Osteomyelitis in Late-Stage Pressure Sore Patients: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Treatment Protocol
2.3. Microbiological Processing and Histopathological Analysis
2.4. Statistical Analysis
3. Results
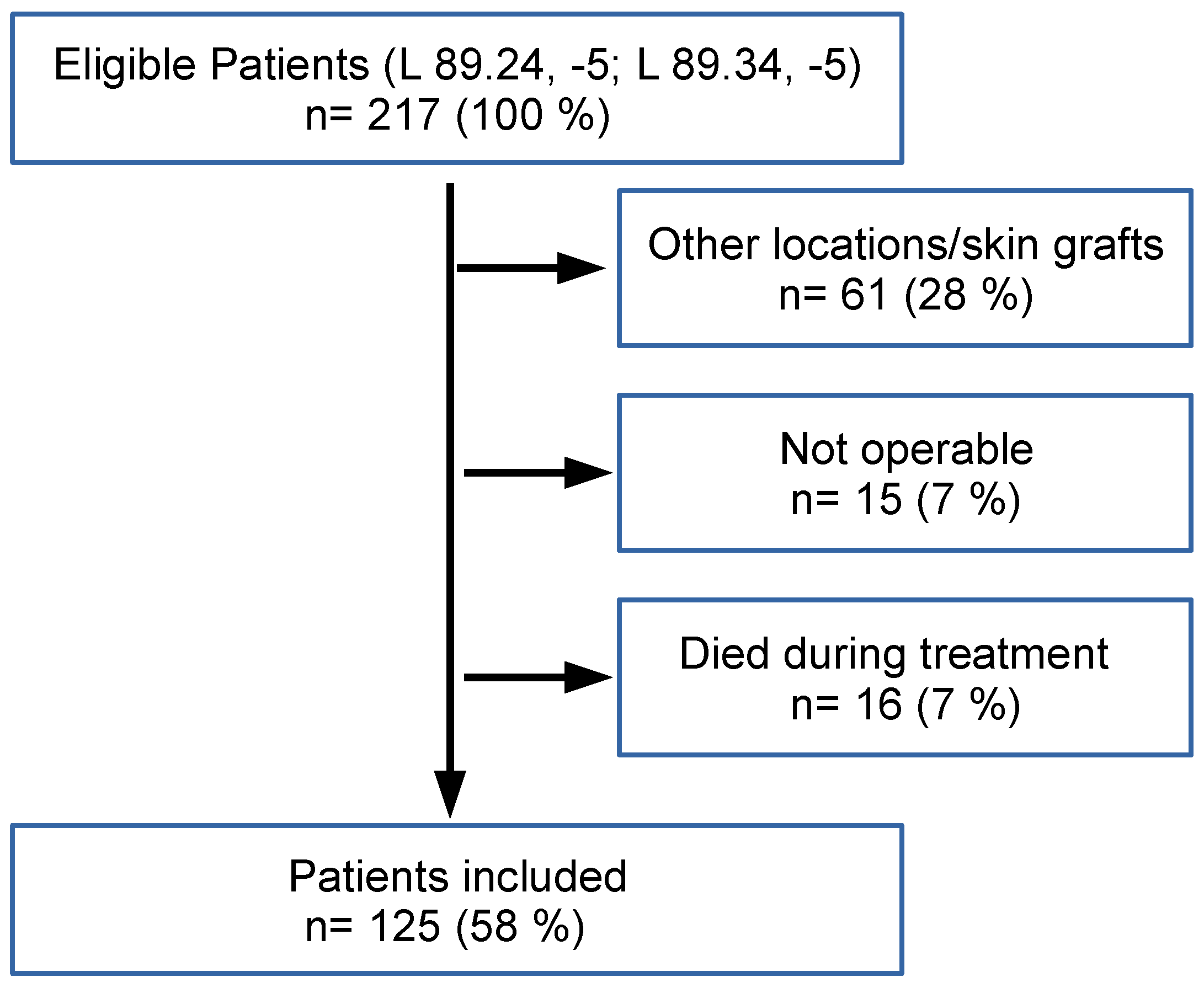
3.1. Patient Cohort
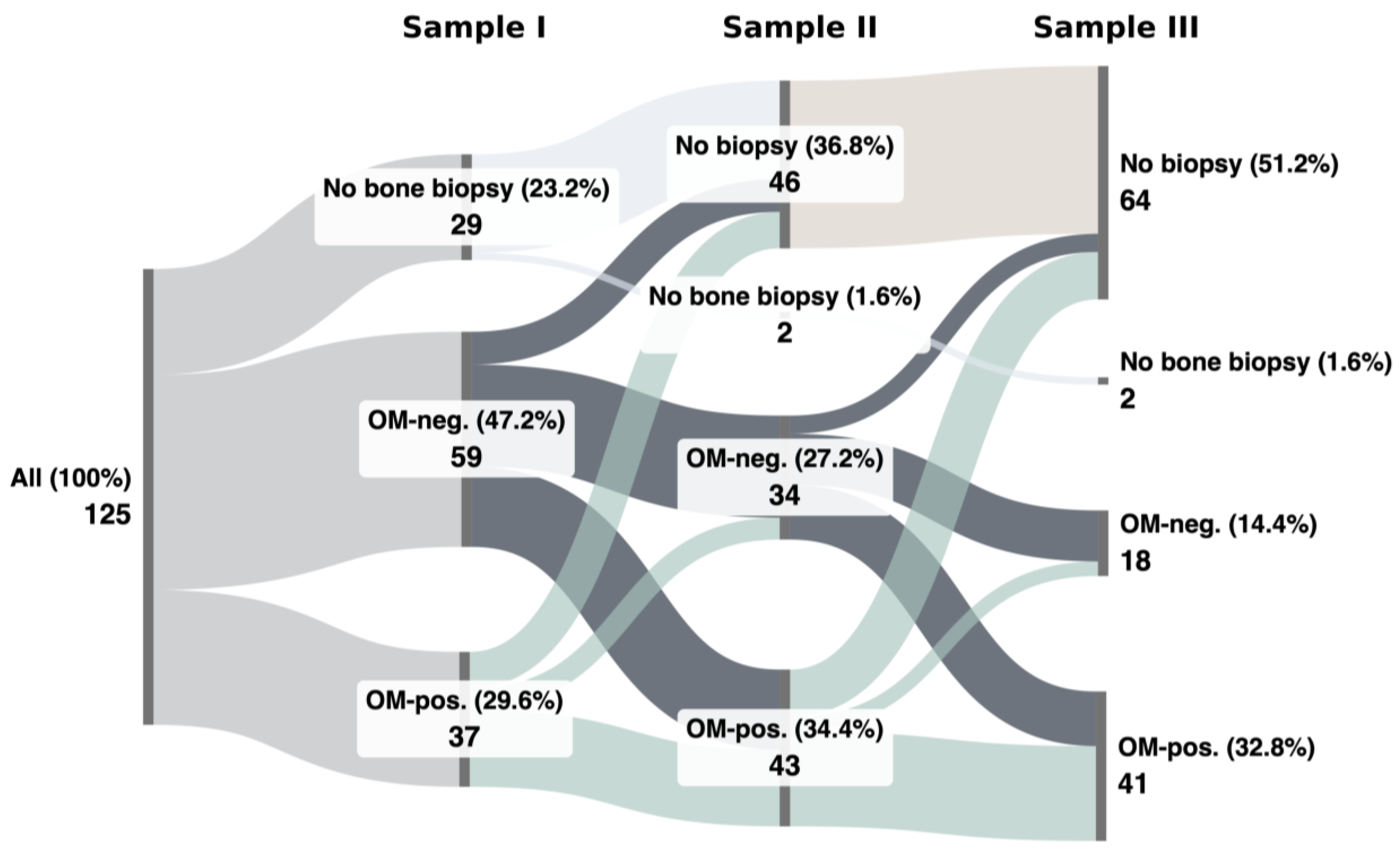
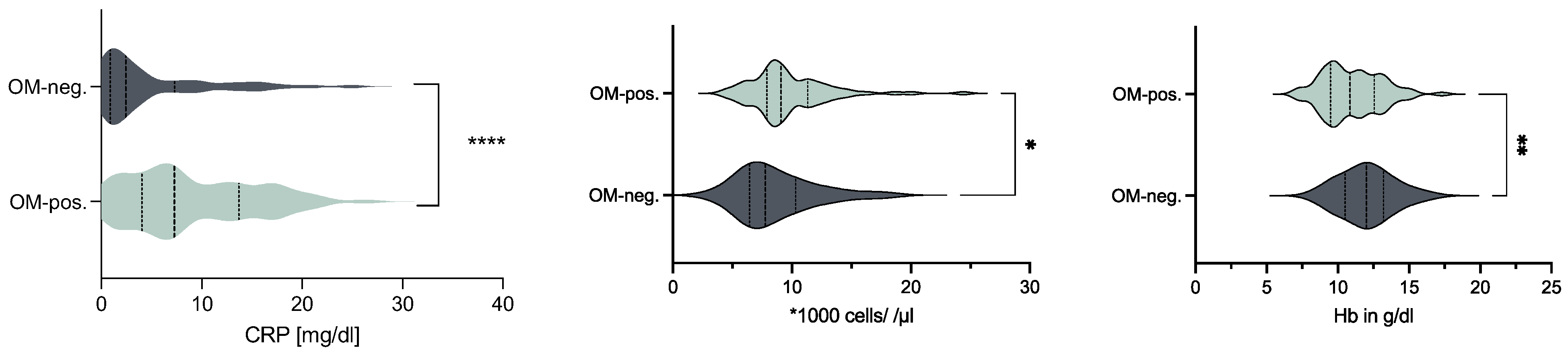
3.2. OM-Positive vs. OM-Negative Biopsies
3.3. Consistent OM-Positivity vs. Consistent OM-Negativity
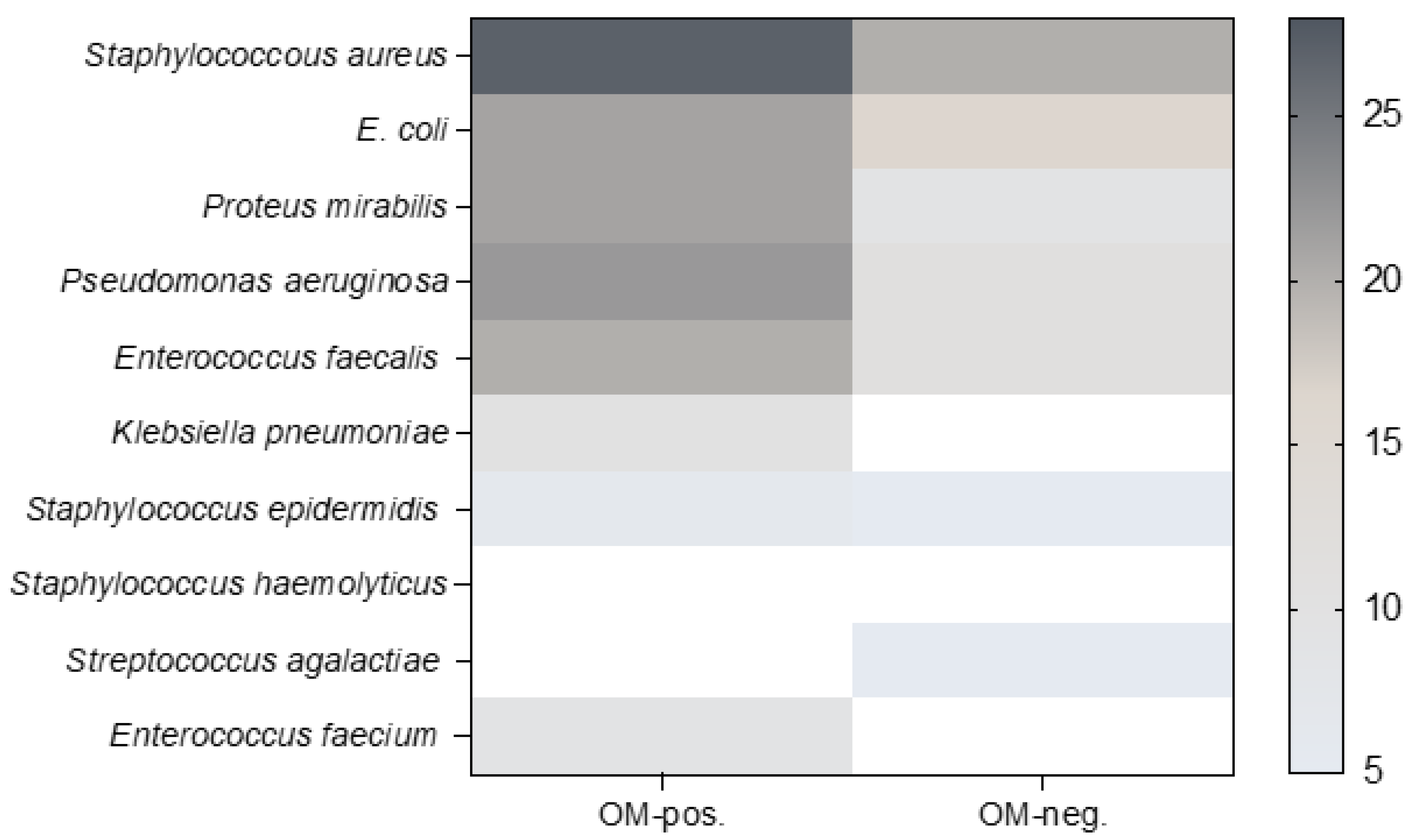
3.4. Microbiological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillespie, B.M.; Chaboyer, W.P.; McInnes, E.; Kent, B.; A Whitty, J.; Thalib, L. Repositioning for pressure ulcer prevention in adults. Cochrane Database Syst. Rev. 2014, 2014, CD009958. [Google Scholar] [CrossRef] [PubMed]
- Kottner, J.; Cuddigan, J.; Carville, K.; Balzer, K.; Berlowitz, D.; Law, S.; Litchford, M.; Mitchell, P.; Moore, Z.; Pittman, J.; et al. Pressure ulcer/injury classification today: An international perspective. J. Tissue Viability 2020, 29, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bialowas, C.; Nguyen, B.; Patel, A.M. Best Solutions for Perineal and Pressure Sore Reconstruction. Plast. Reconstr. Surg. 2021, 148, 1026e–1039e. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Anker, A.M.; Ruewe, M.; Prantl, L.M.; Geis, S.; Kehrer, A.; Baringer, M.; Schiltz, D.; Zeman, F.M.; Vykoukal, J.; Klein, S.M. “A-PePSI LIGhT” Assessment Score to Predict Pressure Sore Impaired Healing Late Recurrence, Immobility, Greater Surface, Inhibited Thrombocytes. Plast. Reconstr. Surg. 2022, 149, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Suissa, D.; Danino, A.; Nikolis, A. Negative-pressure therapy versus standard wound care: A meta-analysis of randomized trials. Plast. Reconstr. Surg. 2011, 128, 498e–503e. [Google Scholar] [CrossRef]
