Pancreatic Cancer: A Review of Risk Factors
Abstract
1. Introduction
2. Methods
3. Risk Factors
3.1. Non-Modifiable Risk Factors
3.1.1. Age
3.1.2. Sex
3.1.3. Height
3.1.4. ABO Group
3.1.5. Genetic Factors
3.1.6. Preneoplasic Pancreatic Lesions
3.2. Modifiable Risk Factors
3.2.1. Smoking
- Electronic cigarettes are battery-operated devices that heat a liquid containing nicotine, a solvent, and one or more flavors. Studies on substances contained in e-cigarette aerosols have shown that they may contain carcinogens such as formaldehyde and acetaldehyde, but in much lower amounts than in the conventional cigarette [129,130], or supersaturated 1,2-propanediol vapor, which causes an increased production of nitric oxide, promoting inflammation [131,132], but there are still no studies evaluating the possible effect on the pancreatic tissue [133]
3.2.2. Diabetes Mellitus (DM)
3.2.3. Obesity, Diet, and Lifestyle
3.2.4. Pancreatitis
3.2.5. Alcohol
3.2.6. Coffee
3.2.7. Hepatitis
3.2.8. Gallbladder Diseases and Cholecystectomy
3.2.9. Periodontal Diseases
3.2.10. Helicobacter pylori (H. pylori)
3.2.11. Autoimmune Diseases
3.2.12. Polycystic Ovary Syndrome (POCS)
3.2.13. Microbiota
3.2.14. Psychological Stress
3.2.15. Renin-Angiotensin Inhibitors
3.2.16. Allergies
3.2.17. Opioids
3.2.18. Proton Pump Inhibitors (PPI)
3.2.19. Nonsteroidal Anti-Inflammatory Drugs (NSAID)
3.2.20. Statins
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACI | Angiotensin I-converting enzyme inhibitors |
| ACR | American College of Radiology |
| AGA | American Gastroenterological Association |
| AP | Acute pancreatitis |
| APC | Adenomatous polyposis coli |
| BD-IPMN | Branch duct intraductal papillary mucinous neoplasm |
| BMI | Body mass index |
| CA 19-9 | Cancer antigen 19-9 |
| CAPS | International Cancer of the Pancreas Screening |
| CEA | Carcinoembryonic antigen |
| CP | Chronic pancreatitis |
| DM | Diabetes mellitus |
| DM1 | Diabetes mellitus type 1 |
| DM2 | Diabetes mellitus type 2 |
| DNA | Deoxyribonucleic acid |
| ECOG | Eastern Cooperative Oncology Group |
| END-PAC | Enriching New-Onset Diabetes for Pancreatic Cancer |
| EOPC | Early onset pancreatic cancer |
| EUS | Endoscopic ultrasound |
| FAMMM-PC | Familial atypical mole melanoma pancreatic carcinoma syndrome |
| FAP | Familial adenomatous polyposis |
| FDR | First-degree relative |
| 68Ga-FAPI PET PET | using 68Ga-labeled fibroblast activation protein inhibitor |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HNPCC | Hereditary nonpolyposis colorectal cancer (Lynch syndrome) |
| IGF | Insulin-like growth factor |
| IPMN | intraductal papillary mucinous neoplasm |
| KRAS | Kirsten-rat sarcoma |
| LOPC | late-onset pancreatic cancer |
| LPS | Lipopolysaccharides |
| MCN | Mucinous cystic neoplasm |
| MD-IPMN | Main duct intraductal papillary mucinous neoplasm |
| MPD | Main pancreatic duct |
| MRCP | Magnetic resonance cholangiopancreatography |
| MRI | Magnet resonance imaging |
| NOD | New-onset diabetes mellitus |
| NSAID | Nonsteroidal Anti-Inflammatory Drugs |
| PC | Pancreatic cancer |
| PCN | Pancreatic cystic neoplasm |
| PCRD | Pancreatic cancer-related diabetes |
| PD-L1 | Programmed cell death ligand 1 |
| PET | Positron Emission Tomography |
| PJS | Peutz-Jeghers syndrome |
| POCS | Polycystic Ovary Syndrome |
| PPDM | Post-pancreatitis diabetes mellitus |
| PPI | Proton Pump Inhibitors |
| SAMF | smoking-associated mortality fraction |
| SDR | second-degree relative |
| SEER | Surveillance, Epidemiology and End Results |
| STK | Serine-threonine kinase |
| WHO | Word Health Organization |
References
- Pancreatic Neuroendocrine Tumors (PNETs). Available online: https://pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/types-of-pancreatic-cancer/endocrine-pancreatic-neuroendocrine-tumors/ (accessed on 19 July 2024).
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014, 20, 11182–11198. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Pancreas—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 12 March 2023).
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Ritchey, J.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer 2007, 110, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, J.-W.; Cheng, Y.-G.; Gao, J.-Y.; Hu, S.-Y.; Wang, L.; Zhan, H.-X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 141, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Petrov, M.S. Pancreatitis, Pancreatic Cancer, and Their Metabolic Sequelae: Projected Burden to 2050. Clin. Transl. Gastroenterol. 2020, 11, e00251. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Lillemoe, K.D.; Yeo, C.J.; Warfield, P.B.; Hruban, R.H. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am. J. Surg. Pathol. 1998, 22, 163–169. [Google Scholar] [CrossRef]
- Brockie, E.; Anand, A.; Albores-Saavedra, J. Progression of atypical ductal hyperplasia/carcinoma in situ of the pancreas to invasive adenocarcinoma. Ann. Diagn. Pathol. 1998, 2, 286–292. [Google Scholar] [CrossRef]
- Berthélemy, P.; Bouisson, M.; Escourrou, J.; Vaysse, N.; Rumeau, J.L.; Pradayrol, L. Identification of K-ras mutations in pancreatic juice in the early diagnosis of pancreatic cancer. Ann. Intern. Med. 1995, 123, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Boadas, J.; Mora, J.; Urgell, E.; Puig, P.; Roca, M.; Cussó, X.; Capellà, G.; Lluís, F.; Farré, A. Clinical usefulness of K-ras gene mutation detection and cytology in pancreatic juice in the diagnosis and screening of pancreatic cancer. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, T.; Qian, J.; Wen, X.; Wu, D. Detecting K-ras and p53 gene mutation from stool and pancreatic juice for diagnosis of early pancreatic cancer. Chin. Med. J. 2002, 115, 1632–1636. [Google Scholar] [PubMed]
- Wakabayashi, T.; Sawabu, N.; Watanabe, H.; Morimoto, H.; Sugioka, G.; Takita, Y. Detection of K-ras point mutation at codon 12 in pure pancreatic juice collected 3 years and 6 months before the clinical diagnosis of pancreatic cancer. Am. J. Gastroenterol. 1996, 91, 1848–1851. [Google Scholar] [PubMed]
- Maire, F.; Micard, S.; Hammel, P.; Voitot, H.; Lévy, P.; Cugnenc, P.-H.; Ruszniewski, P.; Puig, P.L. Differential diagnosis between chronic pancreatitis and pancreatic cancer: Value of the detection of KRAS2 mutations in circulating DNA. Br. J. Cancer 2002, 87, 551–554. [Google Scholar] [CrossRef]
- Ansari, D.; Althini, C.; Ohlsson, H.; Andersson, R. Early-onset pancreatic cancer: A population-based study using the SEER registry. Langenbecks Arch. Surg. 2019, 404, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.P.; Sacks, G.D.; Rochefort, M.M.; Donahue, T.R.; Reber, H.A.; Tomlinson, J.S.; Dawson, D.W.; Eibl, G.; Hines, O.J. Long-term Survival in Patients with Pancreatic Ductal Adenocarcinoma. Surgery 2016, 159, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Japan Data. Available online: https://data.who.int/countries/392 (accessed on 1 October 2023).
