Immature Surfactant Protein Type B and Surfactant Protein Type D Correlate with Coronary Heart Disease in Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Sample Preparation
2.3. Analysis of Surfactant Proteins
2.4. Statistical Evaluation of Data
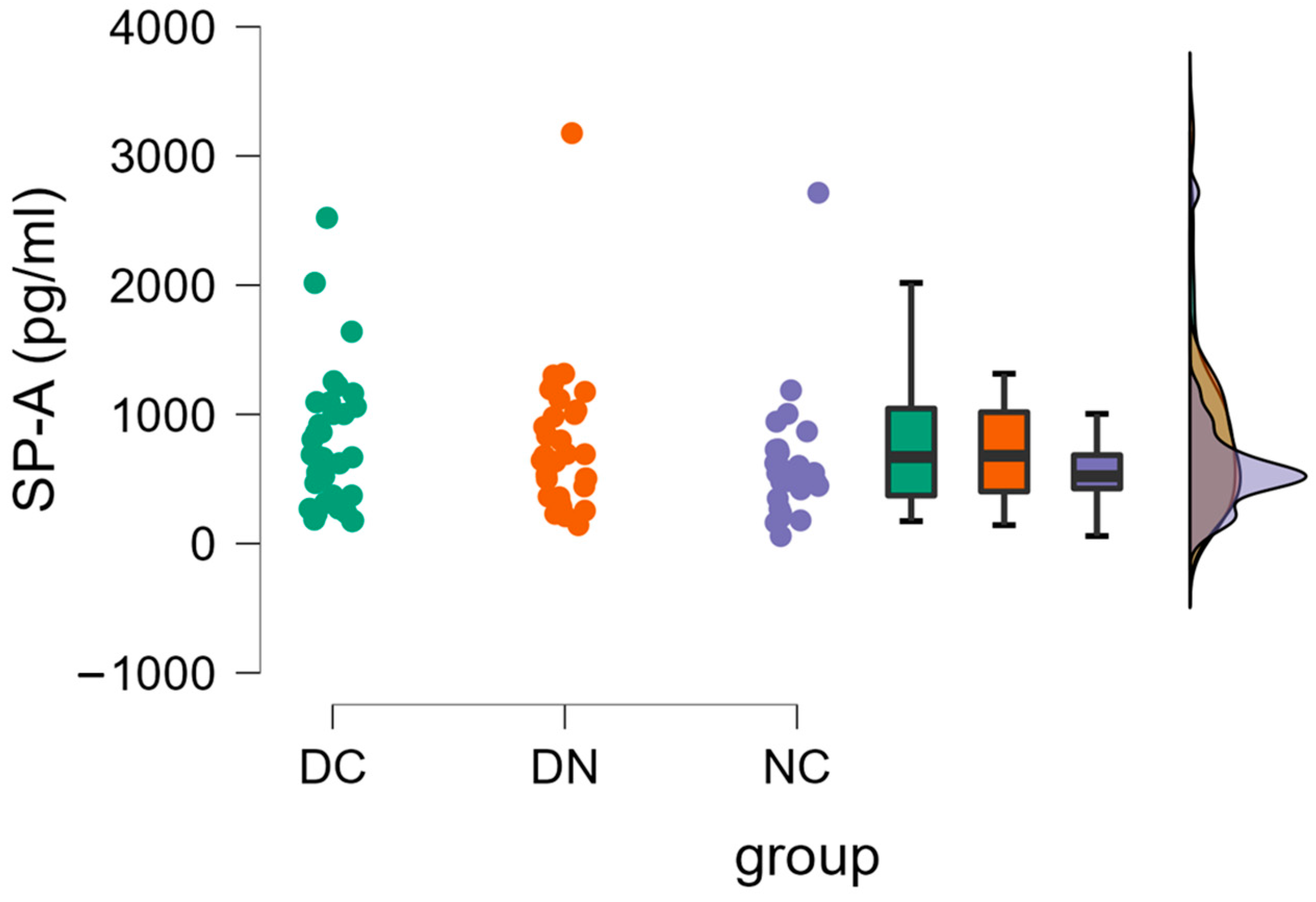
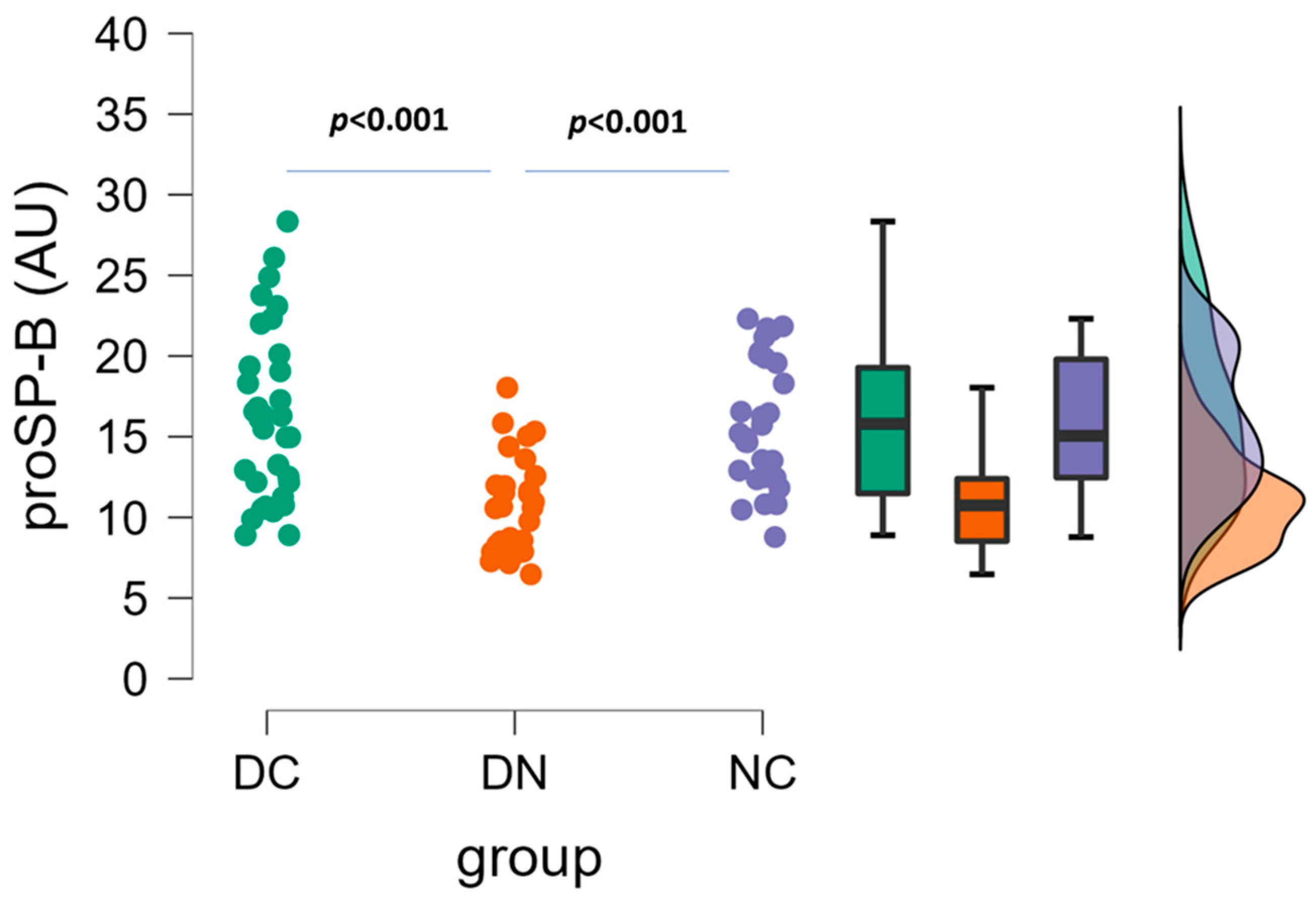
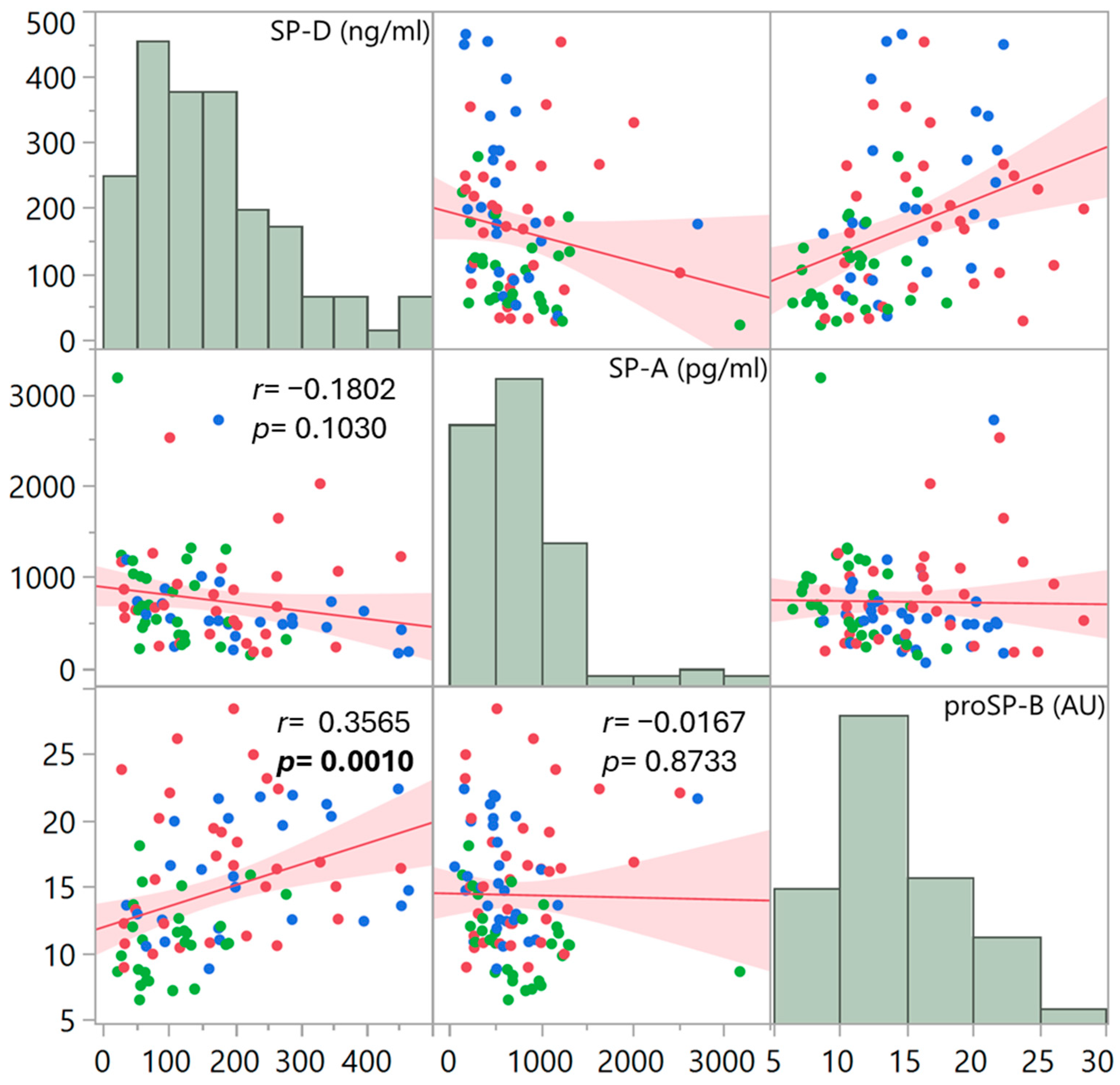
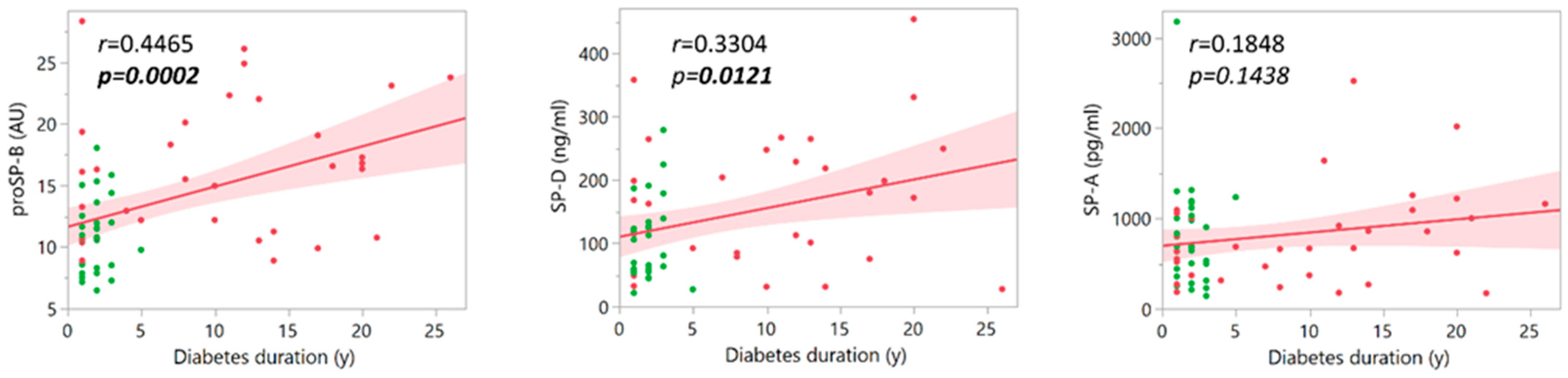
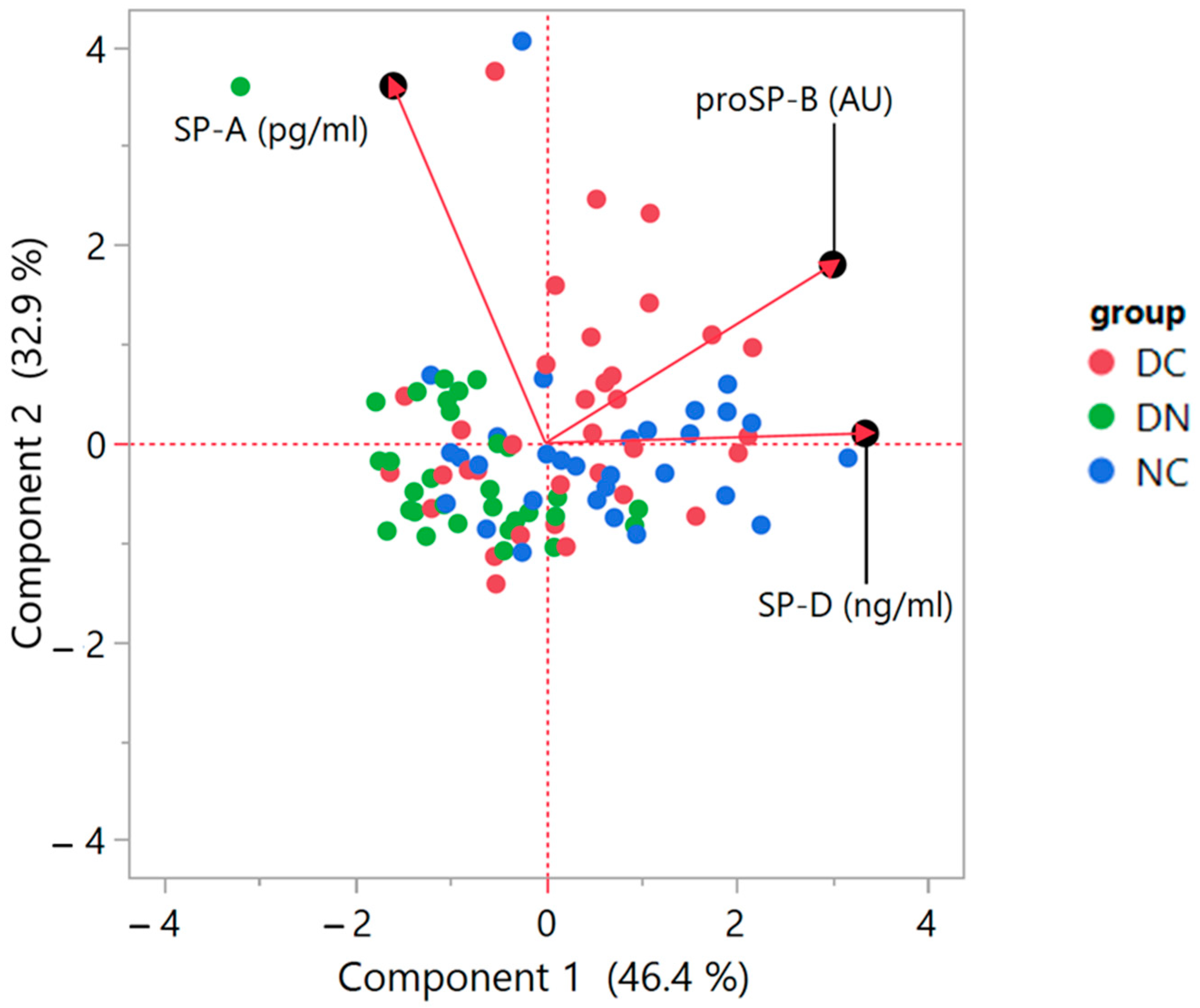
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haagsman, H.P.; Diemel, R.V. Surfactant-associated proteins: Functions and structural variation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Haagsman, H.P. Interactions of surfactant protein A with pathogens. Biochim. Biophys. Acta 1998, 1408, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Kishore, U.; Lim, B.L.; Strong, P.; Reid, K.B. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin. Exp. Immunol. 1996, 106, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kishore, U.; Greenhough, T.J.; Waters, P.; Shrive, A.K.; Ghai, R.; Kamran, M.F.; Bernal, A.L.; Reid, K.B.; Madan, T.; Chakraborty, T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol. Immunol. 2006, 43, 1293–1315. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Cai, D.; King, S.D.; Que, X.; Bath, J.M.; Chen, S.Y. Surfactant Protein A, a Novel Regulator for Smooth Muscle Phenotypic Modulation and Vascular Remodeling-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 808–814. