Liver and Inflammatory Biomarkers Are Related to High Mortality in Hospitalized Patients with COVID-19 in Brazilian Amazon Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Clinical and Epidemiological Profile
3.2. Liver Disorders and Inflammation
3.3. Liver Injury and Mortality
3.4. Biomarkers in Relation to Ventilation Mode
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campana, A.C.; Kuczera, A.C.V.; Meneghetti, B.D.; Daga, D.; Fraccanabbia, D.; Horst, H.; Daghetti, K.C.; Walczewski, L.G.B.; dos Santos, M.L.; Brueckheimer, M.E.K.; et al. Prevalência de Comorbidades em Indivíduos Infectados por COVID-19 em um Município de Porte Médio. Arq. Ciên. Saúde UNIPAR 2023, 27, 121–134. [Google Scholar] [CrossRef]
- Febres-Ramos, R.J. Clinical features of the digestive system in COVID-19. Horiz Med. 2022, 22, e1930. [Google Scholar] [CrossRef]
- Nascimento, J.H.P.; Gomes, B.F.D.O.; Carmo Júnior, P.R.D.; Petriz, J.L.F.; Rizk, S.I.; Costa, I.B.S.D.S.; Lacerda, M.V.G.; Bacal, F.; Hajjar, L.A.; Oliveira, G.M.M.D. COVID-19 and the Hypercoagulable State: A New Therapeutic Perspective. Arq. Bras. Cardiol. 2020, 114, 829–833. [Google Scholar] [CrossRef]
- Diaz-Louzao, C.; Barrera-Lopez, L.; Lopez-Rodriguez, M.; Casar, C.; Vazquez-Agra, N.; Pernas-Pardavila, H.; Marques-Afonso, A.; Vidal-Vazquez, M.; Montoya, J.G.; Andrade, A.H.; et al. Longitudinal relationship of live injury with inflammation biomarkers in hospitalized COVID-19 patients using a joint modeling approach. Sci. Rep. 2022, 12, 5547. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.R.; Basnet, A.; Tamang, B.; Khadka, S.; Maharjan, R.; Maharjan, R.; Chand, A.B.; Thapa, S.; Rai, S.K. Analysis of altered level of blood-based biomarkers in prognosis of COVID-19 patients. PLoS ONE 2023, 18, e0287117. [Google Scholar] [CrossRef]
- Freury, M.K. COVID-19 and the hematology laboratory: A recent literature review. Braz. J. Clin. Anal. 2020. [Google Scholar] [CrossRef]
- Hanlon, P.; Chadwick, F.; Shah, A.; Wood, R.; Minton, J.; McCartney, G.; Fischbacher, C.; Mair, F.S.; Husmeier, D.; Matthiopoulos, J.; et al. COVID-19—Exploring the implications of long-term condition type and extent of multimorbidition on years of life lost: A modeling study. Wellcome Open Res. 2021, 5, 75. [Google Scholar] [CrossRef]
- Phipps, M.M.; Barraza, L.H.; LaSota, E.D.; Sobieszczyk, M.E.; Pereira, M.R.; Zheng, E.X.; Fox, A.N.; Zucker, J.; Verna, E.C. Acute liver injury in COVID-19: Prevalence and association with clinical outcomes in a large US cohort. Hepatology 2020, 72, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.P.; Meyerowitz, E.A.; Reinus, Z.; Daidone, M.; Gustafson, J.; Kim, A.Y.; Schaefer, E.; Chung, R.T. Liver biochemistry in patients hospitalized with COVID-19. Hepatology 2021, 73, 890–900. [Google Scholar] [CrossRef]
