Exploring Lacrimal Gland Tear Production in Sheep under General Anesthesia: Examining the Potential Impact of Utilizing 1% Hyaluronic Acid Ophthalmic Gel
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Effects of the Anesthetic Drugs on Tear Production
4.2. Effect of 1% Hyaluronic Acid Compared with the Eye Closed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelatt, K.N.; Gilger, B.C.; Kern, T.J. Veterinary Ophthalmology, 5th ed.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 568–573, 583–590. [Google Scholar]
- Dalga, S.; Aksu, S.I.; Aslan, K.; Deprem, T.; Uğran, R. Anatomical and Histological Structures of Eye and Lacrimal Gland in Norduz and Morkaraman Sheep. Turk. J. Vet. Anim. Sci. 2022, 46, 336–346. [Google Scholar] [CrossRef]
- Featherstone, H.J.; Heinrich, C.L. Ophthalmic Examination and Diagnostics. In Veteinary Ophthalmology, 5th ed.; Gelatt, K.N., Gilger, B.C., Kern, T.J., Eds.; Two Volume Set; Wiley-Blackwell: Oxford, UK, 2013; pp. 533–702. [Google Scholar]
- Ribeiro, A.; Piso, D.; Padua, I.; Silva, M.; Laus, J. Intraocular Pressure and Tear Secretion in Saanen Goats with Different Ages. Pesqui. Veterinária Bras. 2010, 30, 798–802. [Google Scholar] [CrossRef]
- Van Kampen, K.R.; James, L.F. Ophthalmic lesions in locoweed poisoning of cattle, sheep, and horses. Am. J. Vet. Res. 1971, 32, 1293–1295. [Google Scholar]
- Williams, D.L.; Tighe, A. Immunohistochemical Evaluation of Lymphocyte Populations in the Nictitans Glands of Normal Dogs and Dogs with Keratoconjunctivitis Sicca. Open Vet. J. 2018, 8, 47–52. [Google Scholar] [CrossRef]
- Kurt, B.K.; Bulut, O.; Bozkan, Z.; Şen, Z.B.; Belge, A. Determination of Tear Volume and Intraocular Pressure in Saanen Goat and Sakiz Sheep in Similar Environmental Conditions. Int. J. Vet. Anim. Res. (IJVAR) 2021, 4, 78–82. [Google Scholar]
- Samuelson, D.A. Ophthalmic anatomy. In Veterinary Ophthalmology; Gelatt, K.N., Ed.; Blackwell: Ames, IA, USA, 2007; pp. 37–148. [Google Scholar]