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kanoh, S.; Motoyoshi, K. Serum surfactant protein-A, but not surfactant protein-D or KL-6, can predict preclinical lung damage induced by smoking. Biomarkers 2008, 13, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Heslop, C.; Man, S.F.; Frohlich, J.; Connett, J.E.; Anthonisen, N.R.; Wise, R.A.; Tashkin, D.P.; Sin, D.D. Circulating surfactant protein-D and the risk of cardiovascular morbidity and mortality. Eur. Heart J. 2011, 32, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Atochina-Vasserman, E.N.; Holz, O.; Beers, M.F.; Erpenbeck, V.J.; Krug, N.; Roepcke, S.; Lauer, G.; Elmlinger, M.; Hohlfeld, J.M. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir. Res. 2011, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Otaki, Y.; Watanabe, T.; Takahashi, H.; Sugai, T.; Yokoyama, M.; Nishiyama, S.; Arimoto, T.; Shishido, T.; Miyamoto, T.; Yamanaka, T.; et al. Circulating Surfactant Protein-D is Associated with Clinical Outcomes in Peripheral Artery Disease Patients Following Endovascular Therapy. Circ. J. 2018, 82, 1926–1934. [Google Scholar] [CrossRef]
- Colmorten, K.B.; Nexoe, A.B.; Sorensen, G.L. The Dual Role of Surfactant Protein-D in Vascular Inflammation and Development of Cardiovascular Disease. Front. Immunol. 2019, 10, 2264. [Google Scholar] [CrossRef]
