Sticky Bone as a New Type of Autologous Bone Grafting in Schatzker Type II Tibial Plateau Fracture Case Report
Abstract
:1. Introduction
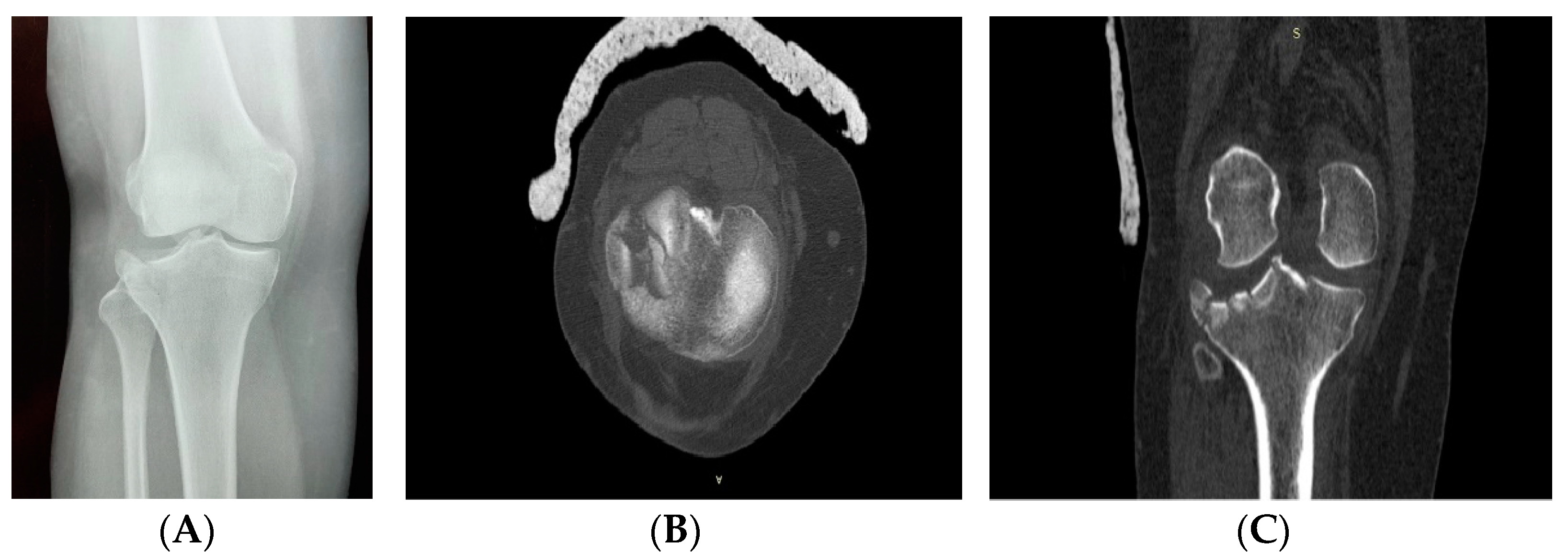
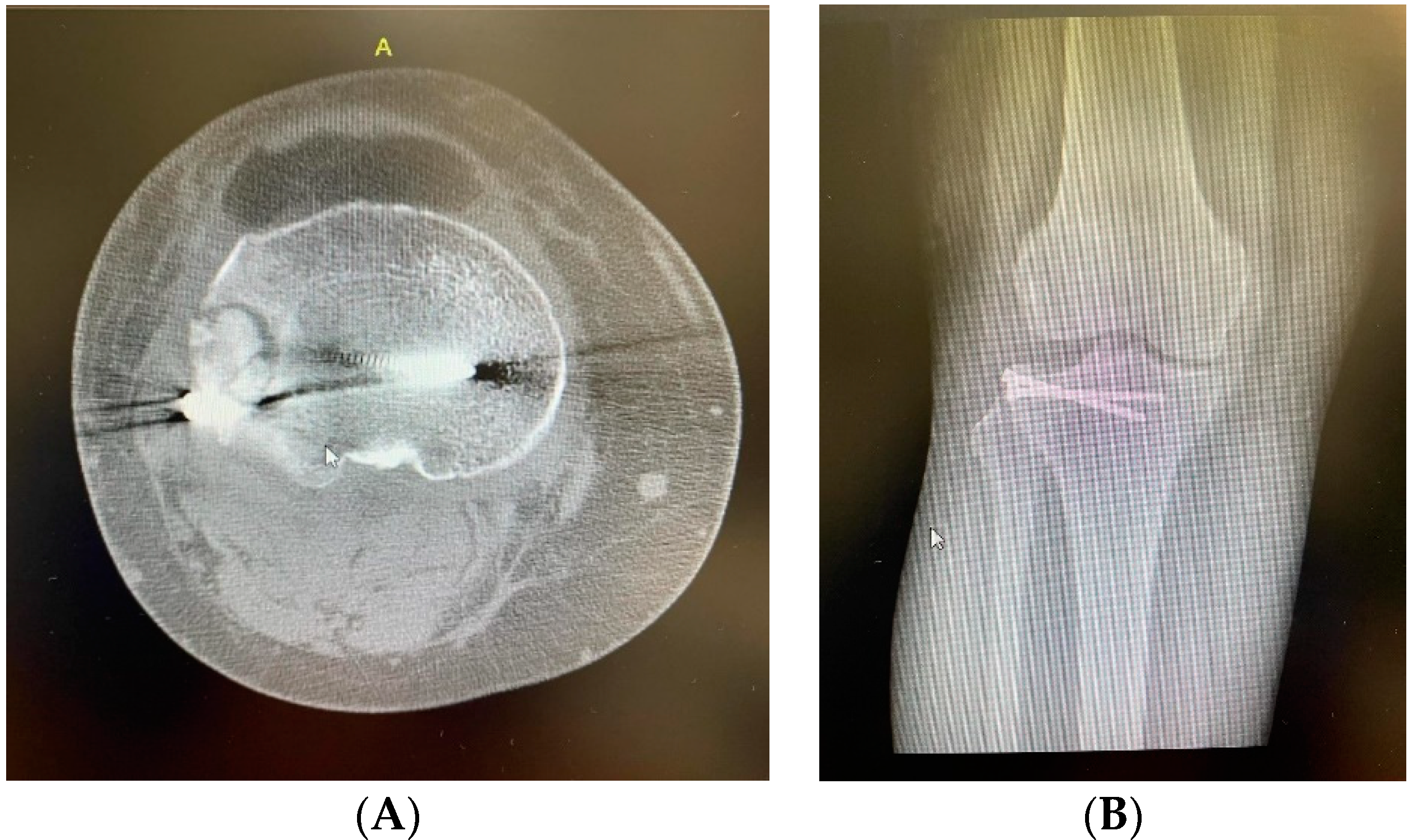
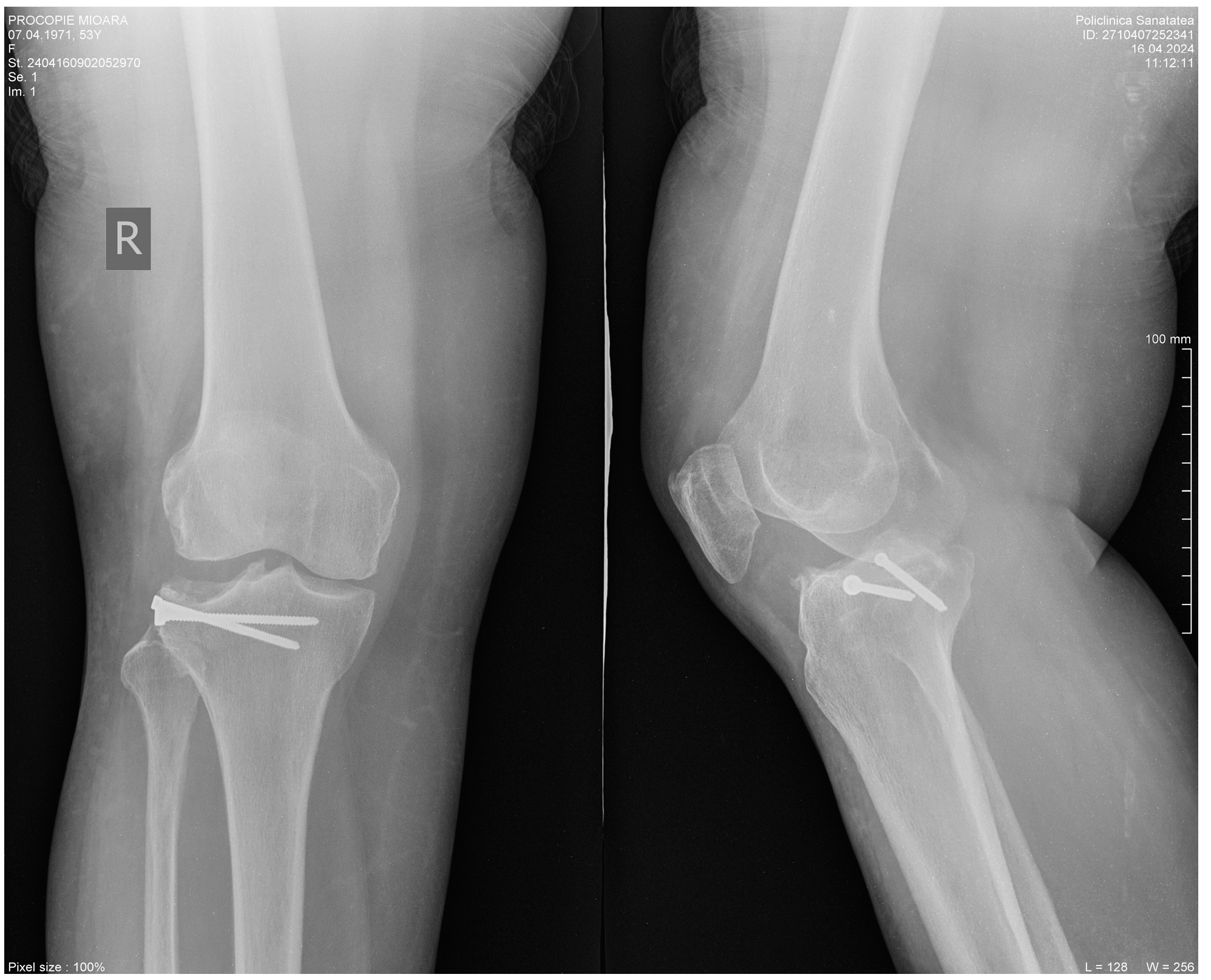
2. Clinical Case Presentation
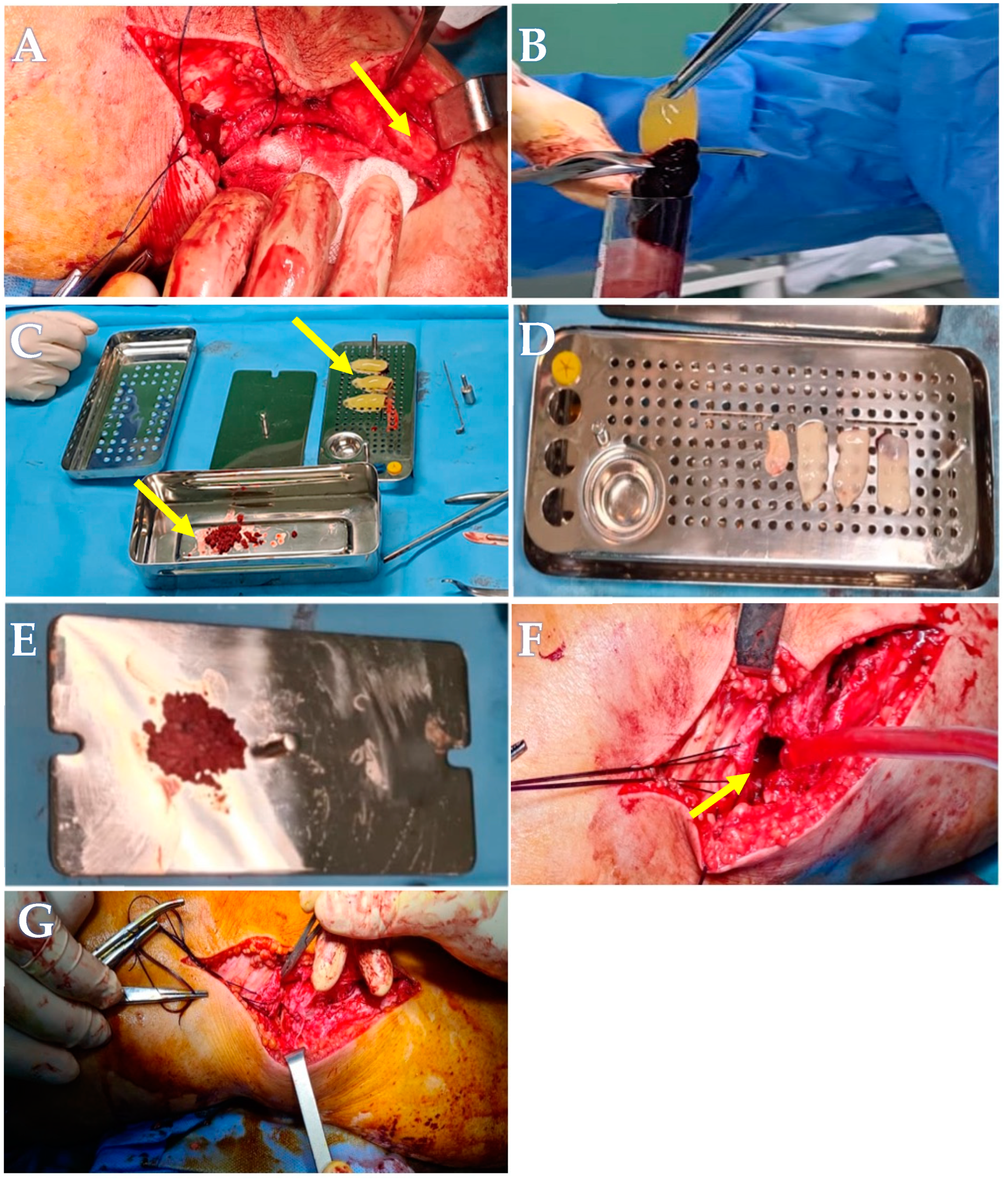
2.1. Advanced Platelets Rich Fibrin (A-PRF) Membrane and Sticky Bone Preparation
Blood Collection
3. Discussion and Review of the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, J.; Bouaicha, W.; Lamouchi, M.; Ammar, A.B.; Jaziri, S.; Daas, S. Comparison of the results of the synthesis of Schatzker II and III tibial plateau fractures by screwing versus plate. Int. Orthop. 2023, 47, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Beuerlein, M.J.; McKee, M.D.; Canadian Orthopaedic Trauma Society. Open reduction and internal fixation compared with circular fixator application for bicondylar tibial plateau fractures. Surgical technique. J. Bone Jt. Surg. 2009, 91, 74–88. [Google Scholar] [CrossRef]
- Egol, K.A.; Tejwani, N.C.; Capla, E.L.; Wolinsky, P.L.; Koval, K.J. Staged management of high-energy proximal tibia fractures (OTA types 41): The results of a prospective, standardized protocol. J. Orthop. Trauma 2005, 19, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.A.; Smith, W.R.; Silva, S.; Agudelo, J.F.; Williams, A.E.; Hak, D.; Morgan, S.J. Treatment of distal femur and proximal tibia fractures with external fixation followed by planned conversion to internal fixation. J. Trauma 2008, 64, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Prat-Fabregat, S.; Camacho-Carrasco, P. Treatment strategy for tibial plateau fractures: An update. EFORT Open Rev. 2017, 1, 225–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M.; CERTiFy Study Group. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surg. Am. Vol. 2020, 102, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, J.T.; Kukkonen, J.; Aho, A.J.; Moisander, S.; Kyyrönen, T.; Mattila, K. Bioactive glass granules: A suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: A prospective, randomized 1 year follow-up study. J. Mater. Sci. Mater. Med. 2011, 22, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Csönge, L.; Bozsik, Á.; Tóth-Bagi, Z.; Gyuris, R.; Kónya, J. Regenerative medicine: Characterization of human bone matrix gelatin (BMG) and folded platelet-rich fibrin (F-PRF) membranes alone and in combination (sticky bone). Cell Tissue Bank. 2021, 22, 711–717. [Google Scholar] [CrossRef]
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltrán, V.; Fuentes, R. Platelet-rich fibrin application in dentistry: A literature review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Everts, P.A.; Knape, J.T.; Weibrich, G.; Schönberger, J.P.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.; van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extra-Corpor. Technol. 2006, 38, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Rupawala, T.A.; Patel, S.M.; Shah, N.H.; Sanghvi, K.B.; Makwana, S.V.; Bhimani, K.K. Efficacy of Sticky Bone as a Novel Autologous Graft for Mandibular Third Molar Extraction Socket Healing—An Evaluative Study. Ann. Maxillofac. Surg. 2020, 10, 335–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sohn, D.S.; Huang, B.; Kim, J.; Park, W.E.; Park, C.C. Utilization of autologous concentrated growth factors (CGF) enriched bone graft matrix (Sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J. Implant. Adv. Clin. Dent. 2015, 7, 11–29. [Google Scholar]
- HalimAyoub, A.; Ramadan, O. Comparative study socket preservation using PRF and MPM platelet concentrates. Int. J. Prev. Clin. Dent. Res. 2015, 2, 1–4. [Google Scholar]
