Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. FA Image Acquisition
2.3. OCTA Image Acquisition
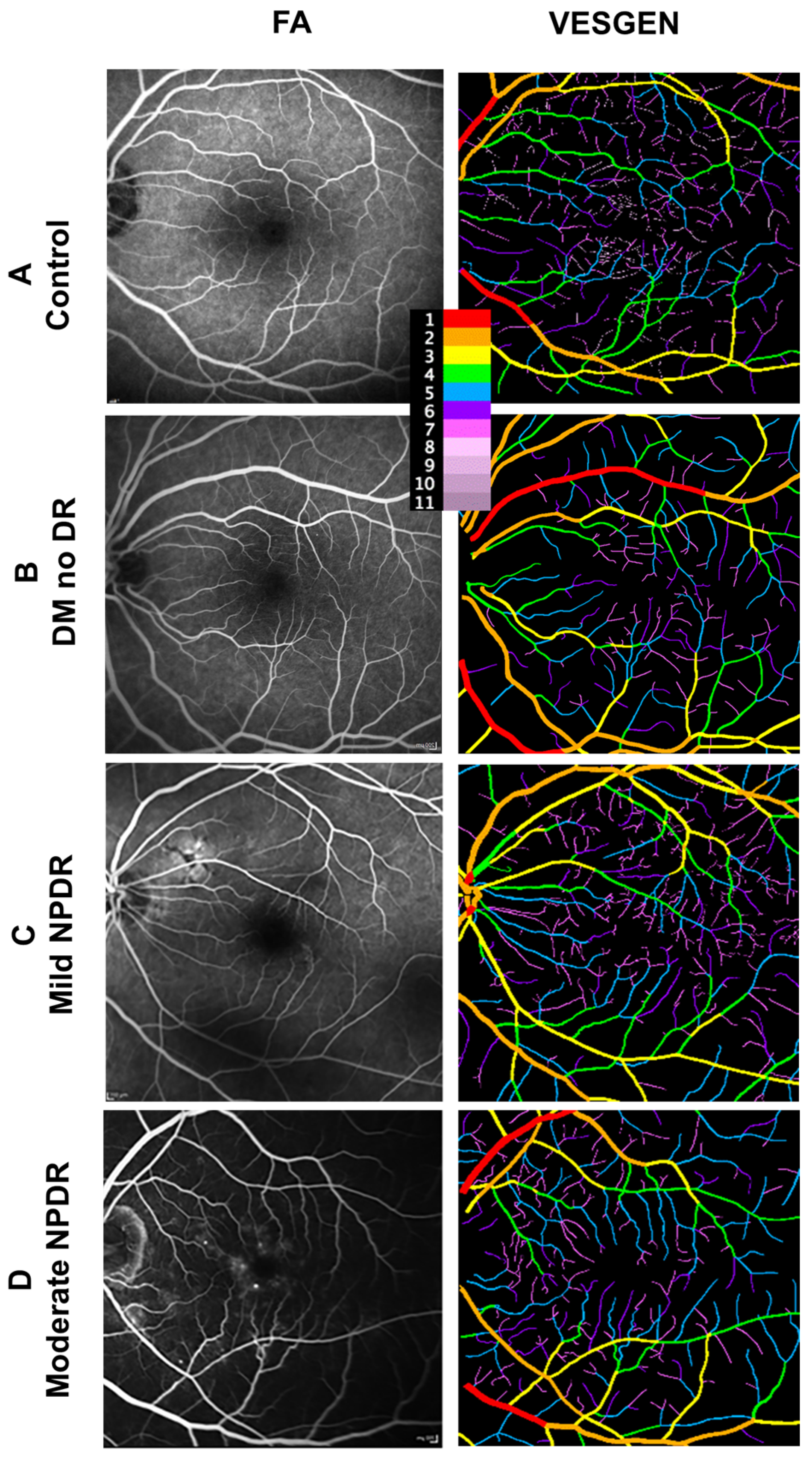
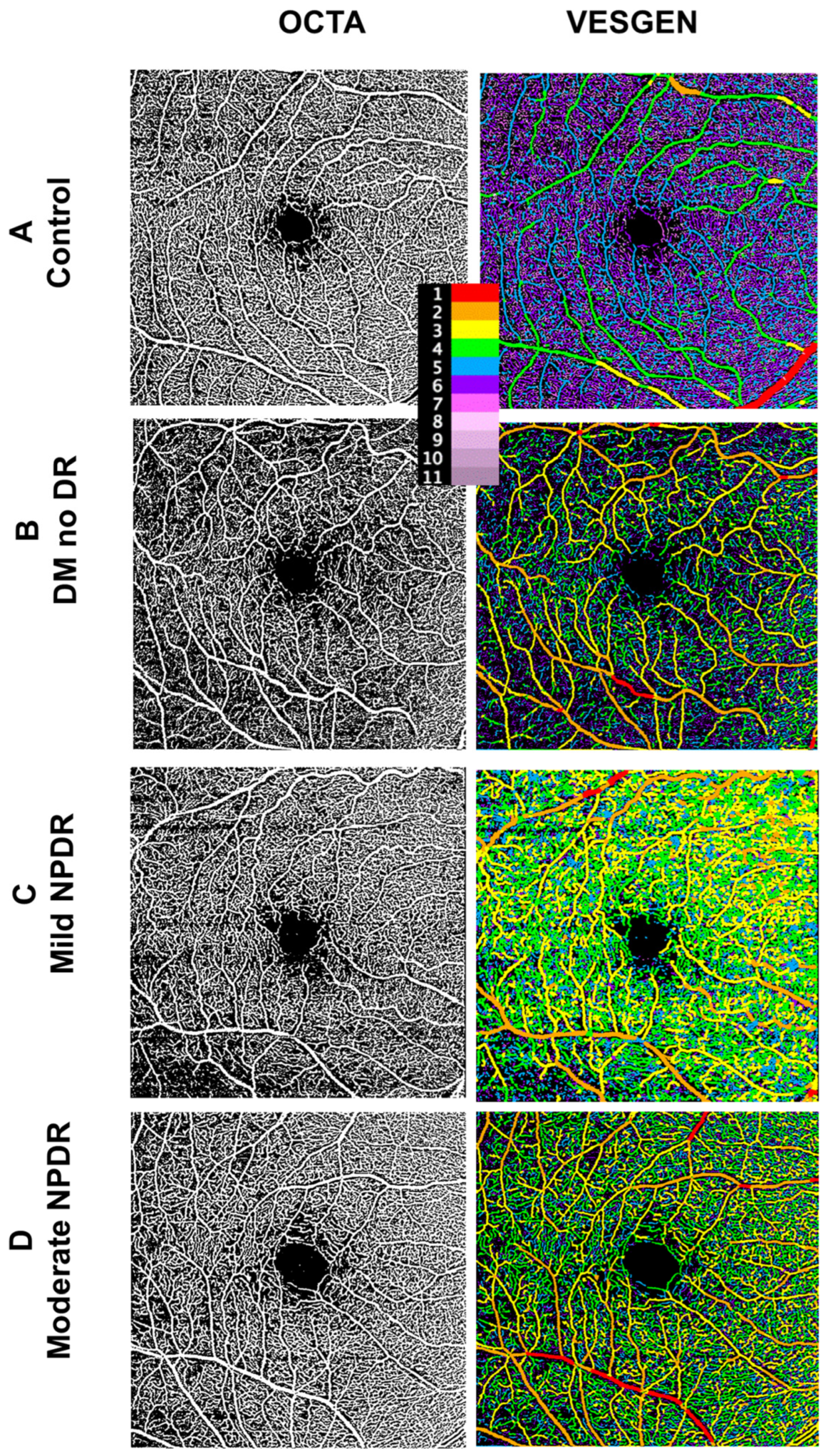
2.4. Image Processing
2.5. Vascular Quantification
2.6. Statistical Analyses
3. Results
3.1. Clinical Characteristics of FA Subjects
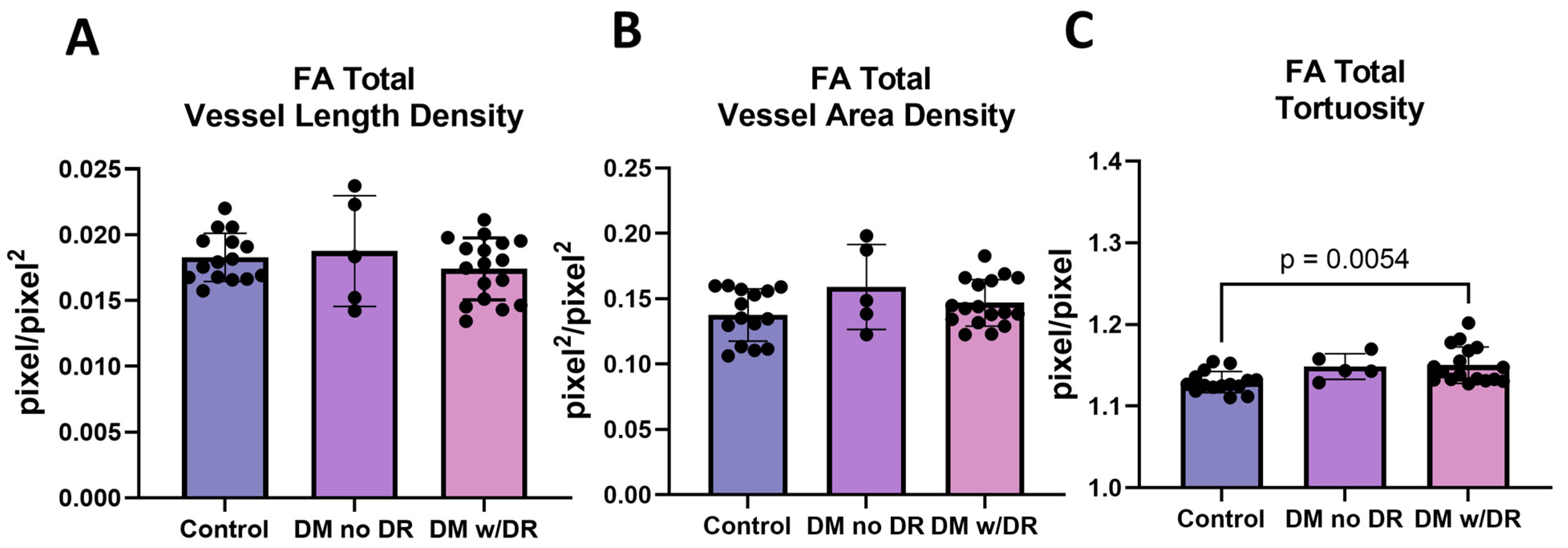
3.2. FA Images Demonstrate Changes in Tortuosity
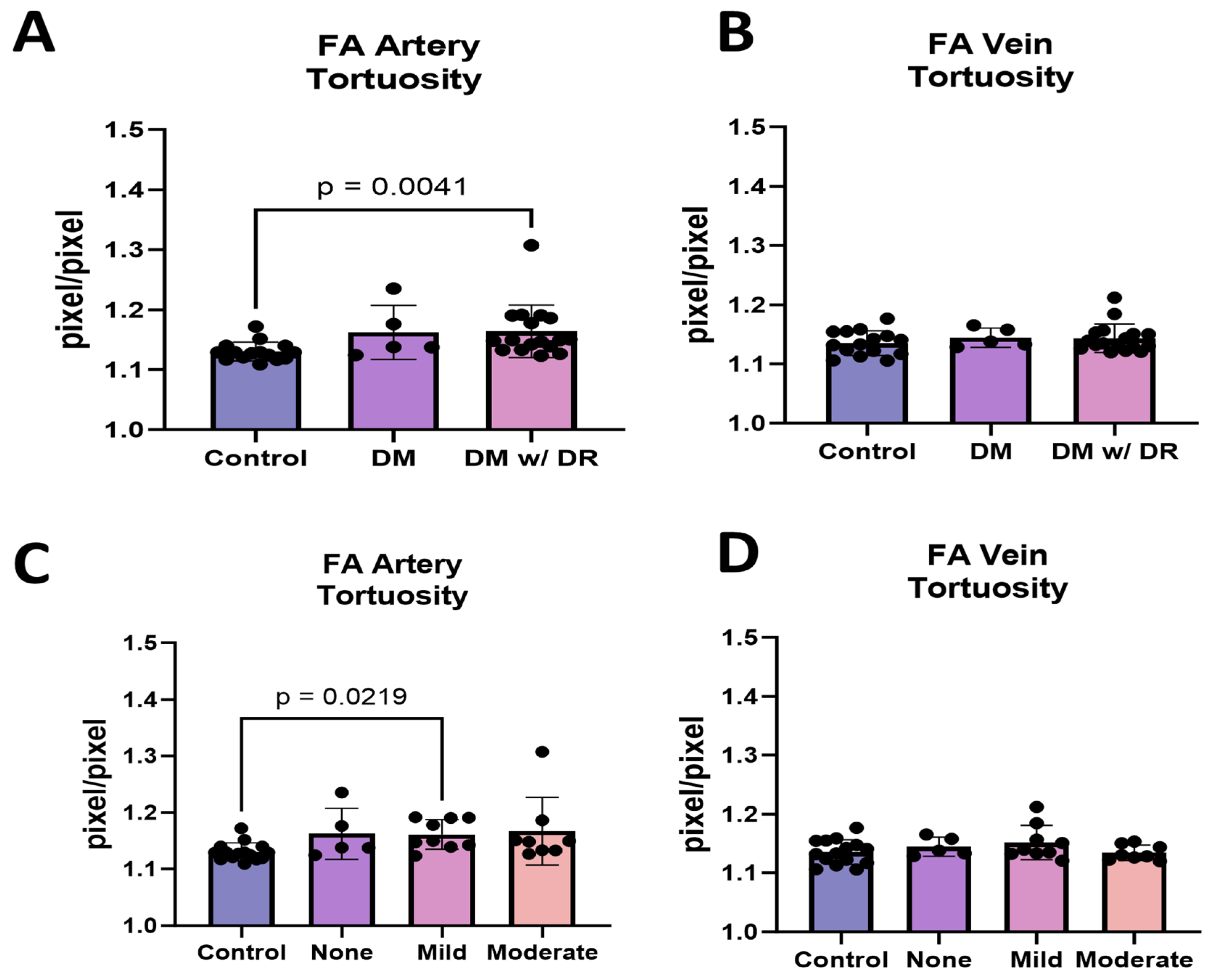
3.3. FA Demonstrates That Arterial but Not Venous Tortuosity Was Increased in Diabetics with DR
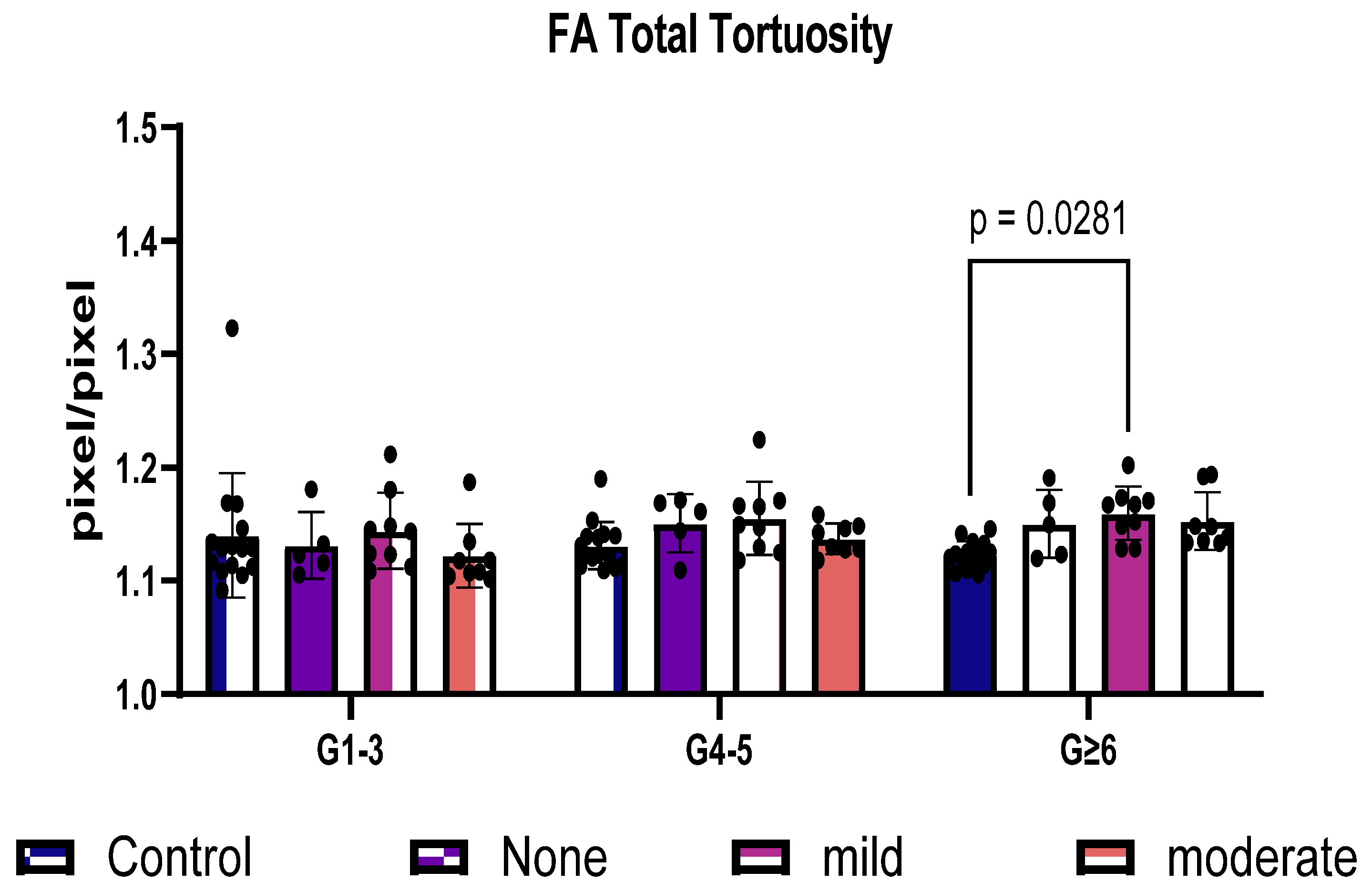
3.4. FA Distinguishes Total Tortuosity by Vessel Generation
3.5. Clinical Characteristics of OCTA Subjects
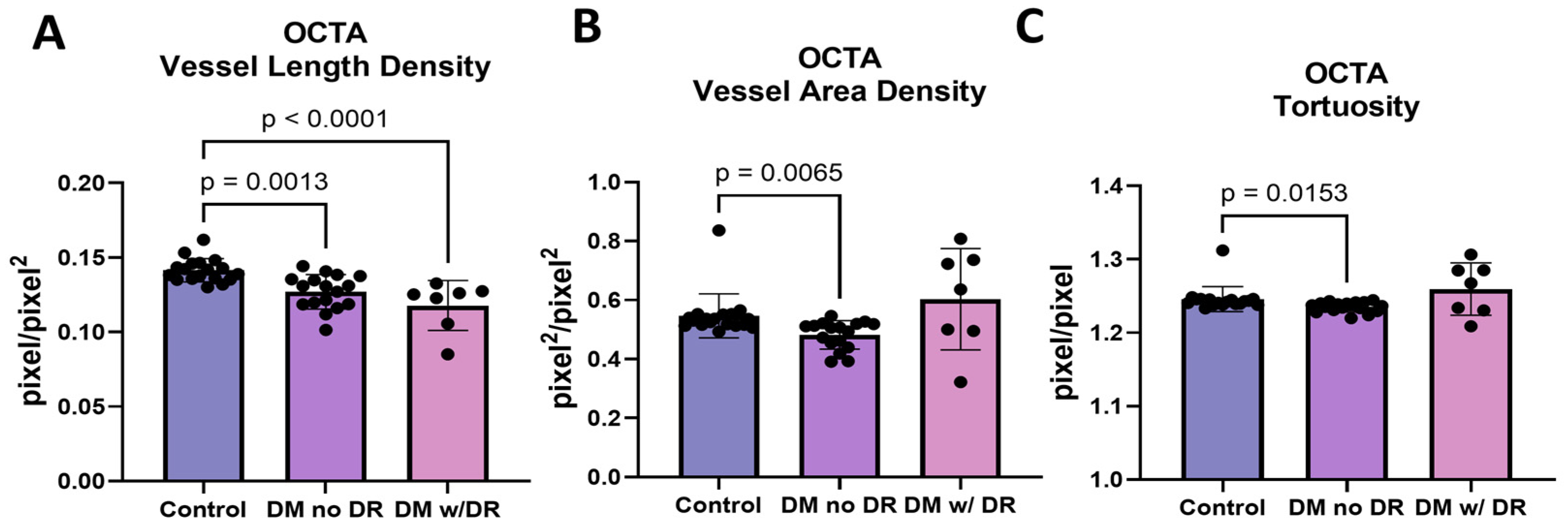
3.6. OCTA Images Demonstrate Changes in Multiple Endpoints
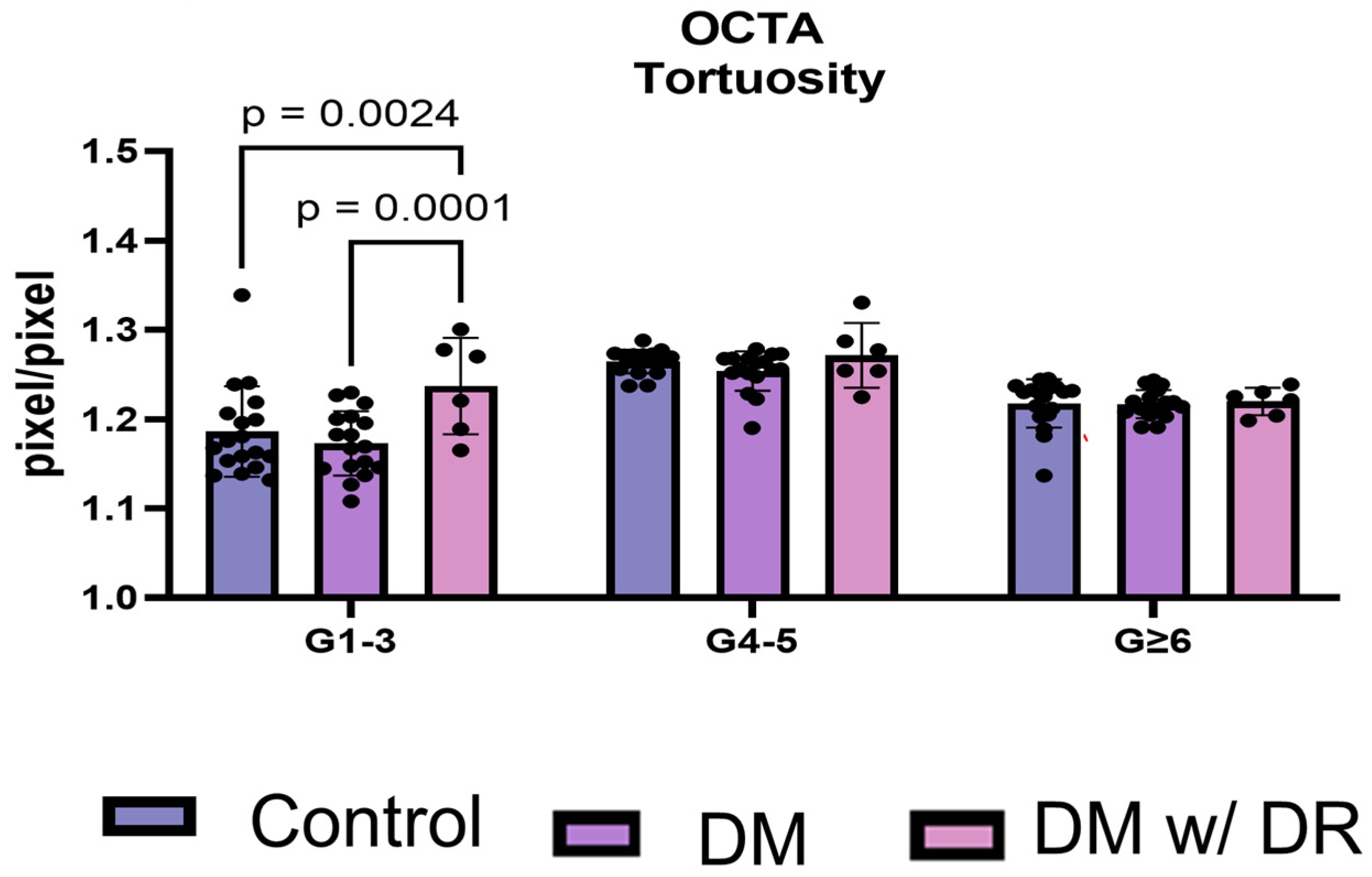