- Newgard, C.B.; Sharpless, N.E. Coming of age: Molecular drivers of aging and therapeutic opportunities. J. Clin. Investig. 2013, 123, 946–950. [Google Scholar] [CrossRef]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; La Torre, M.; et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatology 2015, 15, 151–155. [Google Scholar] [CrossRef]
- Lin, J.-C.; Chan, D.-C.; Chen, P.-J.; Chu, H.-C.; Chueh, T.-H.; Huang, H.-H.; Chang, P.-Y.; Yu, C.-P.; Chang, W.-K.; Hsieh, T.-Y. Clinical Characteristics of Early Onset Pancreatic Adenocarcinoma: A Medical Center Experience and Review of the Literature. Pancreas 2011, 40, 638. [Google Scholar] [CrossRef]
- Ntala, C.; Debernardi, S.; Feakins, R.M.; Crnogorac-Jurcevic, T. Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC Gastroenterol. 2018, 18, 139. [Google Scholar] [CrossRef]
- Raimondi, S.; Maisonneuve, P.; Löhr, J.-M.; Lowenfels, A.B. Early onset pancreatic cancer: Evidence of a major role for smoking and genetic factors. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1894–1897. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 20 July 2024).
- Berrington de González, A.; Spencer, E.A.; Bueno-de-Mesquita, H.B.; Roddam, A.; Stolzenberg-Solomon, R.; Halkjaer, J.; Tjønneland, A.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2006, 15, 879–885. [Google Scholar] [CrossRef][Green Version]
- Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Fuchs, C.S. Physical Activity, Obesity, Height, and the Risk of Pancreatic Cancer. JAMA 2001, 286, 921–929. [Google Scholar] [CrossRef]
- Stevens, R.J.; Roddam, A.W.; Spencer, E.A.; Pirie, K.L.; Reeves, G.K.; Green, J.; Beral, V.; Million Women Study Collaborators. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int. J. Cancer 2009, 124, 2400–2405. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bernstein, L.; van den Brandt, P.A.; Calle, E.E.; English, D.R.; Folsom, A.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer 2011, 129, 1708–1717. [Google Scholar] [CrossRef]
- Green, J.; Cairns, B.J.; Casabonne, D.; Wright, F.L.; Reeves, G.; Beral, V. Height and cancer incidence in the Million Women Study: Prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011, 12, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Iodice, S.; Maisonneuve, P.; Botteri, E.; Sandri, M.T.; Lowenfels, A.B. ABO blood group and cancer. Eur. J. Cancer 2010, 46, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Chan, A.T.; Hartge, P.; Chanock, S.J.; Kraft, P.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 2009, 101, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Walker, A.M. Pancreatic cancer and ABO blood types: A study of cases and controls. Med. Clin. 1991, 96, 761–764. [Google Scholar]
- Hakomori, S. Antigen structure and genetic basis of histo-blood groups A, B and O: Their changes associated with human cancer. Biochim. Biophys. Acta 1999, 1473, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.L.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel Association of ABO Histo-Blood Group Antigen with Soluble ICAM-1: Results of a Genome-Wide Association Study of 6578 Women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef]
- Risch, H.A.; Yu, H.; Lu, L.; Kidd, M.S. ABO Blood Group, Helicobacter pylori Seropositivity, and Risk of Pancreatic Cancer: A Case–Control Study. J. Natl. Cancer Inst. 2010, 102, 502–505. [Google Scholar] [CrossRef]
- Lee, A.A.; Wang, Q.-L.; Kim, J.; Babic, A.; Zhang, X.; Perez, K.; Ng, K.; Nowak, J.; Rifai, N.; Sesso, H.D.; et al. Helicobacter pylori Seropositivity, ABO Blood Type, and Pancreatic Cancer Risk from 5 Prospective Cohorts. Clin. Transl. Gastroenterol. 2023, 14, e00573. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 2002, 8, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E.; ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef]
- Lucas, A.L.; Shakya, R.; Lipsyc, M.D.; Mitchel, E.B.; Kumar, S.; Hwang, C.; Deng, L.; Devoe, C.; Chabot, J.A.; Szabolcs, M.; et al. High Prevalence of BRCA1 and BRCA2 Germline Mutations with Loss of Heterozygosity in a Series of Resected Pancreatic Adenocarcinoma and Other Neoplastic Lesions. Clin. Cancer Res. 2013, 19, 3396–3403. [Google Scholar] [CrossRef]
- Tai, Y.C.; Domchek, S.; Parmigiani, G.; Chen, S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2007, 99, 1811–1814. [Google Scholar] [CrossRef]
- Brose, M.S.; Rebbeck, T.R.; Calzone, K.A.; Stopfer, J.E.; Nathanson, K.L.; Weber, B.L. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl. Cancer Inst. 2002, 94, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Easton, D.F.; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Hahn, S.A.; Greenhalf, B.; Ellis, I.; Sina-Frey, M.; Rieder, H.; Korte, B.; Gerdes, B.; Kress, R.; Ziegler, A.; Raeburn, J.A.; et al. BRCA2 germline mutations in familial pancreatic carcinoma. J. Natl. Cancer Inst. 2003, 95, 214–221. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef]
- Couch, F.J.; Johnson, M.R.; Rabe, K.; Boardman, L.; McWilliams, R.; de Andrade, M.; Petersen, G. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005, 65, 383–386. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Yeo, C.J.; Hruban, R.H.; Kern, S.E. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003, 63, 2585–2588. [Google Scholar]
- Kastrinos, F.; Stoffel, E.M. History, Genetics, and Strategies for Cancer Prevention in Lynch Syndrome. Clin. Gastroenterol. Hepatol. 2014, 12, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K. The genetics of hereditary colon cancer. Genes Dev. 2007, 21, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. The Risk of Pancreatic Cancer in Families with Lynch Syndrome. JAMA 2009, 302, 1790–1795. [Google Scholar] [CrossRef]
- Dunlop, M.G.; Farrington, S.M.; Carothers, A.D.; Wyllie, A.H.; Sharp, L.; Burn, J.; Liu, B.; Kinzler, K.W.; Vogelstein, B. Cancer Risk Associated with Germline DNA Mismatch Repair Gene Mutations. Hum. Mol. Genet. 1997, 6, 105–110. [Google Scholar] [CrossRef]