- Sevencan, A.; Şenol, M.S.; Mısır, A.; Aycan, O.E.; Albayrak, A.; Uçpunar, H. Comparison of cannulated lag screws and lateral locking plate in the treatment of Schatzker type II tibial plateau fractures. Jt. Dis. Relat. Surg. 2020, 31, 130–136. [Google Scholar] [CrossRef]
- Vendeuvre, T.; Gayet, L.-É. Percutaneous treatment of tibial plateau fractures. Orthop. Traumatol. Surg. Researc 2021, 107, 102753. [Google Scholar] [CrossRef]
- Mohan, S.P.; Jaishangar, N.; Devy, S.; Narayanan, A.; Cherian, D.; Madhavan, S.S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Periodontal Regeneration: A Review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. 2), S126–S130. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, N.; Jafarpour, D.; Firoozi, P.; Sahmeddini, S.; Hamedani, S.; de Souza, R.F.; Tayebi, L. The application of injectable platelet-rich fibrin in regenerative dentistry: A systematic scoping review of In vitro and In vivo studies. Jpn. Dent. Sci. Rev. 2022, 58, 89–123. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Patro, B.P.; Jeyaraman, N.; Gangadaran, P.; Rajendran, R.L.; Nallakumarasamy, A.; Jeyaraman, M.; Ramani, P.; Ahn, B.C. Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration. Bioengineering 2023, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Osipov, B.; Emami, A.J.; Christiansen, B.A. Systemic Bone Loss after Fracture. Clin. Rev. Bone Miner. Metab. 2018, 16, 116–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin. Rev. Dent. Chile 2007, 73, 25–28. [Google Scholar] [CrossRef]
- Gassling, V.; Douglas, T.; Warnke, P.H.; Açil, Y.; Wiltfang, J.; Becker, S.T. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin. Oral Implant. Res. 2010, 21, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Sawada, K.; Schaller Miron, R.J. Comparative release of growth factors from PRP, PRF and advanced PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Toffler, M.; Toscano, N.; Holtzclaw, D.; Corso, M.D.; Dohan Ehrenfest, D.M. Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J. Implant. Adv. Clin. Dent. 2009, 1, 21–30. [Google Scholar]