- Gum, G.G.; Gelatt, K.N.; Esson, D.W. Physiology of the eye. In Veterinary Ophthalmology; Gelatt, K.N., Ed.; Blackwell: Ames, IA, USA, 2007; pp. 149–182. [Google Scholar]
- Rolando, M.; Zierhut, M. The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. Surv. Ophthalmol. 2001, 45, S203–S210. [Google Scholar] [CrossRef]
- Ehlers, N. The Precorneal Film. Biomicroscopical, Histological and Chemical Investigations. Acta Ophthalmol. Suppl. 1965, (Suppl. 81), 81–134. [Google Scholar]
- Grixti, A.; Sadri, M.; Watts, M.T. Corneal Protection during General Anesthesia for Nonocular Surgery. Ocul. Surf. 2013, 11, 109–118. [Google Scholar] [CrossRef]
- Kaye, A.D.; Renschler, J.S.; Cramer, K.D.; Anyama, B.O.; Anyama, E.C.; Gayle, J.A.; Armstead-Williams, C.M.; Mosieri, C.N.; Saus, J.A.; Cornett, E.M. Postoperative Management of Corneal Abrasions and Clinical Implications: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 48. [Google Scholar]
- Costea, R.; Pavel, R.; Girdan, G.; Ene, I.; Posastiuc, F.; Micsa, C.; Constantin, T.; Togoe, D.; Diaconescu, A. Partial Intravenous Anesthesia with Isoflurane and Alfaxalone for an Adult Sheep Undergoing Soft Tissue Surgery. Sci. Work. Ser. C Vet. Med. 2023, 69, 57–60. [Google Scholar]
- Costea, R.; Ene, I.; Pavel, R. Pig Sedation and Anesthesia for Medical Research. Animals 2023, 13, 3807. [Google Scholar] [CrossRef]
- Peche, N.; Köstlin, R.; Reese, S.; Pieper, K. Postanaesthetic Tear Production and Ocular Irritation in Cats. Tierärztliche Prax. Kleintiere 2015, 43, 75–82. [Google Scholar] [CrossRef]
- Dodam, B. Effects of Intramuscular Sedative and Opioid Combinations on Tear Production in Dogs. Vet. Ophthalmol. 1998, 1, 57–59. [Google Scholar] [CrossRef]
- Smolle, M.; Keller, C.; Pinggera, G.; Deibl, M.; Rieder, J.; Lirk, P. Clear Hydro-Gel, Compared to Ointment, Provides Improved Eye Comfort after Brief Surgery. Can. J. Anesth. 2004, 51, 126. [Google Scholar] [CrossRef] [PubMed]