- Weaver, T.E.; Conkright, J.J. Function of surfactant proteins B and C. Annu. Rev. Physiol. 2001, 63, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Wert, S.E.; Whitsett, J.A.; Nogee, L.M. Genetic disorders of surfactant dysfunction. Pediatr. Dev. Pathol. 2009, 12, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Agostoni, P. Surfactant protein B: From biochemistry to its potential role as diagnostic and prognostic marker in heart failure. Int. J. Cardiol. 2016, 221, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Doyle, I.R.; Bersten, A.D.; Nicholas, T.E. Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am. J. Respir. Crit. Care Med. 1997, 156, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Bækvad-Hansen, M.; Nordestgaard, B.G.; Dahl, M. Surfactant protein B polymorphisms, pulmonary function and COPD in 10,231 individuals. Eur. Respir. J. 2011, 37, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.B.; Rohatgi, A.; Garcia, C.K.; Ayers, C.R.; Das, S.R.; Lakoski, S.G.; Berry, J.D.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Interactions between smoking, pulmonary surfactant protein B, and atherosclerosis in the general population: The Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Tammemagi, C.M.; Lam, S.; Barnett, M.J.; Duan, X.; Tam, A.; Auman, H.; Feng, Z.; Goodman, G.E.; Hanash, S.; et al. Pro-surfactant protein B as a biomarker for lung cancer prediction. J. Clin. Oncol. 