- Yamamoto, H.; Itoh, F.; Nakamura, H.; Fukushima, H.; Sasaki, S.; Perucho, M.; Imai, K. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001, 61, 3139–3144. [Google Scholar] [PubMed]
- Wilentz, R.E.; Goggins, M.; Redston, M.; Marcus, V.A.; Adsay, N.V.; Sohn, T.A.; Kadkol, S.S.; Yeo, C.J.; Choti, M.; Zahurak, M.; et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am. J. Pathol. 2000, 156, 1641–1651. [Google Scholar] [CrossRef]
- Nakata, B.; Wang, Y.Q.; Yashiro, M.; Nishioka, N.; Tanaka, H.; Ohira, M.; Ishikawa, T.; Nishino, H.; Hirakawa, K. Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin. Cancer Res. 2002, 8, 2536–2540. [Google Scholar]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Galiatsatos, P.; Foulkes, W.D. Familial adenomatous polyposis. Am. J. Gastroenterol. 2006, 101, 385–398. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Offerhaus, G.J.; Lee, D.H.; Krush, A.J.; Tersmette, A.C.; Booker, S.V.; Kelley, N.C.; Hamilton, S.R. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993, 34, 1394–1396. [Google Scholar] [CrossRef]
- Karstensen, J.G.; Bülow, S.; Højen, H.; Jelsig, A.M.; Jespersen, N.; Andersen, K.K.; Wewer, M.D.; Burisch, J.; Pommergaard, H.C. Cancer in Patients with Familial Adenomatous Polyposis: A Nationwide Danish Cohort Study with Matched Controls. Gastroenterology 2023, 165, 573–581.e3. [Google Scholar] [CrossRef]
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Höglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.F.; Biermann, K.; Offerhaus, G.J.A.; Krak, N.; Looman, C.W.N.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: A large cohort study and implications for surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef] [PubMed]
- de Snoo, F.A.; Bishop, D.T.; Bergman, W.; van Leeuwen, I.; van der Drift, C.; van Nieuwpoort, F.A.; Out-Luiting, C.J.; Vasen, H.F.; ter Huurne, J.A.C.; Frants, R.R.; et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin. Cancer Res. 2008, 14, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Borg, A.; Sandberg, T.; Nilsson, K.; Johannsson, O.; Klinker, M.; Måsbäck, A.; Westerdahl, J.; Olsson, H.; Ingvar, C. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J. Natl. Cancer Inst. 2000, 92, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Fraser, M.C.; Struewing, J.P.; Hussussian, C.J.; Ranade, K.; Zametkin, D.P.; Fontaine, L.S.; Organic, S.M.; Dracopoli, N.C.; Clark, W.H. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N. Engl. J. Med. 1995, 333, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Fusaro, R.M.; Lynch, J.F.; Brand, R. Pancreatic cancer and the FAMMM syndrome. Fam. Cancer 2008, 7, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Chan, M.; Harland, M.; Hayward, N.K.; Demenais, F.; Bishop, D.T.; Azizi, E.; Bergman, W.; Bianchi-Scarra, G.; Bruno, W.; et al. Features associated with germline CDKN2A mutations: A GenoMEL study of melanoma-prone families from three continents. J. Med. Genet. 2007, 44, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Brand, R.E.; Hogg, D.; Deters, C.A.; Fusaro, R.M.; Lynch, J.F.; Liu, L.; Knezetic, J.; Lassam, N.J.; Goggins, M.; et al. Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: The familial atypical mole melanoma-pancreatic carcinoma syndrome. Cancer 2002, 94, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.F.; Florell, S.R.; Alexander, A.; DiSario, J.A.; Shami, P.J.; Leachman, S.A. Pancreatic carcinoma surveillance in patients with familial melanoma. Arch. Dermatol. 2003, 139, 1019–1025. [Google Scholar] [CrossRef]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies from Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef]
- Schneider, A.; Suman, A.; Rossi, L.; Barmada, M.M.; Beglinger, C.; Parvin, S.; Sattar, S.; Ali, L.; Khan, A.K.A.; Gyr, N.; et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology 2002, 123, 1026–1030. [Google Scholar] [CrossRef]
- LaRusch, J.; Whitcomb, D.C. Genetics of pancreatitis. Curr. Opin. Gastroenterol. 2011, 27, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Teich, N.; Rosendahl, J.; Tóth, M.; Mössner, J.; Sahin-Tóth, M. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum. Mutat. 2006, 27, 721–730. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahin-Tóth, M.; Tóth, M. Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem. Biophys. Res. Commun. 2000, 278, 286–289. [Google Scholar] [CrossRef]
- Sossenheimer, M.J.; Aston, C.E.; Preston, R.A.; Gates, L.K.; Ulrich, C.D.; Martin, S.P.; Zhang, Y.; Gorry, M.C.; Ehrlich, G.D.; Whitcomb, D.C. Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG). Am. J. Gastroenterol. 1997, 92, 1113–1116. [Google Scholar] [PubMed]
- Gukovsky, I.; Li, N.; Todoric, J.; Gukovskaya, A.; Karin, M. Inflammation, Autophagy, and Obesity: Common Features in the Pathogenesis of Pancreatitis and Pancreatic Cancer. Gastroenterology 2013, 144, 1199–1209.e4. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, A.B.; Maisonneuve, P.; DiMagno, E.P.; Elitsur, Y.; Gates, L.K.; Perrault, J.; Whitcomb, D.C. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl. Cancer Inst. 1997, 89, 442–446. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Whitcomb, D.C.; Lerch, M.M.; DiMagno, E.P. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 2001, 286, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kotsimbos, T. Respiratory Infection and Inflammation in Cystic Fibrosis: A Dynamic Interplay among the Host, Microbes, and Environment for the Ages. Int. J. Mol. Sci. 2023, 24, 4052. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Marshall, B.C.; Lowenfels, A.B. Risk of pancreatic cancer in patients with cystic fibrosis. Gut 2007, 56, 1327–1328. [Google Scholar] [CrossRef]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM mutations in hereditary pancreatic cancer patients. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Swift, M.; Reitnauer, P.J.; Morrell, D.; Chase, C.L. Breast and other cancers in families with ataxia-telangiectasia. N. Engl. J. Med. 1987, 316, 1289–1294. [Google Scholar] [CrossRef]
