Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnostic Scheme for Lung Cancer and IPF
2.3. Treatment Scheme and Surveillance
2.4. Morphometric Complexity Measurements
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics
3.2. Treatment-Related Complications
3.3. Morphometric Complexity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Ley, B.; Elicker, B.M.; Hartman, T.E.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Lee, J.S.; Jones, K.D.; Richeldi, L.; King, T.E., Jr.; et al. Idiopathic pulmonary fibrosis: CT and risk of death. Radiology 2014, 273, 570–579. [Google Scholar] [CrossRef]
- Lee, T.; Park, J.Y.; Lee, H.Y.; Cho, Y.J.; Yoon, H.I.; Lee, J.H.; Jheon, S.; Lee, C.T.; Park, J.S. Lung cancer in patients with idiopathic pulmonary fibrosis: Clinical characteristics and impact on survival. Respir. Med. 2014, 108, 1549–1555. [Google Scholar] [CrossRef]
- Kanaji, N.; Tadokoro, A.; Kita, N.; Murota, M.; Ishii, T.; Takagi, T.; Watanabe, N.; Tojo, Y.; Harada, S.; Hasui, Y.; et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J. Cancer Res. Clin. Oncol. 2016, 142, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Gurioli, C.; Ryu, J.H.; Decker, P.A.; Ravaglia, C.; Tantalocco, P.; Buccioli, M.; Piciucchi, S.; Sverzellati, N.; Dubini, A.; et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015, 147, 157–164. [Google Scholar] [CrossRef]
- Li, F.; Liu, H.; Wu, H.; Liang, S.; Xu, Y. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat. Oncol. 2021, 16, 70. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, Y.S.; Lee, S.N.; Lee, H.C.; Oh, S.J.; Kim, S.J.; Kim, Y.K.; Han, D.H.; Yoo Ie, R.; Kang, J.H.; et al. Interstitial Lung Change in Pre-radiation Therapy Computed Tomography Is a Risk Factor for Severe Radiation Pneumonitis. Cancer Res. Treat. 2015, 47, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: Comparison between X-ray and proton therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.; Pyo, H.; Ahn, Y.C.; Noh, J.M.; Ju, S.G.; Lee, W.; Park, B.; Kim, J.M.; Kang, N.; et al. Impact of Underlying Pulmonary Diseases on Treatment outcomes in Early-Stage Non-Small Cell Lung Cancer Treated with Definitive Radiotherapy. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2273–2281. [Google Scholar] [CrossRef]
- Moon, S.W.; Park, M.S.; Kim, Y.S.; Jang, J.; Lee, J.H.; Lee, C.T.; Chung, J.H.; Shim, H.S.; Lee, K.W.; Kim, S.S.; et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: Impact on survival and acute exacerbation. BMC Pulm. Med. 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ohguri, T.; Ide, S.; Aoki, T.; Imada, H.; Yahara, K.; Narisada, H.; Korogi, Y. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer 2013, 82, 260–265. [Google Scholar] [CrossRef]
- Selman, M. From anti-inflammatory drugs through antifibrotic agents to lung transplantation: A long road of research, clinical attempts, and failures in the treatment of idiopathic pulmonary fibrosis. Chest 2002, 122, 759–761. [Google Scholar] [CrossRef]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef]
- Hadda, V.; Guleria, R. Antifibrotic drugs for idiopathic pulmonary fibrosis: What we should know? Indian J. Med. Res. 2020, 152, 177–180. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, S.S.; Fois, A.G.; Cossu, A.; Palmieri, G.; Pintus, G. Repurposing Anticancer Drugs for the Treatment of Idiopathic Pulmonary Fibrosis and Antifibrotic Drugs for the Treatment of Cancer: State of the Art. Curr. Med. Chem. 2021, 28, 2234–2247. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Oh, Y.M.; Lee, M.; Choi, S.; Seo, J.B.; Lee, S.M.; Kim, N. Low morphometric complexity of emphysematous lesions predicts survival in chronic obstructive pulmonary disease patients. Eur. Radiol. 2019, 29, 176–185. [Google Scholar] [CrossRef]
- Kolb, M.; Collard, H.R. Staging of idiopathic pulmonary fibrosis: Past, present and future. Eur. Respir. Rev. 2014, 23, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, P. On the fractal dimension of the Henon attractor. Phys. Lett. A 1983, 97, 224–226. [Google Scholar] [CrossRef]
- Ott, E. Chaos in Dynamical Systems; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1993. [Google Scholar]
- Karperien, A. User’s Guide for FracLac, V. 2.5: Fractal Analysis: Box Counting. Available online: https://imagej.nih.gov/ij/plugins/fraclac/FLHelp/BoxCounting.htm (accessed on 18 September 2023).