- Buffon, M.R.; Severo, I.M.; Barcellos, R.D.A.; Azzolin, K.D.O.; Lucena, A.D.F. Critical patients with COVID-19: Sociodemographic and clinical profile and associations between variables and workload. Braz. J. Nurs. 2021, 75, e20210119. [Google Scholar] [CrossRef]
- Ejaz, R.; Ashraf, M.T.; Qadeer, S.; Irfan, M.; Azam, A.; Butt, S.; Bibi, S. Gender-based incidence, recovery period and death rate of COVID-19 among the population of Attock District, Pakistan. Braz. J. Biol. 2023, 83, e249125. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Silva, D.S.M.D.; Bacurau, A.G.D.M.; Francisco, P.M.S.B.; Assumpção, D.D.; Neri, A.L.; Borim, F.S.A. Ageism against the elderly in the context of the COVID-19 pandemic: An integrative review. Public Health Mag. 2020, 55, 1–6. [Google Scholar]
- Van Halem, K.; Bruyndonckx, R.; van der Hilst, J.; Cox, J.; Driesen, P.; Opsomer, M.; Van Steenkiste, E.; Stessel, B.; Dubois, J.; Messiaen, P. Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: A retrospective cohort study. BMC Infect. Dis. 2020, 20, 897. [Google Scholar]
- Santos, L.G.; Baggio, J.A.D.O.; Leal, T.C.; Costa, F.A.; Fernandes, T.R.M.D.O.; Silva, R.V.D.; Armstrong, A.; Carmo, R.F.; Souza, C.D.F.D. Prevalence of Systemic Arterial Hypertension and Diabetes Mellitus in Individuals with COVID-19: A Retrospective Study of Deaths in Pernambuco, Brazil. Braz. Arch. Cardiol. 2021, 117, 1–8. [Google Scholar] [CrossRef]
- Pontes, L. Clinical profile and factors associated with death of COVID-19 patients in the first months of the pandemic. Anna Nery Sch. Mag. 2020, 72, 807–817. [Google Scholar]
- Silva, J.P.; Costa, H.P.S.; Silva, L.P. COVID-19: Introduction and measures of coping with the new coronavirus in Santarém (PA), a medium-sized municipality in the Brazilian Amazon. In Proceedings of the National Art Education Association (NAEA), New York, NY, USA, 3–5 March 2022. [Google Scholar]
- Oliveira, W.K.D.; Duarte, E.; França, G.V.A.D.; Garcia, L.P. How Brazil can stop COVID-19. Epidemiol. Health Serv. Mag. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Orellana, J.D.Y.; Cunha, G.M.; Marrero, L.; Horta, B.L.; Leite, I.C. Explosão da mortalidade no epicentro amazônico da epidemia de COVID-19 Explosion in mortality in the Amazonian epicenter of the COVID-19 epidemic. Rep. Public Health 2020, 36, e00120020. [Google Scholar] [CrossRef]