- Taylor, A.M.R.; Byrd, P.J. Molecular pathology of ataxia telangiectasia. J. Clin. Pathol. 2005, 58, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Salvia, R.; Crippa, S.; Warshaw, A.L.; Bassi, C.; Falconi, M.; Thayer, S.P.; Lauwers, G.Y.; Capelli, P.; Mino-Kenudson, M.; et al. Branch-Duct Intraductal Papillary Mucinous Neoplasms: Observations in 145 Patients Who Underwent Resection. Gastroenterology 2007, 133, 72–310. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Castillo, C.F.; Bassi, C.; Thayer, S.P.; Falconi, M.; Mantovani, W.; Pederzoli, P.; Warshaw, A.L. Main-Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2004, 239, 678–687. [Google Scholar] [CrossRef] [PubMed]
- van Huijgevoort, N.C.M.; del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef]
- Brugge, W.R. Role of endoscopic ultrasound in the diagnosis of cystic lesions of the pancreas. Pancreatology 2001, 1, 637–640. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Ren, S.; Guo, K.; Zhang, Y.; Lin, T.; Wang, Y.; Chen, X.; Wang, Z. Threshold of Main Pancreatic Duct Diameter in Identifying Malignant Intraductal Papillary Mucinous Neoplasm by Magnetic Resonance Imaging. Technol. Cancer Res. Treat. 2023, 22, 15330338231170942. [Google Scholar] [CrossRef]
- Ohno, E.; Hirooka, Y.; Kawashima, H.; Ishikawa, T.; Kanamori, A.; Ishikawa, H.; Sasaki, Y.; Nonogaki, K.; Hara, K.; Hashimoto, S.; et al. Natural history of pancreatic cystic lesions: A multicenter prospective observational study for evaluating the risk of pancreatic cancer. J. Gastroenterol. Hepatol. 2018, 33, 320–328. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamaue, H.; Maguchi, H.; Yamao, K.; Hirono, S.; Osanai, M.; Hijioka, S.; Hosoda, W.; Nakamura, Y.; Shinohara, T.; et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013, 42, 883–888. [Google Scholar] [CrossRef]
- Lafemina, J.; Katabi, N.; Klimstra, D.; Correa-Gallego, C.; Gaujoux, S.; Kingham, T.P.; Dematteo, R.P.; Fong, Y.; D’Angelica, M.I.; Jarnagin, W.R.; et al. Malignant progression in IPMN: A cohort analysis of patients initially selected for resection or observation. Ann. Surg. Oncol. 2013, 20, 440–447. [Google Scholar] [CrossRef]
- Tanaka, M. Intraductal Papillary Mucinous Neoplasm of the Pancreas as the Main Focus for Early Detection of Pancreatic Adenocarcinoma. Pancreas 2018, 47, 544. [Google Scholar] [CrossRef] [PubMed]
- Scherer, J.A.; Gebhard, R.; Firkins, S.A.; Shah, Z.K.; Urbina Andersson, I.K.; Barker, S.J.; Fiorillo, L.E.; Hollander, E.; Shaheen, N.; Koay, E.J.; et al. Lower Interobserver Reliability for Nondimensional Intracystic Features among Abdominal Radiologists for Characterizing Intraductal Papillary Mucinous Neoplasms Using Magnetic Resonance Imaging. Pancreas 2022, 51, 1225–1230. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Gao, F.; Chen, Q.; Linghu, E. Diagnostic value of EUS-guided SF6 pancreatography for pancreatic cystic lesions on cyst communication with the pancreatic duct. Endosc. Ultrasound 2023, 12, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Spektor, A.-M.; Hielscher, T.; Hoppner, J.; Glatting, F.M.; Bicu, F.; Hackert, T.; Heger, U.; Pausch, T.; Gutjahr, E.; et al. Static and Dynamic 68Ga-FAPI PET/CT for the Detection of Malignant Transformation of Intraductal Papillary Mucinous Neoplasia of the Pancreas. J. Nucl. Med. 2023, 64, 244–251. [Google Scholar] [CrossRef]
- Levink, I.; Bruno, M.; Cahen, D. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr. Treat. Options Gastroenterol. 2018, 16, 316–332. [Google Scholar] [CrossRef]
- Gentiluomo, M.; Corradi, C.; Arcidiacono, P.G.; Crippa, S.; Falconi, M.; Belfiori, G.; Farinella, R.; Apadula, L.; Lauri, G.; Bina, N.; et al. Role of pancreatic ductal adenocarcinoma risk factors in intraductal papillary mucinous neoplasm progression. Front. Oncol. 2023, 13, 1172606. [Google Scholar] [CrossRef] [PubMed]
- Sofi, A.A.; Ahmad, S.; Peerzada, M.; Hackett, L. Diabetes mellitus and the risk of progression or malignancy of pancreatic cystic neoplasms in patients undergoing surveillance: A systematic review and meta-analysis. Pancreatology 2022, 22, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Schweber, A.B.; Brooks, C.; Agarunov, E.; Sethi, A.; Poneros, J.M.; Schrope, B.A.; Kluger, M.D.; Chabot, J.A.; Gonda, T.A. New onset diabetes predicts progression of low risk pancreatic mucinous cysts. Pancreatology 2020, 20, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Boccia, S.; Salvia, R.; Del Chiaro, M.; Frulloni, L.; Arcidiacono, P.G.; Zerbi, A.; Manta, R.; Fabbri, C.; Ventrucci, M.; et al. Risk Factors for Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas: A Multicentre Case–Control Study. Am. J. Gastroenterol. 2013, 108, 1003. [Google Scholar] [CrossRef]
- Huang, X.; Guo, T.; Zhang, Z.; Cai, M.; Guo, X.; Zhang, J.; Yu, Y. Prediction of malignant intraductal papillary mucinous neoplasm: A nomogram based on clinical information and radiological outcomes. Cancer Med. 2023, 12, 16958–16971. [Google Scholar] [CrossRef]