2013, 31, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Mayo, J.; Tan, W.; Tammemagi, C.M.; Liu, G.; Peacock, S.; Shepherd, F.A.; Goffin, J.; Goss, G.; Nicholas, G.; et al. Plasma pro-surfactant protein B and lung function decline in smokers. Eur. Respir. J. 2015, 45, 1037–1045, Erratum in Eur. Respir. J. 2016, 48, 962. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Banfi, C.; Ghilardi, S.; Magrì, D.; Giovannardi, M.; Bonomi, A.; Salvioni, E.; Battaia, E.; Filardi, P.P.; Tremoli, E.; et al. Surfactant-derived proteins as markers of alveolar membrane damage in heart failure. PLoS ONE 2014, 9, e115030. [Google Scholar] [CrossRef]
- Magrì, D.; Banfi, C.; Maruotti, A.; Farina, S.; Vignati, C.; Salvioni, E.; Morosin, M.; Brioschi, M.; Ghilardi, S.; Tremoli, E.; et al. Plasma immature form of surfactant protein type B correlates with prognosis in patients with chronic heart failure. A pilot single-center prospective study. Int. J. Cardiol. 2015, 201, 394–399. [Google Scholar] [CrossRef]
- Emmens, J.E.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Romaine, S.P.R.; Quinn, P.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; et al. Proteomic diversity of high-density lipoprotein explains its association with clinical outcome in patients with heart failure. Eur. J. Heart Fail. 2018, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Campodonico, J.; Mapelli, M.; Spadafora, E.; Ghilardi, S.; Agostoni, P.; Banfi, C.; Sciomer, S. Surfactant