- Cardoso, P.V.; da Silva Seabra, V.; Bastos, I.B.; de Castro Porto Costa, E. The importance of spatial analysis for decision making: A look at the COVID-19 pandemic. Tamoios Mag. 2020, 16, 125–137. [Google Scholar] [CrossRef]
- Olavegogeascoechea, P.A. Epidemiological characteristics of SARS-CoV-2 infection: A descriptive study. Medwave Mag. 2020, 29, 1–6. [Google Scholar]
- Spearman, C.W.; Aghemo, A.; Valenti, L.; Sonderup, M.W. COVID-19 and the Liver: A 2021 Update. Liver Int. 2021, 41, 1988–1998. [Google Scholar] [CrossRef]
- Gato, S.; Lucena-Valera, A.; Munõz-Hernández, R.; Sousa, J.M.; Romero-Gómez, M.; Ampuero, J. Impact of COVID-19 on liver disease: From the experimental to the clinic perspective. Word J. Virol. 2021, 10, 301. [Google Scholar] [CrossRef]
- Lykowska-Szuber, L.; Wolodzko, K.; Rychter, A.M.; Szymczak-Tomczak, A.; Krela-Kazmierczak, I.; Dobrowolska, A. Liver Injury in Patients with Coronavirus Disease 2019 (COVID-19). A Narrative Review. J. Clin. Med. 2021, 10, 5048. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, J.C.; Jayarajah, U.; Abeysuriya, V.; Riza, R.; Seneviratne, S.L. Liver involvement in COVID-19: A revision systematic. Am. J. Trop. Med. Hyg. 2022, 106, 1026–1041. [Google Scholar] [CrossRef]
- Restrepo-Gutierrez, J.C.; Toro-Montoya, A. I Hepatobiliary disease associated with COVID-19. Hepatol. Mag. 2022, 3, 143–154. [Google Scholar]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2020, 41, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Chang, C.W.D.; Kazahaya, K.; Brereston, J.; Denneny, J.C. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol.-Head Neck Surg. 2020, 163, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef]
- Amin, M. COVID-19 and the liver: Overview. Eur. J. Gastroenterol. Hepatol. 2021, 33, 309–311. [Google Scholar] [CrossRef]
- Ortega-Quiroz, R.J. COVID-19 and Liver Disease: An overview that is being clarified. Rev. Colomb. Gastroenterol. 2022, 37, 131–135. [Google Scholar]
- Harapan, H.; Fajar, J.K.; Supriono, S.; Soegiarto, G.; Wulandari, L.; Seratin, F.; Prayudi, N.G.; Dewi, D.P.; Monica Elsina, M.T.; Atamou, L.; et al. The prevalence, predictors, and outcomes of acute liver injury in patients with COVID-19: A systematic review and meta-analysis. Rev. Med. Virology 2020, 9, 2304. [Google Scholar] [CrossRef]
- Cunha, D.B.A.; Pereira, C.N.P.; Aguiar, Y.C.; Carvalho, S.G.; Borges, J.B.F.; Pedro, J.P.S.; Menezes, P.H.B.; Gonçalves, B.M.; Castro, F.F.S. Use of biological markers for prognostic evaluation of patients with COVID-19: A literature review. Multidiscip. Sci. J. 2021, 2, 1–27. [Google Scholar]
