholar] [CrossRef]
- Guadagni, V.; Byles, H.; Tyndall, A.V.; Parboosingh, J.; Longman, R.S.; Hogan, D.B.; Hanly, P.J.; Younes, M.; Poulin, M.J. Association of sleep spindle characteristics with executive functioning in healthy sedentary middle-aged and older adults. J. Sleep Res. 2021, 30, e13037. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiatry 2020, 33, e100213. [Google Scholar] [CrossRef] [PubMed]
- Sher, L. COVID-19, anxiety, sleep disturbances and suicide. Sleep Med. 2020, 70, 124. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, S.; Trott, M.; Tully, M.; Shin, J.; Barnett, Y.; Butler, L.; McDermott, D.; Schuch, F.; Smith, L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000960. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; van Zeller, M.; Amorim, P.; Pimentel, A.; Dantas, P.; Eusébio, E.; Neves, A.; Pipa, J.; Santa Clara, E.; Santiago, T. Sleep quality in times of Covid-19 pandemic. Sleep Med. 2020, 74, 81–85. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kong, D.; Li, S.; Yang, N. Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (COVID-19) outbreak in January 2020 in China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923921. [Google Scholar] [CrossRef]
- Bacon, A.P.; Carter, R.E.; Ogle, E.A.; Joyner, M.J. VO2max trainability and high intensity interval training in humans: A meta-analysis. PLoS ONE 2013, 8, e73182. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gibson, C.A.; Tran, Z.V.; Osness, W.H. Controlled endurance exercise training and VO2max changes in older adults: A meta-analysis. Prev. Cardiol. 2005, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.K.; Knapp, G.; Reimers, C.-D. Effects of exercise on the resting heart rate: A systematic review and meta-analysis of interventional studies. J. Clin. Med. 2018, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Ehsani, A.A.; Domitrovich, P.P.; Kleiger, R.E.; Rottman, J.N. Effect of exercise training on heart rate variability in healthy older adults. Am. Heart J. 1999, 138, 567–576. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Kail, R.; Salthouse, T.A. Processing speed as a mental capacity. Acta Psychol. 1994, 86, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A.; Kail, R. Memory development throughout the life span: The role of processing rate. In Handbook of Life-Span Development; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Gontkovsky, S.T.; Beatty, W.W. Practical methods for the clinical assessment of information processing speed. Int. J. Neurosci. 2006, 116, 1317–1325. [Google Scholar] [CrossRef]
- de Jager, C.A.; Hogervorst, E.; Combrinck, M.; Budge, M.M. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer’s disease. Psychol. Med. 2003, 33, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Breed, S.; Sacks, A.; Ashman, T.A.; Gordon, W.A.; Dahlman, K.; Spielman, L. Cognitive functioning among individuals with traumatic brain injury, Alzheimer’s disease, and no cognitive impairments. J. Head Trauma Rehabil. 2008, 23, 149–157. [Google Scholar] [CrossRef]
- Mendez, M.F.; Cherrier, M.M.; Perryman, K.M. Differences between Alzheimer’s disease and vascular dementia on information processing measures. Brain Cogn. 1997, 34, 301–310. [Google Scholar] [CrossRef]