- Moog, P.; Jensch, M.; Betzl, J.; Bauer, A.-T.; Cerny, M.K.; Schmauss, D.; Kükrek, H.; Erne, H.; Machens, H.-G.; Megerle, K. Bacterial bioburden of wounds: Influence of debridement and negative-pressure wound therapy (NPWT). J. Wound Care 2021, 30, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.; Golinko, M.S.; Yan, A.; Flattau, A.; Tomic-Canic, M.; Brem, H. Operative debridement of pressure ulcers. World J. Surg. 2009, 33, 1396–1402. [Google Scholar] [CrossRef]
- Rennert, R.; Golinko, M.; Yan, A.; Flattau, A.; Tomic-Canic, M.; Brem, H. Developing and evaluating outcomes of an evidence-based protocol for the treatment of osteomyelitis in Stage IV pressure ulcers: A literature and wound electronic medical record database review. Ostomy Wound Manag. 2009, 55, 42–53. [Google Scholar]
- Dumville, J.C.; Webster, J.; Evans, D.; Land, L. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2015, CD011334. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Holtom, P.; Spellberg, B. Osteomyelitis Complicating Sacral Pressure Ulcers: Whether or Not to Treat with Antibiotic Therapy. Clin. Infect. Dis. 2019, 68, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Brunel, A.-S.; Lamy, B.; Cyteval, C.; Perrochia, H.; Téot, L.; Masson, R.; Bertet, H.; Bourdon, A.; Morquin, D.; Reynes, J.; et al. Diagnosing pelvic osteomyelitis beneath pressure ulcers in spinal cord injured patients: A prospective study. Clin. Microbiol. Infect. 2016, 22, 267.e1–267.e8. [Google Scholar] [CrossRef] [PubMed]