- Sousa, E.L.D.; Gaído, S.B.; Sousa, R.A.D.; Cardoso, O.D.O.; Matos Neto, E.M.D.; Menezes Júnior, J.M.P.D.; Oliveira, B.F.A.D.; Aguiar, B.G.A. Profile of hospital admissions and deaths due to severe acute respiratory syndrome caused by COVID-19 in Piauí: Descriptive study, 2020–2021. Epidemiol. Serviços Saúde 2022, 31, e2021836. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sinica 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Safari, R.; Marofi, P.; Zeinalzadeh, E.; Pagliano, P.; Ganbarov, K.; Esposito, S.; Khodadadi, E.; Yousefi, M.; Samadi Kafil, H. Alteration of Liver Biomarkers in Patients with SARS-CoV-2 (COVID-19). J. Inflamm. Res. 2020, 13, 285–292. [Google Scholar] [CrossRef]
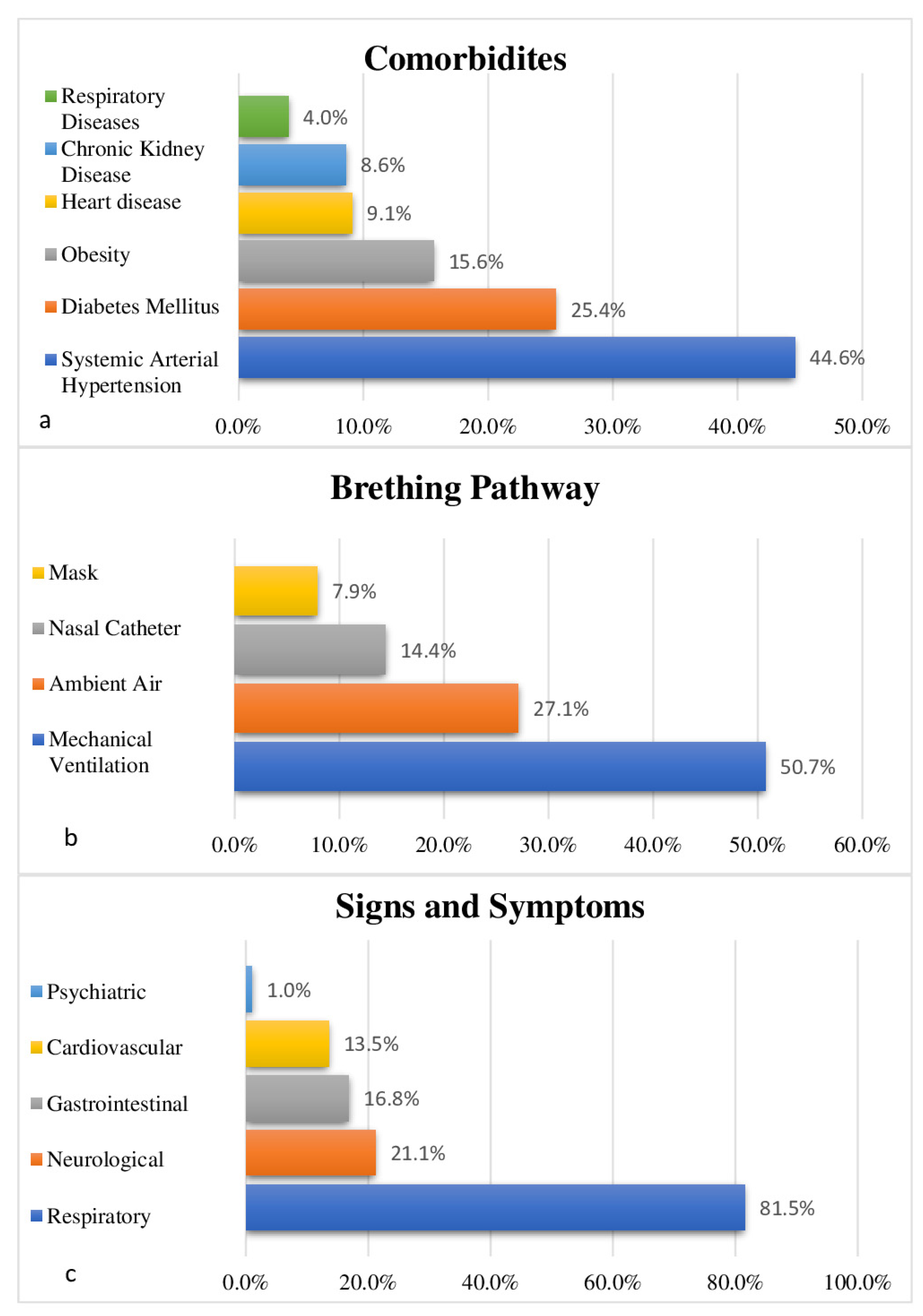
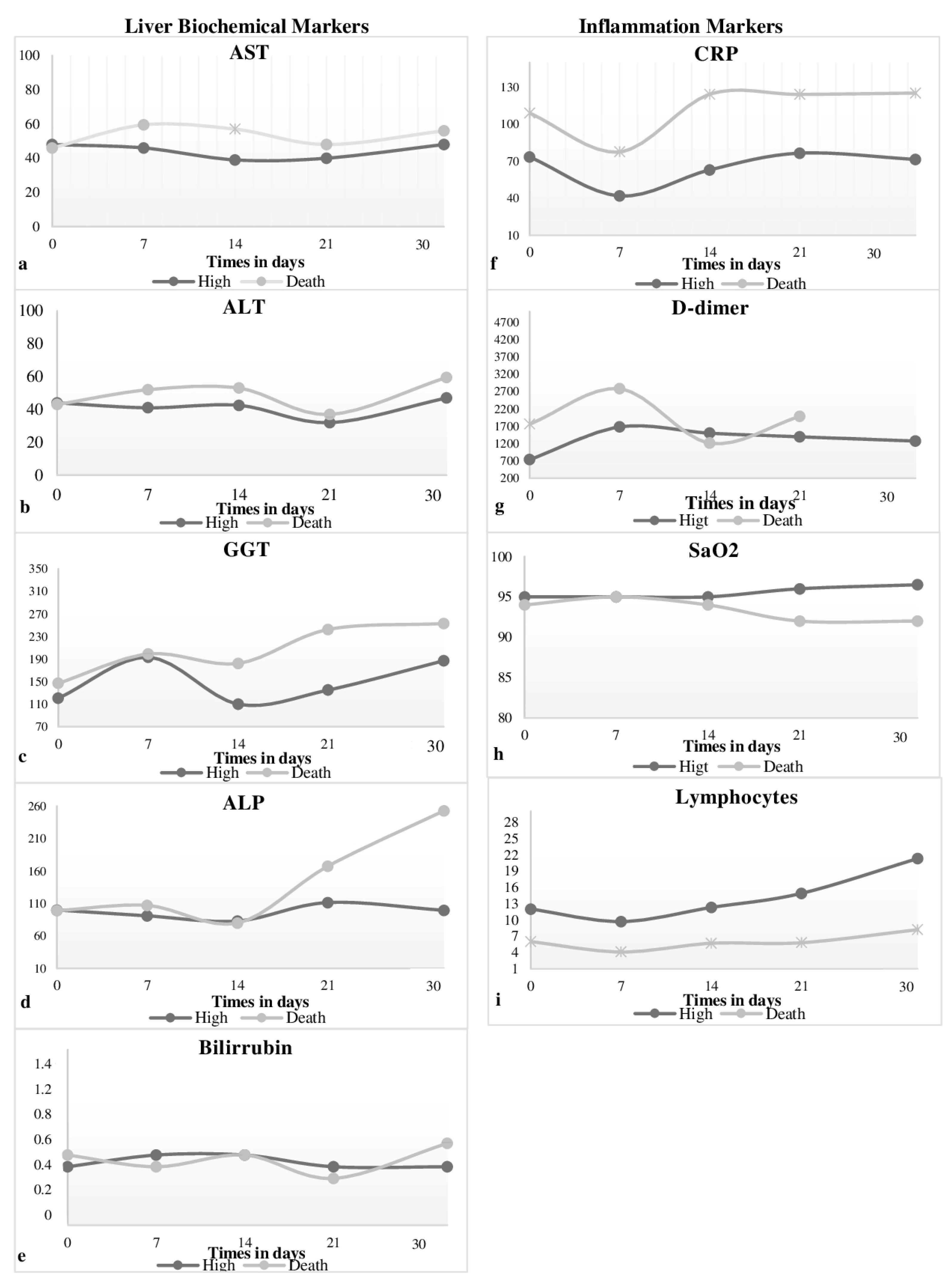
| Variables | N (397) | (%) |
|---|---|---|
| Year | ||
| 2020 | 132 | 33.2 |
| 2021 | 236 | 59.4 |
| 2022 | 29 | 7.3 |
| Sex | ||
| Masculine | 264 | 66.5 |
| Feminine | 133 | 33.5 |
| Age (years) * | ||
| 0–19 years | 14 | 3.6 |
| 20–39 years | 57 | 14.5 |
| 40–59 years | 120 | 30.6 |
| 60 years or older | 201 | 51.3 |
| Color/race * | ||
| White | 28 | 7.7 |
| Black | 333 | 91,2 |
| Indigenous | 4 | 1.1 |
| Education * | ||
| Illiterate/incomplete elementary school | 7 | 3.0 |
| Elementary complete/incomplete secondary | 137 | 57.8 |
| Full medium | 66 | 27.8 |
| Complete or incomplete higher education | 27 | 11.4 |
| Marital status * | ||
| Single/widowed/separated | 149 | 41.4 |
| Married/stable union | 211 | 58.6 |
| City * | ||
| Alenquer | 14 | 3.6 |
| Itaituba | 11 | 2.8 |
| Monte Alegre | 26 | 6.7 |
| Oriximiná | 30 | 7.7 |
| Prainha | 6 | 1.5 |
| Santarém | 262 | 67.2 |
| Others | 41 | 10.5 |
| Admission sector * | ||
| Clinic | 21 | 5.4 |
| ICU ** | 369 | 94.6 |
| Clinical outcome | ||
| High | 191 | 48.1 |
| Death | 206 | 51.9 |
| D1 | D7 | D14 | D21 | D30 | ADH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge | Death | Discharge | Death | Discharge | Death | Discharge | Death | Discharge | Death | Discharge | |||||||
| Variable | Reference Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) |
| Liver Biochemical Markers | |||||||||||||||||
| AST/TGO | 11 to 30 U/L | 0.613 | 0.571 | 0.052 | 0.091 | 0.672 | |||||||||||
| Normal | 51 (35.9) | 63 (38.2) | 23 (37.1) | 20 (32.3) | 28 (50.9) | 10 (26.3) | 19 (47.5) | 4 (23.5) | 12 (44.4) | 2 (28.6) | 18 (66.7) | ||||||
| Discharge | 91 (64.1) | 102 (61.8) | 39 (62.9) | 42 (67.7) | 27 (49.1) | 28 (73.7) | 21 (52.5) | 13 (76.5) | 15 (55.6) | 5 (71.4) | 9 (33.3) | ||||||