- Gurpur, P.P.; Dayakar, M.M.; Mathews, C.A.; Philip, G. The enigma of sticky bone—A review. Int. J. Curr. Adv. Res. 2019, 8, 20751–20756. [Google Scholar]
- Kümmerle, J.M. Suture materials and patterns. In Equine Surgery, 4th ed.; Shaz, B.H., Ed.; WB Saunders: Philadelphia, PA, USA, 2012; pp. 255–280. [Google Scholar]
- Su, L.L.; Lögdberg, L.E. Blood Banking. In Blood Banking and Transfusion Medicine, 2nd ed.; Hillyer, C.D., Ed.; Churchill Livingstone: London, UK, 2007; pp. 861–887. [Google Scholar]
- Kjaergard, H.K.; Velada, J.L.; Pedersen, J.H.; Fleron, H.; Hollingsbee, D.A. Comparative kinetics of polymerisation of three fibrin sealants and influence on timing of tissue adhesion. Thromb. Res. 2000, 98, 221–228. [Google Scholar] [CrossRef]
- Alston, S.M.; Solen, K.A.; Broderick, A.H.; Sukavaneshvar, S.; Mohammad, S.F. New method to prepare autologous fibrin glue on demand. Transl. Res. 2007, 149, 187–195. [Google Scholar] [CrossRef]
- Breen, A.; O’Brien, T.; Pandit, A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Heselhaus, J.; Wozniak, J.; Weinandy, S.; Mela, P.; Tschoeke, B.; Schmitz-Rode, T.; Jockenhoevel, S. Fibrin-based tissue engineering: Comparison of different methods of autologous fibrinogen isolation. Tissue Eng. Part C Methods 2012, 19, 216–226. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A secondgeneration platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cortese, A.; Pantaleo, G.; Borri, A.; Caggiano, M.; Amato, M. Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients. Int. J. Surg. Case Rep. 2016, 28, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Layrolle, P.; Daculsi, G. A review of bioceramics and fibrin sealant. Eur. Cells Mater. 2004, 8, 27–36. [Google Scholar] [CrossRef]