- Gausman, V.; Kandel, P.; Van Riet, P.A.; Moris, M.; Kayal, M.; Do, C.; Poneros, J.M.; Sethi, A.; Gress, F.G.; Schrope, B.A.; et al. Predictors of Progression among Low-Risk Intraductal Papillary Mucinous Neoplasms in a Multicenter Surveillance Cohort. Pancreas 2018, 47, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.T.; Saadat, L.V.; Chou, J.F.; Gönen, M.; Balachandran, V.P.; D’Angelica, M.I.; Drebin, J.A.; Flood, J.; Jarnagin, W.R.; Kingham, T.P.; et al. Risk Factors for Progression in Patients Undergoing Surveillance for Pancreatic Cysts. Ann. Surg. 2024, 279, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Levink, I.J.M.; Jaarsma, S.C.; Koopmann, B.D.M.; van Riet, P.A.; Overbeek, K.A.; Meziani, J.; Sprij, M.L.J.A.; Casadei, R.; Ingaldi, C.; Polkowski, M.; et al. The additive value of CA19.9 monitoring in a pancreatic cyst surveillance program. United Eur. Gastroenterol. J. 2023, 11, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Pezzilli, R.; Bissolati, M.; Capurso, G.; Romano, L.; Brunori, M.P.; Calculli, L.; Tamburrino, D.; Piccioli, A.; Ruffo, G.; et al. Active Surveillance Beyond 5 Years Is Required for Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms Undergoing Non-Operative Management. Am. J. Gastroenterol. 2017, 112, 1153–1161. [Google Scholar] [CrossRef]
- Pergolini, I.; Sahora, K.; Ferrone, C.R.; Morales-Oyarvide, V.; Wolpin, B.M.; Mucci, L.A.; Brugge, W.R.; Mino-Kenudson, M.; Patino, M.; Sahani, D.V.; et al. Long-term Risk of Pancreatic Malignancy in Patients with Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017, 153, 1284–1294.e1. [Google Scholar] [CrossRef]
- Marinelli, V.; Secchettin, E.; Andrianello, S.; Moretti, C.; Donvito, S.; Marchegiani, G.; Esposito, A.; Casetti, L.; Salvia, R. Psychological distress in patients under surveillance for intraductal papillary mucinous neoplasms of the pancreas: The “Sword of Damocles” effect calls for an integrated medical and psychological approach a prospective analysis. Pancreatology 2020, 20, 505–510. [Google Scholar] [CrossRef]
- Duell, E.J. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol. Carcinog. 2012, 51, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.W.; Zhang, D.Y.; Cameron, M.E.; Gerber, M.H.; Delitto, D.; Maduka, M.U.; Cooper, K.J.; Han, S.; Hughes, S.J.; Judge, S.M.; et al. Nicotine Induces IL-8 Secretion from Pancreatic Cancer Stroma and Worsens Cancer-Induced Cachexia. Cancers 2020, 12, 329. [Google Scholar] [CrossRef]
- Iodice, S.; Gandini, S.; Maisonneuve, P.; Lowenfels, A.B. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks Arch. Surg. 2008, 393, 535–545. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Zolotarevsky, E.; Cooper, K.L.; Sherman, S.; Shats, O.; Whitcomb, D.C.; Lynch, H.T.; Ghiorzo, P.; Rubinstein, W.S.; Vogel, K.J.; et al. Alcohol and tobacco lower the age of presentation in sporadic pancreatic cancer in a dose-dependent manner: A multicenter study. Am. J. Gastroenterol. 2012, 107, 1730–1739. [Google Scholar] [CrossRef]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F. Similarities and Differences between Sexes and Countries in the Mortality Imprint of the Smoking Epidemic in 34 Low-Mortality Countries, 1950–2014. Nicotine Tob. Res. 2019, 22, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F.; El Gewily, S.; Bardoutsos, A. Smoking epidemic in Europe in the 21st century. Tob. Control 2021, 30, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tyczynski, J.E.; Bray, F.; Aareleid, T.; Dalmas, M.; Kurtinaitis, J.; Plesko, I.; Pompe-Kirn, V.; Stengrevics, A.; Parkin, D.M. Lung cancer mortality patterns in selected Central, Eastern and Southern European countries. Int. J. Cancer 2004, 109, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Giovannucci, E.; Fuchs, C.S.; Michaud, D.S. Passive Smoking and Pancreatic Cancer in Women: A Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wellenius, G.A.; Michaud, D.S. Environmental tobacco smoke and the risk of pancreatic cancer among non-smokers: A meta-analysis. Occup. Environ. Med. 2012, 69, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Xu, G.; Lu, J.; Snetselaar, L.G.; Wallace, R.B. Changes in Electronic Cigarette Use among Adults in the United States, 2014–2016. JAMA 2018, 319, 2039–2041. [Google Scholar] [CrossRef] [PubMed]
- McMillen, R.C.; Gottlieb, M.A.; Shaefer, R.M.W.; Winickoff, J.P.; Klein, J.D. Trends in Electronic Cigarette Use among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob. Res. 2015, 17, 1195–1202. [Google Scholar] [CrossRef]
- Soneji, S.S.; Sung, H.-Y.; Primack, B.A.; Pierce, J.P.; Sargent, J.D. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS ONE 2018, 13, e0193328. [Google Scholar] [CrossRef] [PubMed]
- Stoklosa, M.; Cahn, Z.; Liber, A.; Nargis, N.; Drope, J. Effect of IQOS introduction on cigarette sales: Evidence of decline and replacement. Tob. Control 2020, 29, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Rosaria Galanti, M.; Lundberg, M.; Lager, A.; Engström, G.; Alfredsson, L.; Knutsson, A.; Norberg, M.; Sund, M.; Wennberg, P.; et al. Use of moist oral snuff (snus) and pancreatic cancer: Pooled analysis of nine prospective observational studies. Int. J. Cancer 2017, 141, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Thompson, K.; Weaver, S.; Thompson, J.; O’Connell, G. Snus: A compelling harm reduction alternative to cigarettes. Harm Reduct. J. 2019, 16, 62. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M.L. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Stephens, W.E. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob. Control 2018, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Schober, W.; Szendrei, K.; Matzen, W.; Osiander-Fuchs, H.; Heitmann, D.; Schettgen, T.; Jörres, R.A.; Fromme, H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 2014, 217, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.; Jaccard, G.; Tafin Djoko, D.; Korneliou, A.; Esposito, M.; Belushkin, M. Cancer potencies and margin of exposure used for comparative risk assessment of heated tobacco products and electronic cigarettes aerosols with cigarette smoke. Arch. Toxicol. 2021, 95, 283–298. [Google Scholar] [CrossRef]
- Schaller, J.-P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81, S27–S47. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Jiang, X.; Zhang, H.; Zhu, F.; Hu, S.; Hou, H.; Hu, Q.; Pang, Y. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine Tob. Res. 2019, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 14 April 2023).