| ALT/TGP | 11 to 45 U/L | 0.858 | 0.178 | 0.563 | 0.685 | 0.605 | |||||||||||
| Normal | 74 (52.1) | 85 (52.2) | 34 (55.7) | 23 (39.0) | 27 (54.0) | 16 (45.7) | 21 (55.7) | 10 (62.5) | 10 (40.0) | 3 (50.0) | 17 (63) | ||||||
| Discharge | 68 (47.9) | 78 (47.9) | 27 (44.3) | 36 (61.0) | 23 (46.0) | 19 (54.3) | 17 (44.7) | 6 (37.5) | 15 (60.0) | 3 (50.0) | 10 (37.0) | ||||||
| GGT | 7 to 58 U/L | 0.104 | 0.193 | 1.000 | - | 1.000 | |||||||||||
| Normal | 10 (14.7) | 5 (5.4) | 2 (11.8) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (11.1) | ||||||
| Discharge | 58 (85.3) | 88 (94.6) | 15 (88.2) | 21 (100.0) | 9 (90.0) | 6 (100.0) | 7 (100.0) | 2 (100.0) | 6 (85.7) | 1 (100.0) | 8 (88.9) | ||||||
| ALP | 27 to 100 U/L | 0.297 | 0.268 | 1.000 | 1.000 | 0.464 | |||||||||||
| Normal | 38 (51.4) | 49 (50.5) | 8 (57.1) | 8 (38.1) | 5 (55.6) | 3 (60.0) | 3 (37.5) | 1 (50.0) | 3 (50.0) | 0 (0.0) | 3 (100.0) | ||||||
| Discharge | 36 (48.6) | 48 (49.5) | 6 (42.9) | 13 (61.9) | 4 (44.4) | 2 (40.0) | 5 (62.5) | 1 (50.0) | 3 (50.0) | 2 (100.0) | 0 (0.0) | ||||||
| total bilirubin | ≤1.2 mg/dL | 0.173 | 0.096 | 0.695 | 1.000 | 1.000 | |||||||||||
| Normal | 95 (88.0) | 106 (81.5) | 18 (69.2) | 32 (86.5) | 14 (66.7) | 18 (72.0) | 9 (81.8) | 5 (83.3) | 7 (87.5) | 4 (80.0) | 6 (75.0) | ||||||
| Discharge | 13 (12.0) | 24 (18.5) | 8 (30.8) | 5 (13.5) | 7 (33.3) | 7 (28.0) | 2 (18.2) | 1 (16.7) | 1 (12.5) | 1 (20.0) | 2 (25.0) | ||||||
| Direct Bilirubin | ≤1.2 mg/dL | 0.239 | 0.231 | 0.357 | 1.000 | 0.385 | |||||||||||
| Normal | 104 (95.4) | 120 (91.6) | 23 (85.2) | 35 (94.6) | 20 (95.2) | 21 (84.0) | 10 (90.9) | 6 (100.0) | 8 (100.0) | 4 (80.0) | 7 (87.5) | ||||||
| Discharge | 5 (4.6) | 11 (8.4) | 4 (14.8) | 2 (5.4) | 1 (4.8) | 4 (16.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (12.5) | ||||||
| Indirect Bilirubin | ≤1.2 mg/dL | 0.650 | 0.302 | 0.346 | - | 0.385 | |||||||||||
| Normal | 104 (94.5) | 122 (93.1) | 24 (88.9) | 36 (97.3) | 19 (90.5) | 24 (96.0) | 11 (100.0) | 6 (100.0) | 8 (100.0) | 4 (80.0) | 7 (87.5) | ||||||
| Discharge | 6 (5.5) | 9 (6.9) | 3 (11.1) | 1 (2.7) | 2 (9.5) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (12.5) | ||||||
| D1 | D7 | D14 | D21 | D30 | ADH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge | Death | Discharge | Death | Discharge | Death | Discharge | Death | Discharge | Death | Discharge | |||||||
| Variable | Reference Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) |
| Inflammation Markers | |||||||||||||||||
| CRP | ≤5 mg/dL | 0.056 | 0.008 | 0.213 | 0.367 | 1.000 | |||||||||||