- Siffring, P.; Poulton, T. Prevention of Ophthalmic Complications during General Anesthesia. J. Am. Soc. Anesthesiol. 1987, 66, 569–570. [Google Scholar] [CrossRef]
- Schmidt, P.; Bøggild-Madsen, N.B. Protection of the Eyes with Ophthalmic Ointments during General Anaesthesia. Acta Ophthalmol. 1981, 59, 422–427. [Google Scholar] [CrossRef]
- Orlin, S.E.; Kurata, F.K.; Krupin, T.; Schneider, M.; Glendrange, R.R. Ocular Lubricants and Corneal Injury during Anesthesia. Anesth. Analg. 1989, 69, 384–385. [Google Scholar]
- Grover, V.K.; Kumar, K.V.; Sharma, S.; Sethi, N.; Grewal, S.P. Comparison of Methods of Eye Protection under General Anaesthesia. Can. J. Anaesth. 1998, 45, 575–577. [Google Scholar] [CrossRef]
- Bøggild-Madsen, N.B.; Bundgarrd-Nielsen, P.; Hammer, U.; Jakobsen, B. Comparison of Eye Protection with Methylcellulose and Paraffin Ointments during General Anaesthesia. Can. Anaesth. Soc. J. 1981, 28, 575–578. [Google Scholar] [CrossRef]
- Kovalcuka, L.; Malniece, A. Measurement of Tear Production and Intraocular Pressure in Clinically Conscious Normal Captive Red Deer (Cervus elaphus). Animals 2024, 14, 940. [Google Scholar] [CrossRef]
- Orhan, A.; Bozkan, Z. Evaluation of Normal Tear Volume and Intraocular Pressure in Saanen Goats at Different Periods. Int. J. Vet. Anim. Res. (IJVAR) 2022, 5, 154–158. [Google Scholar]
- Dedousi, A.; Karatzia, M.A.; Katsoulos, P.D. Reference Values of Schirmer Tear Test in Sheep and the Effect of Season on the Test Results. Acta Vet. Hung. 2019, 67, 553–560. [Google Scholar] [CrossRef]
- Beech, J.; Zappala, R.; Smith, G.; Lindborg, S. Schirmer Tear Test Results in Normal Horses and Ponies: Effect of Age, Season, Environment, Sex, Time of Day and Placement of Strips. Vet. Ophthalmol. 2003, 6, 251–254. [Google Scholar] [CrossRef]