- Ghoderao, D.; Rathod, S.; Kolte, A.P.; Bawankar, P.; Jadhav, A. Randomized, controlled clinical trial to evaluate efficacy of sticky bone and concentrated growth factor in the management of intrabony defects: 12 months follow-up study. Dent. Res. J. 2022, 19, 67. [Google Scholar] [PubMed] [PubMed Central]
- Egol, K.A.; Nauth, A.; Lee, M.; Pape, H.C.; Watson, J.T.; Borrelli, J., Jr. Bone Grafting: Sourcing, Timing, Strategies, and Alternatives. J. Orthop. Trauma 2015, 29, S10–S14. [Google Scholar] [CrossRef]
- Tayapongsak, P.; O’Brien, D.A.; Monteiro, C.B.; Arceo-Diaz, L.Y. Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J. Oral Maxillofac. Surg. 1994, 52, 161–165. [Google Scholar] [CrossRef]
- Yoon, E.; Dhar, S.; Chun, D.E.; Gharibjanian, N.A.; Evans, G.R. In vivo osteogenic potential of human adiposederived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007, 13, 619–627. [Google Scholar] [CrossRef]
- Haghighat, A.; Akhavan, A.; Hashemi-Beni, B.; Deihimi, P.; Yadegari, A.; Heidari, F. Adipose derived stem cells for treatment of mandibular bone defects: An autologous study in dogs. Dent. Res. J. 2011, 8, S51. [Google Scholar]
- Cui, L.; Liu, B.; Liu, G.; Zhang, W.; Cen, L.; Sun, J.; Yin, S.; Liu, W.; Cao, Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007, 28, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Ficai, A.; Marin, S.; Marin, M.; Ene, A.M.; Pătraşcu, J.M. Collagen/Bioactive Glass Ceramic/Doxycycline Composites for Bone Defects. Rev. Romana Mater. Rom. J. Mater. RRM 2015, 45, 307–314. [Google Scholar]
- de la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Titan, A.L.; Stafford, M.; hua Zheng, C.; Levenston, M.E. Variations in chondrogenesis of human bone marrow-derived mesenchymal stem cells in fibrin/alginate blended hydrogels. Acta Biomater. 2012, 8, 3754–3764. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Annual review of materials research. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- McDuffee, L.A.; Gonzalez, B.P.; Nino-Fong, R.; Aburto, E. Evaluation of an in vivo heterotopic model of osteogenic differentiation of equine bone marrow and muscle mesenchymal stem cells in fibrin glue scaffold. Cell Tissue Res. 2014, 355, 327–335. [Google Scholar] [CrossRef]
- Wang, K.; Guan, Y.; Liu, Y.; Zhu, M.; Li, T.; An, D.; Ou, L.; Che, Y.; Zhang, G.; Zhang, J.; et al. Fibrin glue with autogenic bone marrow mesenchymal stem cells for urethral injury repair in rabbit model. Tissue Eng. Part A 2012, 18, 2507–2517. [Google Scholar] [CrossRef]
- Hasan, S.; Weinberg, M.; Khatib, O.; Jazrawi, L.; Strauss, E.J. The effect of platelet-rich fibrin matrix on rotator cuff healing in a rat model. Int. J. Sport. Med. 2016, 37, 36–42. [Google Scholar] [CrossRef]

| Type of Tubes | Product | Time of Centrifugation in Min | Revolutions per Minute (rpm) |
|---|---|---|---|
| A-PRF Choukroun 10 mL | A-PRF | 12 | 800 |
| Specific PRP Gel separation tube | PRP | 10 | 3500 |
| Fibrin | Fibrin Glue | 3 | 2700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciugelu, S.I.; Patrascu, J.M., Jr.; Florescu, S.; Marian, C. Sticky Bone as a New Type of Autologous Bone Grafting in Schatzker Type II Tibial Plateau Fracture Case Report. Life 2024, 14, 1042. https://doi.org/10.3390/life14081042
Stanciugelu SI, Patrascu JM Jr., Florescu S, Marian C. Sticky Bone as a New Type of Autologous Bone Grafting in Schatzker Type II Tibial Plateau Fracture Case Report. Life. 2024; 14(8):1042. https://doi.org/10.3390/life14081042
Chicago/Turabian StyleStanciugelu, Stefan Iulian, Jenel Marian Patrascu, Jr., Sorin Florescu, and Catalin Marian. 2024. "Sticky Bone as a New Type of Autologous Bone Grafting in Schatzker Type II Tibial Plateau Fracture Case Report" Life 14, no. 8: 1042. https://doi.org/10.3390/life14081042