| Normal | 9 (5.5) | 3 (1.7) | 10 (7.2) | 1 (0.8) | 5 (4.3) | 1 (0.9) | 4 (5.6) | 1 (1.5) | 1 (1.9) | 0 (0.0) | 8 (22.9) | ||||||
| Discharge | 156 (94.5) | 176 (98.3) | 129 (92.8) | 129 (99.2) | 111 (95.7) | 114 (99.1) | 67 (94.4) | 66 (98.5) | 53 (98.1) | 29 (100.0) | 27 (77.1) | ||||||
| D-dimer | ≤500 ng/mL | 0.004 | 0.103 | 0.675 | 0.490 | 0.143 | |||||||||||
| Normal | 40 (43.5) | 28 (24.6) | 6 (23.1) | 1 (4.3) | 6 (31.6) | 2 (20.0) | 1 (7.7) | 1 (20.0) | 0 (0.0) | 1 (100.0) | 4 (57.1) | ||||||
| Discharge | 52 (56.5) | 86 (75.4) | 20 (76.9) | 22 (95.7) | 13 (68.4) | 8 (80.0) | 12 (92.3) | 4 (80.0) | 6 (100.0) | 0 (0.0)) | 3 (42.9) | ||||||
| SaO2 | 90 to 99% | 0.189 | 0.639 | 0.356 | 0.002 | 0.071 | |||||||||||
| Normal | 122 (75.8) | 131 (68.9) | 95 (78.5) | 114 (80.9) | 78 (78.0) | 90 (72.0) | 58 (82.9) | 43 (58.1) | 34 (81.0) | 20 (60.6) | 11 (61.1) | ||||||
| Low | 39 (24.2) | 59 (31.1) | 26 (21.5) | 27 (19.1) | 22 (22.0) | 35 (28.0) | 12 (17.1) | 31 (41.9) | 8 (19.0) | 13 (39.4) | 7 (38.9) | ||||||
| Lymphocytes | 22 to 45/mm3 | 0.002 | 0.039 | 0.000 | 0.000 | 0.002 | |||||||||||
| Normal | 34 (19.3) | 14 (7.2) | 19 (12.8) | 7 (4.8) | 27 (21.6) | 3 (2.4) | 22 (26.5) | 2 (2.6) | 24 (42.1) | 3 (8.8) | 24 (51.1) | ||||||
| Low | 136 (77.3) | 175 (89.7) | 125 (83.9) | 136 (93.2) | 92 (73.6) | 121 (96.0) | 60 (72.3) | 71 (93.4) | 32 (56.1) | 31 (91.2) | 18 (38.3) | ||||||
| Discharge | 6 (3.4) | 6 (3.1) | 5 (3.4) | 3 (2.1) | 6 (4.8) | 2 (1.6) | 1 (1.2) | 3 (3.9) | 1 (1.8) | 0 (0.0) | 5 (10.6) | ||||||
| D1 | D7 | D14 | D21 | D30 | ADH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIV | IMV | NIV | IMV | NIV | IMV | NIV | IMV | NIV | IMV | NIV | |||||||
| Variable | Reference Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) |
| Liver Biochemical Markers | |||||||||||||||||
| AST/TGO | 11 to 30 U/L | 0.545 | 0.444 | 0.008 | 0.425 | 0.868 | |||||||||||
| Normal | 52 (38.0) | 52 (35.4) | 25 (38.5) | 17 (30.4) | 28 (56.0) | 10 (24.4) | 14 (46.7) | 9 (34.6) | 8 (40.0) | 6 (42.9) | 14 (66.7) | ||||||
| Discharge | 85 (62.0) | 95 (64.6) | 40 (61.5) | 39 (69.6) | 22 (44.0) | 31 (75.6) | 16 (53.3) | 17 (65.4) | 12 (55.6) | 8 (57.1) | 7 (33.3) | ||||||
| ALT/TGP | 11 to 45 U/L | 0.516 | 0.239 | 0.649 | 0.135 | 0.160 | |||||||||||
| Normal | 70 (50) | 76 (53.5) | 34 (53.1) | 22 (41.5) | 24 (52.2) | 18 (0.0) | 14 (48.2) | 17 (70.8) | 5 (27.8) | 8 (61.5) | 11 (52.4) | ||||||
| Discharge | 70 (50.0) | 66 (46.5) | 30 (46.9) | 31 (58.5) | 22 (47.8) | 19 (51.4) | 15 (51.7) | 7 (29.2) | 13 (72.2) | 5 (38.5) | 10 (47.6) | ||||||
| GGT | 7 to 58 U/L | 0.010 | 0.486 | 0.464 | - | 0.571 | |||||||||||