- Krueger, J.M.; Obál Jr, F.; Fang, J.; Kubota, T.; Taishi, P. The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 2001, 933, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, T.M.; Nono, J.K.; De Gouveia, K.S.; Makena, N.; Darby, M.; Womersley, J.; Tamgue, O.; Brombacher, F. IL-13–mediated regulation of learning and memory. J. Immunol. 2017, 198, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Van Breukelen, G.J. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J. Clin. Epidemiol. 2006, 59, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Sun, H.; Paixao, L.; Westmeijer, M.; Sikka, P.; Jin, J.; Tesh, R.; Cardoso, M.; Cash, S.S.; Akeju, O. Night-to-night variability of sleep electroencephalography-based brain age measurements. Clin. Neurophysiol. 2021, 132, 1–12. [Google Scholar] [CrossRef]
- Schaun, G.Z. The maximal oxygen uptake verification phase: A light at the end of the tunnel? Sports Med. Open 2017, 3, 44. [Google Scholar] [CrossRef]



| Pre-Exercise | Post-Exercise | |
|---|---|---|
| Age | 60.11 ± 7.37 | - |
| Sex (F, M) | 26 (20/77%, 6/23%) | - |
| Race | 1/3% (American Indian or Native Alaskan) | - |
| 3/12% (Asian) | ||
| 1/3% (Black or African) | ||
| 23/88% (White) | ||
| YOE (SD) | 17.19 ± 2.80 | - |
| BMI (SD) | 25.86 ± 4.16 | 25.83 ± 3.97 |
| AHI | 4.05 ± 3.92 | 3.06 ± 3.94 |
| Exercise days | - | 47 ± 13 |
| Exercise time (minutes) | - | 55 ± 9.8 |
| Features | Pearson’s r | p Value | |
|---|---|---|---|
| VO2max vs. | SHR | −0.47 | 0.0160 |
| FLD | 0.65 | 0.0003 | |
| cognition total | 0.53 | 0.0050 | |
| DCCS | 0.62 | 0.0007 | |
| FICA | 0.49 | 0.0115 | |
| LSWM | 0.70 | <0.0001 | |
| PCPS | 0.40 | 0.0441 | |
| BAI | 0.03 | 0.8914 | |
| BAI vs. | sleep efficiency | −0.53 | 0.0099 |
| WASO | 0.47 | 0.0252 | |
| Wake | 0.53 | 0.0099 | |
| N2 | −0.44 | 0.0368 | |
| N3 | −0.42 | 0.0440 | |
| NREM | −0.58 | 0.0035 | |
| IL-13 | −0.60 | 0.0023 | |
| IL-4 | −0.47 | 0.0240 | |
| DCCS vs. | N2 | 0.50 | 0.0150 |
| NREM | 0.44 | 0.0370 | |
| IL-13 vs. | PVT | −0.47 | 0.0246 |
| sleep efficiency | 0.46 | 0.0262 | |
| Wake | −0.46 | 0.0262 | |
| N3 | 0.55 | 0.0062 | |
| NREM | 0.54 | 0.0081 | |
| IL-4 vs. | PCPS | 0.51 | 0.0134 |
| ORR | 0.43 | 0.0412 | |
| delta band power in N3 | 0.42 | 0.0435 | |
| IL-8 vs. | awakening index | 0.44 | 0.0341 |
| Dependent var. | Independent var. | p Value | 95%CI | Coefficient |
|---|---|---|---|---|
| VO2max | BAI | 0.564 | −0.14, 0.07 | −0.03 |
| Resting HR | 0.03 | −0.29, −0.05 | −0.15 | |
| BAI | VO2max | 0.868 | −0.66, 0.55 | −0.05 |
| Resting HR | 0.087 | −0.5, 0.75 | 0.35 | |
| FLD | BAI | 0.009 | −0.5, −0.06 | −0.28 |
| PCPS | 0.002 | −0.32, −0.05 | −0.18 | |
| LSWM | 0.103 | −0.4, 0.07 | −0.17 | |
| IL−4 | 0.009 | −22.83, −3.89 | −13.36 | |
| IL−8 | 0.035 | 0.04, 2.17 | 1.1 | |
| IL−13 | <0.0001 | −1.64, −0.51 | −1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, A.; Zhang, C.; Adra, N.; Tesh, R.A.; Sun, H.; Lei, D.; Jing, J.; Fan, P.; Paixao, L.; Ganglberger, W.; et al. Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial. Life 2024, 14, 855. https://doi.org/10.3390/life14070855
Ouyang A, Zhang C, Adra N, Tesh RA, Sun H, Lei D, Jing J, Fan P, Paixao L, Ganglberger W, et al. Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial. Life. 2024; 14(7):855. https://doi.org/10.3390/life14070855
Chicago/Turabian StyleOuyang, An, Can Zhang, Noor Adra, Ryan A. Tesh, Haoqi Sun, Dan Lei, Jin Jing, Peng Fan, Luis Paixao, Wolfgang Ganglberger, and et al. 2024. "Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial" Life 14, no. 7: 855. https://doi.org/10.3390/life14070855
APA StyleOuyang, A., Zhang, C., Adra, N., Tesh, R. A., Sun, H., Lei, D., Jing, J., Fan, P., Paixao, L., Ganglberger, W., Briggs, L., Salinas, J., Bevers, M. B., Wrann, C. D., Chemali, Z., Fricchione, G., Thomas, R. J., Rosand, J., Tanzi, R. E., & Westover, M. B. (2024). Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial. Life, 14(7), 855. https://doi.org/10.3390/life14070855