- Chicco, M.; Singh, P.; Beitverda, Y.; Williams, G.; Hirji, H.; Rao, G.G. Diagnosing pelvic osteomyelitis in patients with pressure ulcers: A systematic review comparing bone histology with alternative diagnostic modalities. J. Bone Jt. Infect. 2020, 6, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Marriott, R.; Rubayi, S. Successful Truncated Osteomyelitis Treatment for Chronic Osteomyelitis Secondary to Pressure Ulcers in Spinal Cord Injury Patients. Ann. Plast. Surg. 2008, 61, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.L.M.; Hudak, K.A.M.; Waring, W.P.M.; Orr, M.R.M.; Simonelic, K.M. Protocol Management of Late-Stage Pressure Ulcers: A 5-Year Retrospective Study of 101 Consecutive Patients with 179 Ulcers. Plast. Reconstr. Surg. 2012, 129, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Jugun, K.; Richard, J.-C.; Lipsky, B.A.M.; Kressmann, B.; Pittet-Cuenod, B.; Suvà, D.; Modarressi, A.; Uçkay, I. Factors Associated with Treatment Failure of Infected Pressure Sores. Ann. Surg. 2016, 264, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Türk, E.E.; Tsokos, M.; Delling, G. Autopsy-Based Assessment of Extent and Type of Osteomyelitis in Advanced-Grade Sacral Decubitus Ulcers: A Histopathologic Study. Arch. Pathol. Lab. Med. 2003, 127, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Arowojolu, O.A.; Wirth, G.A.M. Sacral and Ischial Pressure Ulcer Management with Negative-Pressure Wound Therapy with Instillation and Dwell. Plast. Reconstr. Surg. 2021, 147, 61S–67S. [Google Scholar] [CrossRef]