- Wang, F.; Herrington, M.; Larsson, J.; Permert, J. The relationship between diabetes and pancreatic cancer. Mol. Cancer 2003, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and Clinical Profile of Pancreatic Cancer-associated Diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Klee, G.G.; Miller, L.J.; Raimondo, M.; DiMagno, E.P. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology 2001, 121, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M. Pancreatic Cancer-associated Diabetes Mellitus: Prevalence and Temporal Association with Diagnosis of Cancer. Gastroenterology 2008, 134, 95–101. [Google Scholar] [CrossRef]
- Cui, Y.; Andersen, D.K. Diabetes and pancreatic cancer. Endocr. Relat. Cancer 2012, 19, F9–F26. [Google Scholar] [CrossRef] [PubMed]
- White, M.J.; Sheka, A.C.; LaRocca, C.J.; Irey, R.L.; Ma, S.; Wirth, K.M.; Benner, A.; Denbo, J.W.; Jensen, E.H.; Ankeny, J.S.; et al. The association of new-onset diabetes with subsequent diagnosis of pancreatic cancer—Novel use of a large administrative database. J. Public Health 2022, 45, e266–e274. [Google Scholar] [CrossRef]
- Dankner, R.; Boffetta, P.; Balicer, R.D.; Boker, L.K.; Sadeh, M.; Berlin, A.; Olmer, L.; Goldfracht, M.; Freedman, L.S. Time-Dependent Risk of Cancer after a Diabetes Diagnosis in a Cohort of 2.3 Million Adults. Am. J. Epidemiol. 2016, 183, 1098–1106. [Google Scholar] [CrossRef]
- Li, D. Diabetes and pancreatic cancer. Mol. Carcinog. 2012, 51, 64–74. [Google Scholar] [CrossRef]
- Li, D.; Tang, H.; Hassan, M.M.; Holly, E.A.; Bracci, P.M.; Silverman, D.T. Diabetes and risk of pancreatic cancer: A pooled analysis of three large case-control studies. Cancer Causes Control 2011, 22, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients with New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Patalon, T.; Webb, M.; Margalit, O.; Beller, T.; Yang, Y.-X.; Chodick, G. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model: A Retrospective Cohort Study Using Real-World Data. Pancreas 2022, 51, 196–199. [Google Scholar] [CrossRef]
- Chen, W.; Butler, R.K.; Lustigova, E.; Chari, S.T.; Wu, B.U. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model in a Diverse and Integrated Healthcare Setting. Dig. Dis. Sci. 2021, 66, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Safarudin, R.F.; Kupec, J.T. Validation of the ENDPAC model: Identifying new-onset diabetics at risk of pancreatic cancer. Pancreatology 2021, 21, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Mellenthin, C.; Balaban, V.D.; Dugic, A.; Cullati, S. Risk Factors for Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4684. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gu, Y.; Hou, X.; Zhang, L.; Bao, Y.; Hu, C.; Jia, W. Association between uric acid, cancer incidence and mortality in patients with type 2 diabetes: Shanghai diabetes registry study. Diabetes/Metab. Res. Rev. 2016, 32, 325–332. [Google Scholar] [CrossRef]
- Batty, G.D.; Shipley, M.J.; Marmot, M.; Smith, G.D. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: Findings from the original Whitehall study. Cancer Causes Control 2004, 15, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Egger, M.; Shipley, M.J.; Marmot, M.G. Post-challenge glucose concentration, impaired glucose tolerance, diabetes, and cancer mortality in men. Am. J. Epidemiol. 1992, 136, 1110–1114. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Gann, P.H.; Lowe, W.; Liu, K.; Colangelo, L.; Dyer, A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000, 283, 2552–2558. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Li, D.; Silverman, D.; Petersen, G.M.; Bracci, P.M.; Neale, R.E.; Muscat, J.; Anderson, K.; Gallinger, S.; et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2014, 25, 2065–2072. [Google Scholar] [CrossRef]
- Ding, X.Z.; Fehsenfeld, D.M.; Murphy, L.O.; Permert, J.; Adrian, T.E. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas 2000, 21, 310–320. [Google Scholar] [CrossRef]
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parikh, A.A.; Bucana, C.D.; Evans, D.B.; Semenza, G.L.; Ellis, L.M. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am. J. Pathol. 2003, 163, 1001–1011. [Google Scholar] [CrossRef]
- Zeng, H.; Datta, K.; Neid, M.; Li, J.; Parangi, S.; Mukhopadhyay, D. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem. Biophys. Res. Commun. 2003, 302, 46–55. [Google Scholar] [CrossRef]
- Tao, L.V.; Topham, J.T.; Karasinska, J.M.; Tsang, E.S.; Metcalfe, A.; Ali, H.; Ashforth, D.; Goodwin, R.; Tang, P.A.; Bathe, O.F.; et al. Abstract B066: Elucidating the role of insulin receptor isoform expression in metastatic pancreatic ductal adenocarcinoma. Cancer Res. 2022, 82, B066. [Google Scholar] [CrossRef]
- Schneider, M.B.; Matsuzaki, H.; Haorah, J.; Ulrich, A.; Standop, J.; Ding, X.Z.; Adrian, T.E.; Pour, P.M. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001, 120, 1263–1270. [Google Scholar] [CrossRef]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef]
- Li, D.; Yeung, S.-C.J.; Hassan, M.M.; Konopleva, M.; Abbruzzese, J.L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009, 137, 482–488. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar] [CrossRef]
- Dowling, R.J.O.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, B.; Read, S.H.; Friis, S.; Sund, R.; Keskimäki, I.; Svensson, A.-M.; Ljung, R.; Wild, S.H.; Kerssens, J.J.; Harding, J.L.; et al. Cancer incidence in persons with type 1 diabetes: A five-country study of 9000 cancers in type 1 diabetic individuals. Diabetologia 2016, 59, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Roddam, A.W.; Beral, V. Pancreatic cancer in type 1 and young-onset diabetes: Systematic review and meta-analysis. Br. J. Cancer 2007, 96, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Wideroff, L.; Gridley, G.; Mellemkjaer, L.; Chow, W.H.; Linet, M.; Keehn, S.; Borch-Johnsen, K.; Olsen, J.H. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J. Natl. Cancer Inst. 1997, 89, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 April 2023).