proteins changes after acute hemodynamic improvement in patients with advanced chronic heart failure treated with Levosimendan. Respir. Physiol. Neurobiol. 2018, 252, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Mattavelli, I.; Salvioni, E.; Banfi, C.; Ghilardi, S.; De Martino, F.; Gugliandolo, P.; Mantegazza, V.; Volpato, V.; Basile, C.; et al. Impact of Sacubitril/Valsartan on surfactant binding proteins, central sleep apneas, lung function tests and heart failure biomarkers: Hemodynamic or pleiotropism? Front. Cardiovasc. Med. 2022, 9, 971108. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L. Cardiovascular disease incidence and mortality in older men with diabetes and in men with coronary heart disease. Heart 2004, 90, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, F.; Banfi, C.; Brioschi, M.; Altomare, A.; Ragazzi, E.; Cosma, C.; Sartore, G.; Lapolla, A. The Burden of Impaired Serum Albumin Antioxidant Properties and Glyco-Oxidation in Coronary Heart Disease Patients with and without Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; States, D.J.; Adamski, M.; Blackwell, T.W.; Menon, R.; Hermjakob, H.; Apweiler, R.; Haab, B.B.; Simpson, R.J.; Eddes, J.S.; et al. Overview of the HUPO Plasma Proteome Project: Results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics 2005, 5, 3226–3245. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Jolliffe, I.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Aitchison, J.; Greenacre, M. Biplots of Compositional Data. J. R. Stat. Soc. C Appl. Stat. 2002, 51, 375–392. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Wulf-Johansson, H.; Thinggaard, M.; Tan, Q.; Johansson, S.L.; Schlosser, A.; Christensen, K.; Holmskov, U.; Sorensen, G.L. Circulating surfactant protein D is associated to mortality in elderly women: A twin study. Immunobiology 2013, 218, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhong, Q.; Gong, J.; Qin, Y.; Cui, L.; Yuan, H. Serum Surfactant Protein D is Associated with Atherosclerosis of the Carotid Artery in Patients on Maintenance Hemodialysis. Clin. Lab. 2016, 62, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Madsen, J.; Clark, H.W. SP-A and SP-D: Dual Functioning Immune Molecules with Antiviral and Immunomodulatory Properties. Front. Immunol. 2021, 11, 622598. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, F.; Sartore, G.; Lapolla, A. Glyco-oxidation and cardiovascular complications in type 2 diabetes: A clinical update. Acta Diabetol. 2013, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Baek, J.Y.; Shin, W.S.; Kim, D.B.; Jang, S.W.; Shin, D.I.; Koh, Y.S.; Seo, S.M.; Uhm, J.S.; Kim, T.H.; et al. Soluble receptor of advanced glycated endproducts is associated with plaque vulnerability in patients with acute myocardial infarction. Circ. J. 2011, 75, 1685–1690. [Google Scholar] [CrossRef]