- Cataldo, M.C.; Bonura, C.; Caputo, G.; Aleo, A.; Rizzo, G.; Geraci, D.M.; Calà, C.; Fasciana, T.; Mattaliano, A.R.; Mammina, C. Colonization of pressure ulcers by multidrug-resistant microorganisms in patients receiving home care. Scand. J. Infect. Dis. 2011, 43, 947–952. [Google Scholar] [CrossRef]
- Norman, G.; Dumville, J.C.; Moore, Z.E.; Tanner, J.; Christie, J.; Goto, S. Antibiotics and antiseptics for pressure ulcers. Cochrane Database Syst. Rev. 2016, 4, CD011586. [Google Scholar] [CrossRef] [PubMed]
- Braga, I.A.; Pirett, C.C.N.S.; Ribas, R.M.; Filho, P.G.; Filho, A.D. Bacterial colonization of pressure ulcers: Assessment of risk for bloodstream infection and impact on patient outcomes. J. Hosp. Infect. 2013, 83, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Kanhere, A.P.; Dowell, E.; Sabbagh, R.S.; Bonamer, J.; Franklin, A.; Sanders, D.T.; Sagi, H.C. Risk Factors and Characteristics of Recalcitrant Osteomyelitis After Initial Surgical and Antibiotic Treatment. J. Orthop. Trauma. 2023, 37, 423. [Google Scholar] [CrossRef]
- Sapico, F.L.; Ginunas, V.J.; Thornhill-Joynes, M.; Canawati, H.N.; Capen, D.A.; Klein, N.E.; Khawam, S.; Montgomerie, J.Z. Quantitative microbiology of pressure sores in different stages of healing. Diagn. Microbiol. Infect. Dis. 1986, 5, 31–38. [Google Scholar] [CrossRef]
| All (n = 125) | OM-neg. (n = 51) | OM-pos. (n = 74) | p | ||
|---|---|---|---|---|---|
| Sex | Female | 57 (45.6%) | 23 (45.1%) | 34 (45.9%) | 0.925 |
| Male | 68 (54.4%) | 28 (54.9%) | 40 (54.1%) | ||
| Reason for bedrest | Frailty | 24 (19.2%) | 11 (21.6%) | 13 (17.6%) | 0.683 |
| Paraplegia | 42 (33.6%) | 15 (29.4%) | 27 (36.5%) | ||
| Other | 59 (47.2%) | 25 (49%) | 34 (45.9%) | ||