3.7. OCTA Distinguishes Total Tortuosity by Vessel Generation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, K.-Y.; Hsih, W.-H.; Lin, Y.-B.; Wen, C.-Y.; Chang, T.-J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Modi, P.; Arsiwalla, T. Hypertensive Retinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ghamdi, A.H.A. Clinical predictors of diabetic retinopathy progression: A systematic review. Curr. Diabetes Rev. 2020, 16, 242–247. [Google Scholar] [PubMed]
- Grunwald, J.E.; Alexander, J.; Ying, G.S.; Maguire, M.; Daniel, E.; Whittock-Martin, R.; Parker, C.; McWilliams, K.; Lo, J.C.; Go, A.; et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch. Ophthalmol. 2012, 130, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kandula, V.; Miller, R.E. Monitoring retinal pathology and cerebral injury in sickle cell disease using spectral-domain optical coherence tomography in pediatric patients. Pediatr. Blood Cancer 2021, 68, e29028. [Google Scholar] [CrossRef] [PubMed]
- Farrah, T.E.; Dhillon, B.; Keane, P.A.; Webb, D.J.; Dhaun, N. The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons. Kidney Int. 2020, 98, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Alghadyan, A.A. Diabetic retinopathy—An update. Saudi J. Ophthalmol. 2011, 25, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- De Madrid, D.A.P.; Berástegui, I. Diabetic retinopathy. An. Sist. Sanit. Navar. 2008, 31 (Suppl. S3), 23–34. [Google Scholar] [PubMed]
- Moreno, A.; Lozano, M.; Salinas, P. Diabetic retinopathy. Nutr. Hosp. 2013, 28 (Suppl. S2), 53–56. [Google Scholar] [PubMed]
- Kanski, J.J. Diabetic retinopathy—A preventable cause of blindness. Practitioner 1985, 229, 343–348. [Google Scholar] [PubMed]
- Parsons-Wingerter, P.; Radhakrishnan, K.; Vickerman, M.B.; Kaiser, P.K. Oscillation of angiogenesis with vascular dropout in diabetic retinopathy by VESsel GENeration analysis (VESGEN). Investig. Ophthalmol. Vis. Sci. 