- Del Sole, M.J.; Sande, P.H.; Bernades, J.M.; Aba, M.A.; Rosenstein, R.E. Circadian Rhythm of Intraocular Pressure in Cats. Vet. Ophthalmol. 2007, 10, 155–161. [Google Scholar] [CrossRef]
- Giannetto, C.; Piccione, G.; Giudice, E. Daytime Profile of the Intraocular Pressure and Tear Production in Normal Dog. Vet. Ophthalmol. 2009, 12, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.S.; Shojaei, M.; Sabzevari, A.; Khorami, N. Reference Values for Intraocular Pressure and Schirmer Tear Test in Clinically Normal Sanjabi Sheep. Small Rumin. Res. 2011, 97, 101–103. [Google Scholar] [CrossRef]
- Akgul, M.B. The effect of late pregnancy on tear secretıon ın romanov sheep. Int. J. Inf. Res. Rev. 2019, 6, 6078–6080. [Google Scholar]
- Işler, C.; Altuğ, M.; Kilic, S. Evaluation of Tear Fluid Secretion and Intraocular Pressure in Normal Merinos Sheep and Saanen Goats. Rev. Med. Vet. 2013, 164, 278–282. [Google Scholar]
- Rothschild, C.M.; Sellon, D.C.; Bryan, G.M.; Gay, J.M.; Hines, M.T. Effects of Trimethoprim–Sulfadiazine on Tear Production and the Fluctuations of Schirmer Tear Test Values in Horses. Vet. Ophthalmol. 2004, 7, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, F.; Costa, G.L.; Stagnoli, A.; Zubin, E.; Boschi, P.; Sabbioni, A.; Simonazzi, B. The Effect of Intramuscular Dexmedetomidine-Butorphanol Combination on Tear Production in Dogs. Can. Vet. J. 2019, 60, 55. [Google Scholar] [PubMed]
- Di Palma, C.; Micieli, F.; Lamagna, B.; Nieddu, A.; Uccello, V.; Fatone, G.; Vesce, G. Schirmer Tear Test Value and Corneal Lesions’ Incidence during General Anesthesia for Non-Ophthalmic Surgery in Non-Brachycephalic Dogs: A Pilot Study Comparing Three Different Lubricant Eye Drop Formulations. Vet. Sci. 2020, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Di Stefano, G.; Ferreri, F.; Spinella, R.; Stilo, A. Sodium Hyaluronate Eye Drops of Different Osmolarity for the Treatment of Dry Eye in Sjögren’s Syndrome Patients. Br. J. Ophthalmol. 2002, 86, 879–884. [Google Scholar] [PubMed]