| Region | Sacral | 80 (64.0%) | 29 (56.9%) | 51 (68.9%) | 0.200 |
| Ischial | 34 (27.2%) | 15 (29.4%) | 19 (25.7%) | ||
| Trochanteric | 11 (8.8%) | 7 (13.7%) | 4 (5.4%) | ||
| Distance to anus | <3 cm | 27 (21.6%) | 10 (19.6%) | 17 (23%) | 0.376 |
| >3 cm | 79 (63.2%) | 37 (72.5%) | 42 (56.8%) | ||
| Missing | 19 (15.2%) | 4 (7.8%) | 15 (20.2%) | ||
| Colostomy | None | 95 (76.0%) | 45 (88.2%) | 50 (67.2%) | 0.014 |
| Present at admission | 23 (18.4%) | 6 (11.8%) | 17 (23%) | ||
| Newly applied | 7 (0%) | 0 (0%) | 7 (9.8%) | ||
| Grading | 3 | 25 (20.2%) | 20 (39.2%) | 5 (6.8%) | <0.001 |
| 4 | 100 (79.8%) | 31 (60.8%) | 69 (93.2%) | ||
| Area | Small (<5 cm) | 53 (42.4%) | 34 (66.7%) | 19 (26.8%) | <0.001 |
| Medium (5–10 cm) | 50 (40.0%) | 15 (29.4%) | 35 (49.3%) | ||
| Large (>10 cm) | 19 (15.2%) | 2 (3.9%) | 17 (23.9%) | ||
| Missing | 3 (2.4%) | 0 (0%) | 3 (4.1%) | ||
| Histologic samples | 1 | 33 (26.4%) | 23 (45.1%) | 10 (13.5%) | <0.001 |
| 2 | 21 (16.8%) | 5 (9.8%) | 16 (21.6%) | ||
| 3 | 71 (56.8%) | 23 (45.1%) | 48 (64.9%) |
| Total (n = 125) | OM-neg. (n = 51) | OM-pos. (n = 74) | p | |
|---|---|---|---|---|
| Age (years) | 66 (13–95) | 66 (13–95) | 67 (16–95) | 0.714 |
| BMI (kg/m2) | 24.9 ± 6.9 (n = 121) | 24.8 ± 7.4 (n = 49) | 23.8 ± 6.5 (n = 71) | 0.804 |
| LOS (days) | 27 (6–119) | 22 (6–98) | 33 (15–119) | <0.001 |
| Creatinine (mg/dL) | 0.7 (0.09–2.73) | 0.69 (0.09–2.63) | 0.68 (0.24–2.73) | 0.888 |
| GFR (mL/min) | 101 (17–140) | 101 (17–140) | 99 (18–140) | 0.909 |
| Consistent OM-neg. (n = 11) | Consistent OM-pos. (n = 15) | p | |
|---|---|---|---|
| Age (years) | 64 (14–91) | 65 (27–89) | 0.54 |