2010, 51, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, M.B.; Keith, P.A.; McKay, T.L.; Gedeon, D.J.; Watanabe, M.; Montano, M.; Karunamuni, G.; Kaiser, P.K.; Sears, J.E.; Ebrahem, Q.; et al. VESGEN 2D: Automated, user-interactive software for quantification and mapping of angiogenic and lymphangiogenic trees and networks. Anat. Rec. 2009, 292, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Classification and treatment of diabetic retinopathy. Diabetes Res. Clin. Pract. 1994, 24, S171–S176. [Google Scholar] [CrossRef] [PubMed]
- The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch. Ophthalmol. 1995, 113, 36–51. [CrossRef] [PubMed]
- Arthur, E.; Ravichandran, S.; Snyder, P.J.; Alber, J.; Strenger, J.; Bittner, A.K.; Khankan, R.; Adams, S.L.; Putnam, N.M.; Lypka, K.R.; et al. Retinal mid-peripheral capillary free zones are enlarged in cognitively unimpaired older adults at high risk for Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.; Alber, J.; Thompson, L.I.; Sinoff, S.; Snyder, P.J. OCTA reveals remodeling of the peripheral capillary free zones in normal aging. Sci. Rep. 2021, 11, 15593. [Google Scholar] [CrossRef]
- DuPont, M.; Lambert, S.; Rodriguez-Martin, A.; Hernandez, O.; Lagatuz, M.; Yilmaz, T.; Foderaro, A.; Baird, G.L.; Parsons-Wingerter, P.; Lahm, T.; et al. Retinal vessel changes in pulmonary arterial hypertension. Pulm. Circ. 2022, 12, e12035. [Google Scholar] [CrossRef] [PubMed]
- Lagatuz, M.; Vyas, R.J.; Predovic, M.; Lim, S.; Jacobs, N.; Martinho, M.; Valizadegan, H.; Kao, D.; Oza, N.; Theriot, C.A.; et al. Vascular patterning as integrative readout of complex molecular and physiological signaling by vessel generation analysis. J. Vasc. Res. 2021, 58, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Pramil, V.; Levine, E.S.; Waheed, N.K. Macular vessel density in diabetic retinopathy patients: How can we accurately measure and what can it tell us? Clin. Ophthalmol. 