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Series B Stat. Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, M.; Lee, S.M.; Oh, S.Y.; Oh, Y.M.; Kim, N.; Seo, J.B. A size-based emphysema severity index: Robust to the breath-hold-level variations and correlated with clinical parameters. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1835–1841. [Google Scholar] [CrossRef]
- Glenny, R.W.; Robertson, H.T. Fractal properties of pulmonary blood flow: Characterization of spatial heterogeneity. J. Appl. Physiol. 1990, 69, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.; Tanabe, N.; Tan, W.C.; Zhou, G.; Obeidat, M.; Hague, C.J.; Leipsic, J.; Bourbeau, J.; Sin, D.D.; Hogg, J.C.; et al. Total Airway Count on Computed Tomography and the Risk of Chronic Obstructive Pulmonary Disease Progression. Findings from a Population-based Study. Am. J. Respir. Crit. Care Med. 2018, 197, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. Fractal geometry: A design principle for living organisms. Am. J. Physiol. 1991, 261, L361–L369. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. It takes more than cells to make a good lung. Am. J. Respir. Crit. Care Med. 2013, 187, 342–346. [Google Scholar] [CrossRef]

| Characteristics | Number | % |
|---|---|---|
| Age [years; median (range)] | 74 (53–86) | |
| Sex | ||
| Female | 1 | 5.3% |
| Male | 18 | 94.7% |
| Smoking Status | ||
| Never smoker | 2 | 10.5% |
| Current or Ex-smoker | 17 | 89.5% |
| Histology | ||
| Adenocarcinoma | 8 | 42.1% |
| Squamous cell carcinoma | 11 | 57.9% |
| Anti-fibrotic agent treatment | ||
| No | 9 | 47.4% |
| Yes | 10 | 52.6% |
| Pretreatment DLCO | ||
| >60% | 5 | 26.3% |
| ≤60% | 10 | 52.6% |