| BMI (kg/m2) | 23.2 ± 5.1 | 24.5 ± 4.9 | 0.344 |
| LOS (days) | 28 (16–42) | 44 (15–119) | 0.015 |
| CRP (mg/dL) | 4.2 (0.32–18) | 10.1 (1.75–21.9) | 0.144 |
| Leukocytes (*1000/µL) | 7.7 (3–17.3) | 8.6 (6.2–18.6) | 0.474 |
| HB (g/dL) | 13.3 (8.5–15.7) | 10.3 (8.5–15.2) | 0.077 |
| Creatinine (mg/dL) | 0.72 (0.39–2.5) | 0.71 (0.31–2.73) | 0.878 |
| GFR (mL/min) | 99 (17–140) | 100 (18–140) | 1 |
| Consistent OM-pos. (n = 15) | Consistent OM neg. (n = 11) | p | ||
|---|---|---|---|---|
| Flap revision | No revision | 7 (46.7%) | 11 (100%) | 0.007 |
| Revision | 8 (53.3%) | 0 (0%) | ||
| Wound status at discharge | Closed | 12 (80%) | 10 (90.9%) | 0.614 |
| Open | 3 (20%) | 1 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruewe, M.; Siegmund, A.; Rupp, M.; Prantl, L.; Anker, A.M.; Klein, S.M. Osteomyelitis in Late-Stage Pressure Sore Patients: A Retrospective Analysis. Life 2024, 14, 973. https://doi.org/10.3390/life14080973
Ruewe M, Siegmund A, Rupp M, Prantl L, Anker AM, Klein SM. Osteomyelitis in Late-Stage Pressure Sore Patients: A Retrospective Analysis. Life. 2024; 14(8):973. https://doi.org/10.3390/life14080973
Chicago/Turabian StyleRuewe, Marc, Andreas Siegmund, Markus Rupp, Lukas Prantl, Alexandra M. Anker, and Silvan M. Klein. 2024. "Osteomyelitis in Late-Stage Pressure Sore Patients: A Retrospective Analysis" Life 14, no. 8: 973. https://doi.org/10.3390/life14080973
APA StyleRuewe, M., Siegmund, A., Rupp, M., Prantl, L., Anker, A. M., & Klein, S. M. (2024). Osteomyelitis in Late-Stage Pressure Sore Patients: A Retrospective Analysis. Life, 14(8), 973. https://doi.org/10.3390/life14080973