2021, 15, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-C. Twisted blood vessels: Symptoms, etiology, and biomechanical mechanisms. J. Vasc. Res. 2012, 49, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Vingopoulos, F.; Le, R.; Laíns, I.; Wu, D.M.; et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef]
- Weinhaus, R.S.; Burke, J.M.; Delori, F.C.; Snodderly, D.M. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp. Eye Res. 1995, 61, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sambhav, K.; Abu-Amero, K.K.; Chalam, K.V. Deep Capillary Macular Perfusion Indices Obtained with OCT Angiography Correlate with Degree of Nonproliferative Diabetic Retinopathy. Eur. J. Ophthalmol. 2017, 27, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, T.; Kim, T.-H.; Le, D.; Son, T.; Ma, G.; Yao, X. Depth-resolved vascular profile features for artery-vein classification in OCT and OCT angiography of human retina. Biomed. Opt. Express 2022, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
| FA | ||
|---|---|---|
| Control | Type 2 Diabetics | |
| N | 8 | 12 |
| Total eyes | 15 | 21 |
| Sex * | ||
| Male | 4 | 9 |
| Female | 11 | 5 |
| Age (range) ** | 25–52 | 32–69 |
| Race *** | ||
| White | 7 | 5 |
| Black | 2 | 7 |
| Asian | 4 | 0 |
| Other | 2 | 0 |
| Retinopathy type | ||
| None | - | 5 |
| Mild | - | 8 |
| Moderate | - | 8 |
| HbA1c% (Mean ± SD) **** | - | 8.3 ± 1.8 |
| OCTA | ||
|---|---|---|
| Control | Type 2 Diabetics | |
| N | 17 | 13 |
| Total eyes | 18 | 23 |
| Sex | ||
| Male | 5 | 1 |
| Female | 13 | 22 |
| Age (range) | 46–74 | 43–65 |
| Race | ||
| White | 15 | 2 |
| Black | 3 | 21 |
| Asian | 0 | 0 |
| Other | 0 | 0 |
| Retinopathy type | ||
| None | - | 17 |
| Mild | - | 0 |
| Moderate | - | 6 |
| HbA1c% (Mean ± SD) * | - | 7.54 ± 1.80 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuPont, M.; Arthur, E.; Shihab, Y.; Kenny, M.; Ravichandran, S.; Parsons-Wingerter, P.; Vyas, R.; Murray, M.C.; Predovic, M.; Lim, S.; et al. Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy. Life 2024, 14, 893. https://doi.org/10.3390/life14070893
DuPont M, Arthur E, Shihab Y, Kenny M, Ravichandran S, Parsons-Wingerter P, Vyas R, Murray MC, Predovic M, Lim S, et al. Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy. Life. 2024; 14(7):893. https://doi.org/10.3390/life14070893
Chicago/Turabian StyleDuPont, Mariana, Edmund Arthur, Yazen Shihab, Madelyn Kenny, Swetha Ravichandran, Patricia Parsons-Wingerter, Ruchi Vyas, Matthew C. Murray, Marina Predovic, Shiyin Lim, and et al. 2024. "Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy" Life 14, no. 7: 893. https://doi.org/10.3390/life14070893