- Prasad, K. AGE-RAGE Stress and Coronary Artery Disease. Int. J. Angiol. 2021, 30, 4–14. [Google Scholar] [CrossRef]
- Piarulli, F.; Banfi, C.; Ragazzi, E.; Gianazza, E.; Munno, M.; Carollo, M.; Traldi, P.; Lapolla, A.; Sartore, G. Multiplexed MRM-based proteomics for identification of circulating proteins as biomarkers of cardiovascular damage progression associated with diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banfi, C.; Piarulli, F.; Ragazzi, E.; Ghilardi, S.; Greco, A.; Lapolla, A.; Sartore, G. Immature Surfactant Protein Type B and Surfactant Protein Type D Correlate with Coronary Heart Disease in Patients with Type 2 Diabetes. Life 2024, 14, 886. https://doi.org/10.3390/life14070886
Banfi C, Piarulli F, Ragazzi E, Ghilardi S, Greco A, Lapolla A, Sartore G. Immature Surfactant Protein Type B and Surfactant Protein Type D Correlate with Coronary Heart Disease in Patients with Type 2 Diabetes. Life. 2024; 14(7):886. https://doi.org/10.3390/life14070886
Chicago/Turabian StyleBanfi, Cristina, Francesco Piarulli, Eugenio Ragazzi, Stefania Ghilardi, Arianna Greco, Annunziata Lapolla, and Giovanni Sartore. 2024. "Immature Surfactant Protein Type B and Surfactant Protein Type D Correlate with Coronary Heart Disease in Patients with Type 2 Diabetes" Life 14, no. 7: 886. https://doi.org/10.3390/life14070886