| Normal | 11 (18.0) | 3 (3.0) | 2 (10.5) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 5 (100.0) | 4 (100.0) | 1 (14.3) | 0 (0.0) | 1 (12.5) | ||||||
| Discharge | 50 (82.0) | 85 (94.6) | 11 (89.5) | 18 (100.0) | 9 (90.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | 6 (85.7) | 2 (100.0) | 7 (87.5) | ||||||
| ALP | 27 to 100 U/L | 0.883 | 1.000 | 0.872 | 0.065 | 0.673 | |||||||||||
| Normal | 37 (54.4) | 45 (50.5) | 7 (43.8) | 9 (47.4) | 5 (55.6) | 3 (60.0) | 1 (16.7) | 3 (75.0) | 2 (33.3) | 1 (50.0) | 3 (100.0) | ||||||
| Discharge | 31 (45.6) | 44 (49.4) | 9 (56.2) | 10 (52.6) | 4 (44.4) | 2 (40.0) | 5 (83.3) | 1 (25.0) | 4 (66.7) | 1 (50.0) | 0 (0.0) | ||||||
| Total Bilirubin | ≤1.2 mg/dL | 1.000 | 0.758 | 1.000 | 0.182 | 0.155 | |||||||||||
| Normal | 87 (86.1) | 103 (85.8) | 23 (76.7) | 27 (81.8) | 16 (69.6) | 16 (69.6) | 7 (100) | 7 (77.8) | 5 (71.4) | 6 (100.0) | 4 (66.7) | ||||||
| Discharge | 14 (13.9) | 17 (14.2) | 7 (23.3) | 6 (18.2) | 7 (30.4) | 7 (30.4) | 0 (0.0) | 2 (22.2) | 1 (28.6) | 0 (0.0) | two () | ||||||
| Direct Bilirubin | ≤1.2 mg/dL | 0.598 | 0.419 | 0.636 | 0.362 | 0.335 | |||||||||||
| Normal | 94 (92.2) | 114 (94.2) | 27 (87.1) | 31 (93.9) | 20 (87.0) | 21 (91.3) | 7 (100.0) | 8 (88.9) | 6 (85.7) | 6 (100.0) | 5 (83.3) | ||||||
| Discharge | 8 (7.8) | 7 (5.8) | 4 (12.9) | 2 (6.1) | 3 (13.0) | 2 (8.7) | 0 (0.0) | 1 (11.1) | 1 (14.3) | 0 (0.0) | 1 (16.7) | ||||||
| Indirect Bilirubin | ≤1.2 mg/dL | 0.419 | 0.050 | 0.073 | - | 0.335 | |||||||||||
| Normal | 95 (92.2) | 115 (95.0) | 27 (87.1) | 33 (100.0) | 20 (87.0) | 23 (100) | 7 (100.0) | 9 (100.0) | 6 (85.7) | 6 (100.0) | 5 (83.3) | ||||||
| Discharge | 8 (7.8) | 6 (5.0) | 4 (12.9) | 0 (0.0) | 3 (13.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (16.7) | ||||||
| D1 | D7 | D14 | D21 | D30 | ADH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIV | IMV | NIV | IMV | NIV | IMV | NIV | IMV | NIV | IMV | NIV | |||||||
| Variable | Reference Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) | N (%) | p-Value | N (%) |
| Inflammation Markers | |||||||||||||||||
| CRP | ≤ 5 mg/dL | 0.001 | 0.010 | 0.058 | 0.184 | 0.284 | |||||||||||
| Normal | 11 (6.8) | 0 (0.0) | 10 (7.6) | 1 (0.8) | 5 (4.9) | 1 (0.8) | 3 (5.2) | 1 (1.3) | 1 (2.6) | 0 (0.0) | 5 (20.8) | ||||||
| Discharge | 150 (93.2) | 176 (100.0) | 122 (92.4) | 131 (99.2) | 98 (95.1) | 123 (99.2) | 55 (94.8) | 77 (98.7) | 37 (97.4) | 43 (100.0) | 19 (79.2) | ||||||
| D-dimer | ≤ 500 ng/mL | 0.035 | 0.080 | 0.334 | 0.929 | 0.212 | |||||||||||