- Larsson, S.C.; Orsini, N.; Wolk, A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int. J. Cancer 2007, 120, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Stocks, T.; Rapp, K.; Bjørge, T.; Manjer, J.; Ulmer, H.; Selmer, R.; Lukanova, A.; Johansen, D.; Concin, H.; Tretli, S.; et al. Blood Glucose and Risk of Incident and Fatal Cancer in the Metabolic Syndrome and Cancer Project (Me-Can): Analysis of Six Prospective Cohorts. PLoS Med. 2009, 6, e1000201. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Swanson, C.A.; Gridley, G.; Wacholder, S.; Greenberg, R.S.; Brown, L.M.; Hayes, R.B.; Swanson, G.M.; Schoenberg, J.B.; Pottern, L.M.; et al. Dietary and nutritional factors and pancreatic cancer: A case-control study based on direct interviews. J. Natl. Cancer Inst. 1998, 90, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef]
- Abate, N. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care 1996, 19, 292–294. [Google Scholar] [CrossRef]
- Butler, A.E.; Galasso, R.; Matveyenko, A.; Rizza, R.A.; Dry, S.; Butler, P.C. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia 2010, 53, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Adams, K.; Leitzmann, M.; Schairer, C.; Michaud, D.S.; Hollenbeck, A.; Schatzkin, A.; Silverman, D.T. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am. J. Epidemiol. 2008, 167, 586–597. [Google Scholar] [CrossRef]
- Arslan, A.A.; Helzlsouer, K.J.; Kooperberg, C.; Shu, X.-O.; Steplowski, E.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; Gross, M.D.; Jacobs, E.J.; LaCroix, A.Z.; et al. Anthropometric Measures, Body Mass Index and Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 2010, 170, 791–802. [Google Scholar] [CrossRef]
- Sreedhar, U.L.; DeSouza, S.V.; Park, B.; Petrov, M.S. A Systematic Review of Intra-pancreatic Fat Deposition and Pancreatic Carcinogenesis. J. Gastrointest. Surg. 2020, 24, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Seino, T.; Fukuhara, S.; Minami, K.; Horibe, M.; Iwasaki, E.; Takaishi, H.; Itoh, K.; Sugino, Y.; Inoue, N.; et al. Pancreatic Fat Content Detected by Computed Tomography and Its Significant Relationship with Intraductal Papillary Mucinous Neoplasm. Pancreas 2018, 47, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.; Seeman, T.E.; Stachenfeld, N.S.; Katz, L.D.; Nadel, E.R. Moderate-Intensity Aerobic Training Improves Glucose Tolerance in Aging Independent of Abdominal Adiposity. J. Am. Geriatr. Soc. 1998, 46, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z. Obesity-associated cancer risk: The role of intestinal microbiota in the etiology of the host proinflammatory state. Transl. Res. 2017, 179, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Prospective Study of Diet and Pancreatic Cancer in Male Smokers. Am. J. Epidemiol. 2002, 155, 783–792. [Google Scholar] [CrossRef]
- Casari, I.; Falasca, M. Diet and Pancreatic Cancer Prevention. Cancers 2015, 7, 2309–2317. [Google Scholar] [CrossRef]
- Garcia, C.C.; Lawres, L.; Agabiti, S.; Singh, J.; Tong, A.; Venkat, A.; Burkhardt, D.B.; Cardone, R.; Kibbey, R.G.; Krishnaswamy, S.; et al. Abstract C050: Elucidating mechanisms of endocrine-exocrine signaling in obesity-driven pancreatic cancer. Cancer Res. 2022, 82, C050. [Google Scholar] [CrossRef]
- Jarosz, M.; Sekuła, W.; Rychlik, E. Influence of Diet and Tobacco Smoking on Pancreatic Cancer Incidence in Poland in 1960–2008. Gastroenterol. Res. Pract. 2012, 2012, 682156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paluszkiewicz, P.; Smolińska, K.; Dębińska, I.; Turski, W.A. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. 2012, 36, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Lugo, A.; Lagiou, P.; Zucchetto, A.; Polesel, J.; Serraino, D.; Negri, E.; Trichopoulos, D.; Vecchia, C.L. Proanthocyanidins and other flavonoids in relation to pancreatic cancer: A case–control study in Italy. Ann. Oncol. 2012, 23, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Heinen, M.M.; Verhage, B.A.J.; Goldbohm, R.A.; van den Brandt, P.A. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int. J. Cancer 2012, 130, 147–158. [Google Scholar] [CrossRef]
- Falasca, M.; Casari, I.; Maffucci, T. Cancer chemoprevention with nuts. J. Natl. Cancer Inst. 2014, 106, dju238. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S. Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. 1), A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef]
- Jeurnink, S.M.; Ros, M.M.; Leenders, M.; van Duijnhoven, F.J.B.; Siersema, P.D.; Jansen, E.H.J.M.; van Gils, C.H.; Bakker, M.F.; Overvad, K.; Roswall, N.; et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: A nested case-control study: Plasma micronutrients and pancreatic cancer risk. Int. J. Cancer 2015, 136, E665–E676. [Google Scholar] [CrossRef]