| ≤40% | 4 | 21.1% |
| Radiotherapy aim | ||
| Definitive radiotherapy alone | 6 | 31.6% |
| Definitive CCRT | 6 | 31.6% |
| Consolidative radiotherapy alone | 7 | 36.8% |
| Radiotherapy technique | ||
| IMRT | 13 | 68.4% |
| SABR | 6 | 31.6% |
| GAP IPF stage | ||
| I | 7 | 36.8% |
| II | 11 | 57.9% |
| III | 1 | 5.3% |
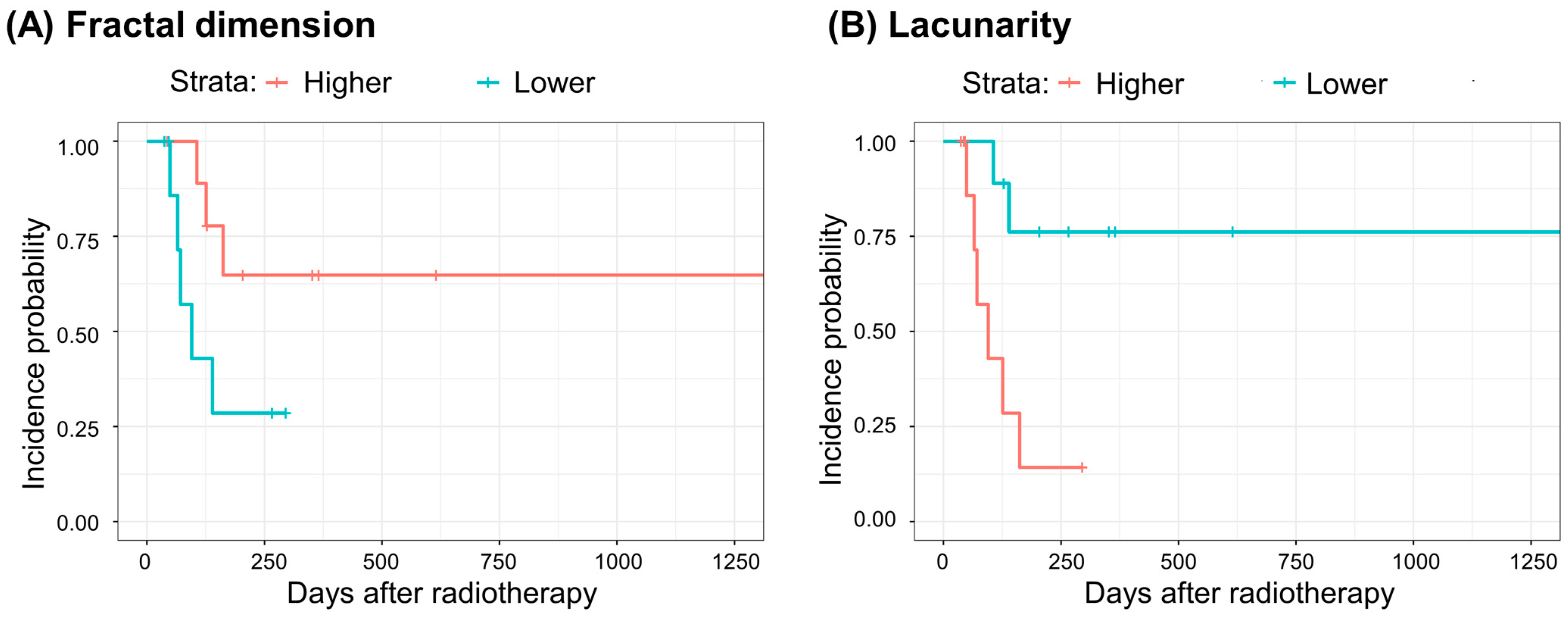
| Fractal Dimension | Lacunarity | |||||
|---|---|---|---|---|---|---|
| 2.175–2.363 (N = 10) | 1.914–2.157 (N = 9) | 0.455–0.908 (N = 10) | 0.317–0.454 (N = 9) | |||
| Grade≥3 (N = 8) | Unadjusted model | HR b (95% CI c) | Reference | 3.536 (0.833–15.01) | Reference | 0.144 (0.029–0.725) |
| p | 0.087 | 0.019 | ||||
| C-index d | 0.689 | 0.750 | ||||
| p of Log-rank test | 0.070 | 0.007 | ||||
| Adjusted model a | HR (95% CI) | Reference | 7.755 (1.168–51.51) | Reference | 0.022 (0.002–0.306) | |
| p | 0.034 | 0.004 | ||||
| C-index | 0.744 | 0.878 | ||||
| p of Log-rank test | 0.200 | 0.020 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Kim, H.; Kim, S.M.; Yang, D.S. Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis. Life 2024, 14, 897. https://doi.org/10.3390/life14070897
Hwang J, Kim H, Kim SM, Yang DS. Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis. Life. 2024; 14(7):897. https://doi.org/10.3390/life14070897
Chicago/Turabian StyleHwang, Jeongeun, Hakyoung Kim, Sun Myung Kim, and Dae Sik Yang. 2024. "Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis" Life 14, no. 7: 897. https://doi.org/10.3390/life14070897
APA StyleHwang, J., Kim, H., Kim, S. M., & Yang, D. S. (2024). Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis. Life, 14(7), 897. https://doi.org/10.3390/life14070897