| Normal | 41 (41.8) | 26 (26.8) | 7 (21.9) | 0 (4.3) | 7 (33.3) | 1 (14.3) | 1 (11.1) | 1 (12.5) | 0 (0.0) | 1 (33.3) | 4 (66.7) | ||||||
| Discharge | 57 (58.2) | 81 (73.2) | 25 (78.1) | 17 (100.0) | 14 (66.7) | 6 (85.7) | 8 (88.9) | 7 (87.5) | 4 (100.0) | 2 (66.7) | 2 (33.3) | ||||||
| SaO2 | 90 to 99% | 0.064 | 0.278 | 0.875 | 0.897 | 1.000 | |||||||||||
| Normal | 118 (77.1) | 118 (67.4) | 86 (76.1) | 119 (82.1) | 67 (73.6) | 98 (75.4) | 38 (69.1) | 61 (70.1) | 21 (72.4) | 32 (71.1) | 5 (55.6) | ||||||
| Low | 35 (22.9) | 57 (32.6) | 27 (23.9) | 26 (17.9) | 24 (26.4) | 32 (24.6) | 17 (30.9) | 26 (29.9) | 8 (27.6) | 13 (28.9) | 4 (44.4) | ||||||
| Lymphocytes | 22 to 45/mm3 | 0.000 | 0.003 | 0.000 | 0.116 | 0.164 | |||||||||||
| Normal | 33 (19.3) | 12 (6.9) | 21 (14.7) | 5 (3.4) | 26 (23.0) | 4 (3.0) | 15 (22.1) | 9 (10.1) | 15 (36.6) | 11 (22.4) | 16 (51.6) | ||||||
| Low | 130 (76.0) | 161 (92.0) | 118 (82.5 | 138 (93.9) | 81 (71.7) | 128 (95.5) | 52 (76.5) | 78 (87.6) | 25 (61.0) | 38 (77.6) | 11 (35.5) | ||||||
| Discharge | 8 (4.7) | 2 (1.1) | 4 (2.8) | 4 (2.7) | 6 (5.3) | 2 (1.5) | 1 (1.5) | 2 (2.2) | 1 (2.4) | 0 (0.0) | 4 (12.9) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.S.d.; Martinelli, K.G.; Viana, M.W.M.; Soares, D.d.S.; Corrêa, Y.G.S.; Silva, L.L.d.; Paula, V.S.d.; Rodrigues, L.L.S.; Villar, L.M. Liver and Inflammatory Biomarkers Are Related to High Mortality in Hospitalized Patients with COVID-19 in Brazilian Amazon Region. Life 2024, 14, 869. https://doi.org/10.3390/life14070869
Silva CSd, Martinelli KG, Viana MWM, Soares DdS, Corrêa YGS, Silva LLd, Paula VSd, Rodrigues LLS, Villar LM. Liver and Inflammatory Biomarkers Are Related to High Mortality in Hospitalized Patients with COVID-19 in Brazilian Amazon Region. Life. 2024; 14(7):869. https://doi.org/10.3390/life14070869
Chicago/Turabian StyleSilva, Carla Sousa da, Katrini Guidolini Martinelli, Marlison Wesley Miranda Viana, Deliane dos Santos Soares, Yasmin Garcia Silva Corrêa, Lucas Lima da Silva, Vanessa Salete de Paula, Luana Lorena Silva Rodrigues, and Livia Melo Villar. 2024. "Liver and Inflammatory Biomarkers Are Related to High Mortality in Hospitalized Patients with COVID-19 in Brazilian Amazon Region" Life 14, no. 7: 869. https://doi.org/10.3390/life14070869
APA StyleSilva, C. S. d., Martinelli, K. G., Viana, M. W. M., Soares, D. d. S., Corrêa, Y. G. S., Silva, L. L. d., Paula, V. S. d., Rodrigues, L. L. S., & Villar, L. M. (2024). Liver and Inflammatory Biomarkers Are Related to High Mortality in Hospitalized Patients with COVID-19 in Brazilian Amazon Region. Life, 14(7), 869. https://doi.org/10.3390/life14070869