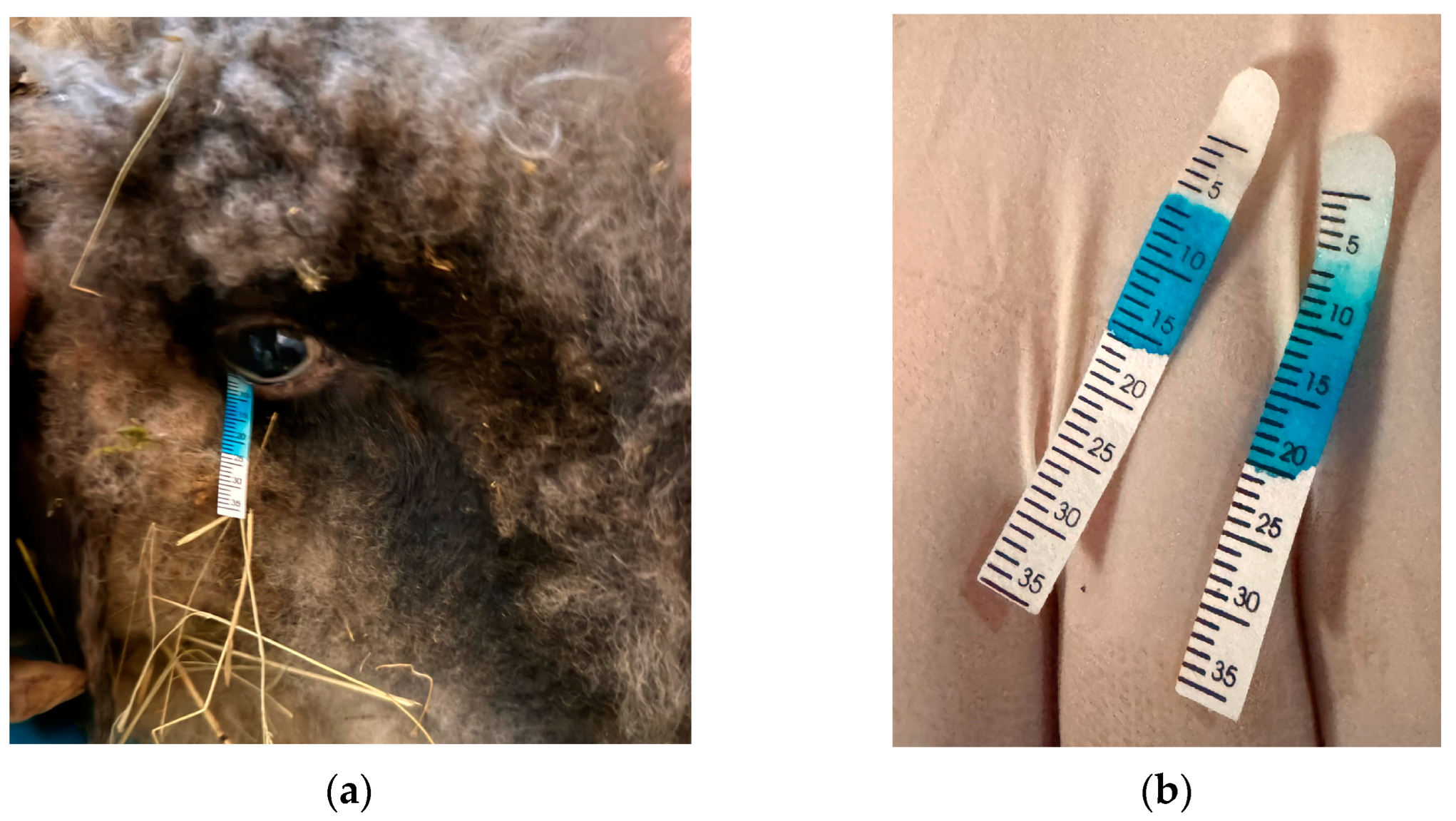





| STT Values in Both Eyes at Different Time Periods | Mean ± SD | |
|---|---|---|
| T0 | OD before premedication | 19.09 ± 3.96 mm/min |
| OS before premedication | 17.33 ± 2.53 mm/min | |
| T1 | OD 15 min after premedication | 13.08 ± 3.31 mm/min |
| OS 15 min after premedication | 12 ± 3.43 mm/min | |
| T2 | OD 15 min after taping the eye and intubation | 8.75 ± 2.70 mm/min |
| OS 15 min after instilating the ocular lubricant and intubation | 3.58 ± 2.71 mm/min | |
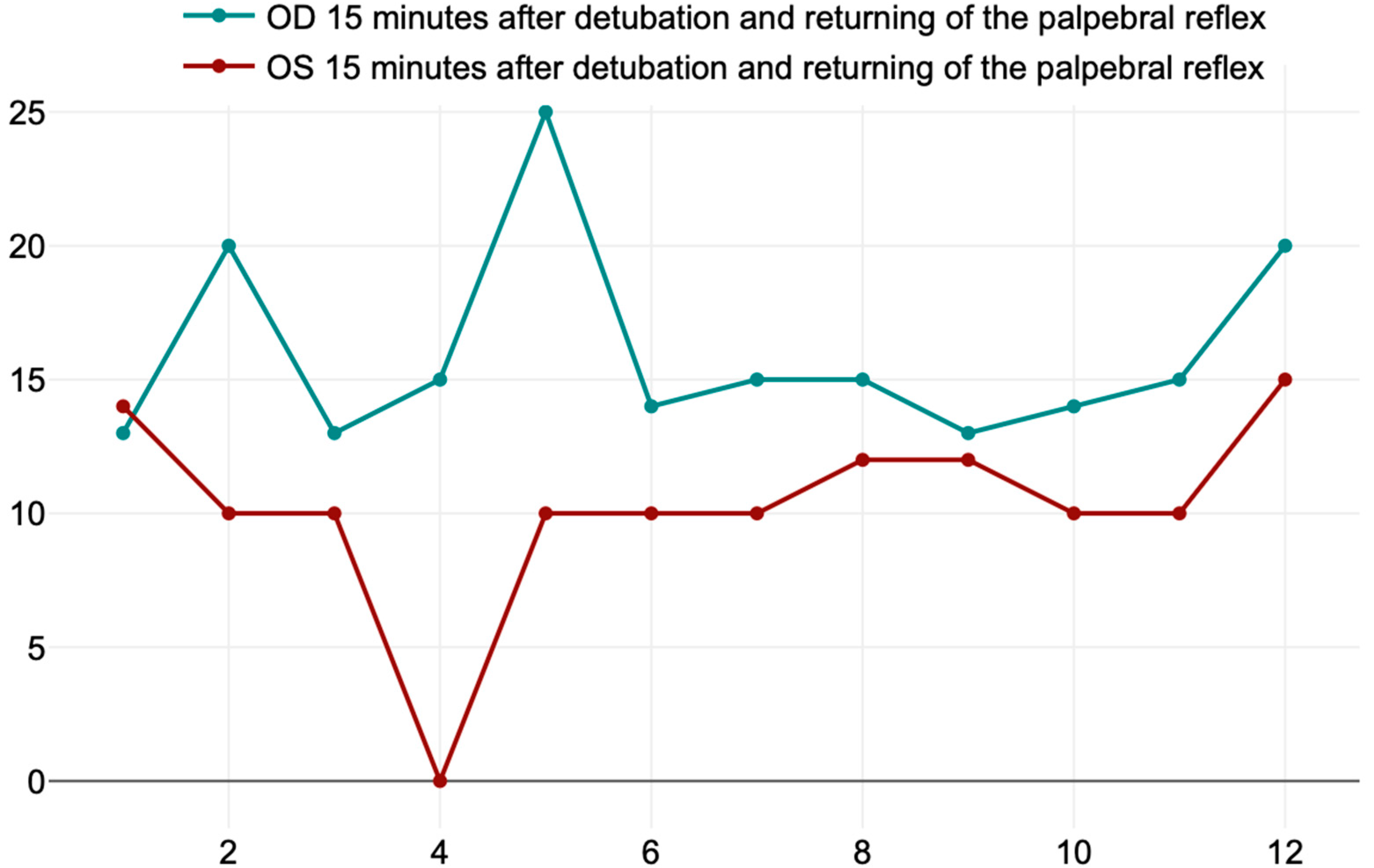
| T3 | OD 15 min after detubation and returning of the palpebral reflex | 16 ± 3.71 mm/min |
| OS 15 min after detubation and returning of the palpebral reflex | 10.25 ± 3.67 mm/min | |
| T4 | OD after 24 h | 18.08 ± 2.42 mm/min |
| OS after 24 h | 14.16 ± 1.89 mm/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, R.; Ene, I.; Costea, R. Exploring Lacrimal Gland Tear Production in Sheep under General Anesthesia: Examining the Potential Impact of Utilizing 1% Hyaluronic Acid Ophthalmic Gel. Life 2024, 14, 1038. https://doi.org/10.3390/life14081038
Pavel R, Ene I, Costea R. Exploring Lacrimal Gland Tear Production in Sheep under General Anesthesia: Examining the Potential Impact of Utilizing 1% Hyaluronic Acid Ophthalmic Gel. Life. 2024; 14(8):1038. https://doi.org/10.3390/life14081038
Chicago/Turabian StylePavel, Ruxandra, Ioana Ene, and Ruxandra Costea. 2024. "Exploring Lacrimal Gland Tear Production in Sheep under General Anesthesia: Examining the Potential Impact of Utilizing 1% Hyaluronic Acid Ophthalmic Gel" Life 14, no. 8: 1038. https://doi.org/10.3390/life14081038
APA StylePavel, R., Ene, I., & Costea, R. (2024). Exploring Lacrimal Gland Tear Production in Sheep under General Anesthesia: Examining the Potential Impact of Utilizing 1% Hyaluronic Acid Ophthalmic Gel. Life, 14(8), 1038. https://doi.org/10.3390/life14081038