- Sahu, R.P.; Zhang, R.; Batra, S.; Shi, Y.; Srivastava, S.K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis 2009, 30, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Haldar, S. Anti-proliferative and proapoptotic effects of benzyl isothiocyanate on human pancreatic cancer cells is linked to death receptor activation and RasGAP/Rac1 down-modulation. Int. J. Oncol. 2009, 35, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.G.; Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Vieth, R.; Azad, A.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. A Prospective Nested Case-Control Study of Vitamin D Status and Pancreatic Cancer Risk in Male Smokers. Cancer Res. 2006, 66, 10213–10219. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Calvo, M.S. Dietary recommendations to meet both endocrine and autocrine needs of Vitamin D. J. Steroid Biochem. Mol. Biol. 2005, 97, 7–12. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Eads, D.; Rao, A.; Cramer, S.D.; Willingham, M.C.; Chen, T.C.; Jamieson, D.P.; Wang, L.; Burnstein, K.L.; Holick, M.F.; et al. Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis 2004, 25, 1015–1026. [Google Scholar] [CrossRef]
- Pettersson, F.; Colston, K.W.; Dalgleish, A.G. Differential and antagonistic effects of 9-cis-retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br. J. Cancer 2000, 83, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Yoshizawa, K.; Tokoo, M.; Imai, H.; Oguchi, H.; Kiyosawa, K.; Homma, T.; Nikaido, T.; Furihata, K. Inhibitory effect of 220-oxa-1,25-dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology 1996, 110, 1605–1613. [Google Scholar] [CrossRef]
- Maestro, B.; Dávila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gukovsky, I.; Algül, H.; Habtezion, A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 2017, 153, 1212–1226. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Yu, Y. Meta-analysis reveals an association between acute pancreatitis and the risk of pancreatic cancer. World J. Clin. Cases 2020, 8, 4416–4430. [Google Scholar] [CrossRef]
- Duell, E.J.; Lucenteforte, E.; Olson, S.H.; Bracci, P.M.; Li, D.; Risch, H.A.; Silverman, D.T.; Ji, B.T.; Gallinger, S.; Holly, E.A.; et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2964–2970. [Google Scholar] [CrossRef]
- Ahmed Ali, U.; Issa, Y.; Hagenaars, J.C.; Bakker, O.J.; van Goor, H.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; Brink, M.A.; et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis after a First Episode of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. 2016, 14, 738–746. [Google Scholar] [CrossRef]
- Whitcomb, D.C. Value of genetic testing in the management of pancreatitis. Gut 2004, 53, 1710–1717. [Google Scholar] [CrossRef]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best. Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Chen, Q.; Yu, W.; Dong, E.Y.; Chung, J.; Pandol, S.J.; Yadav, D.; Conwell, D.L.; Wu, B.U. Identification of Individuals at Increased Risk for Pancreatic Cancer in a Community-Based Cohort of Patients with Suspected Chronic Pancreatitis. Clin. Transl. Gastroenterol. 2020, 11, e00147. [Google Scholar] [CrossRef]
- Kong, X.; Sun, T.; Kong, F.; Du, Y.; Li, Z. Chronic Pancreatitis and Pancreatic Cancer. Gastrointest. Tumors 2014, 1, 123–134. [Google Scholar] [CrossRef]
- Gandhi, S.; de la Fuente, J.; Murad, M.H.; Majumder, S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases with Duration of Disease: A Systematic Review and Meta-analysis. Clin. Transl. Gastroenterol. 2022, 13, e00463. [Google Scholar] [CrossRef]
- Munigala, S.; Kanwal, F.; Xian, H.; Agarwal, B. New diagnosis of chronic pancreatitis: Risk of missing an underlying pancreatic cancer. Am. J. Gastroenterol. 2014, 109, 1824–1830. [Google Scholar] [CrossRef]
- Ma, D.-M.; Dong, X.-W.; Han, X.; Ling, Z.; Lu, G.-T.; Sun, Y.-Y.; Yin, X.-D. Pancreatitis and Pancreatic Cancer Risk. Technol. Cancer Res. Treat. 2023, 22, 15330338231164875. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Blaho, M.; Dítě, P.; Kunovský, L.; Kianička, B. Autoimmune pancreatitis—An ongoing challenge. Adv. Med. Sci. 2020, 65, 403–408. [Google Scholar] [CrossRef]
- Gupta, R.; Khosroshahi, A.; Shinagare, S.; Fernandez, C.; Ferrone, C.; Lauwers, G.Y.; Stone, J.H.; Deshpande, V. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma?: A retrospective analysis of pancreatic resections. Pancreas 2013, 42, 506–510. [Google Scholar] [CrossRef]
- Ikeura, T.; Miyoshi, H.; Uchida, K.; Fukui, T.; Shimatani, M.; Fukui, Y.; Sumimoto, K.; Matsushita, M.; Takaoka, M.; Okazaki, K. Relationship between autoimmune pancreatitis and pancreatic cancer: A single-center experience. Pancreatology 2014, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Kamisawa, T.; Brugge, W.R.; Chung, J.B.; Culver, E.L.; Czakó, L.; Frulloni, L.; Go, V.L.W.; Gress, T.M.; Kim, M.-H.; et al. Long-term outcomes of autoimmune pancreatitis: A multicentre, international analysis. Gut 2013, 62, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, H.; Tabeya, T.; Suzuki, C.; Naishiro, Y.; Ishigami, K.; Yajima, H.; Shimizu, Y.; Obara, M.; Yamamoto, H.; et al. Risk of malignancies in IgG4-related disease. Mod. Rheumatol. 2012, 22, 414–418. [Google Scholar] [CrossRef]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Woodmansey, C.; McGovern, A.P.; McCullough, K.A.; Whyte, M.B.; Munro, N.M.; Correa, A.C.; Gatenby, P.A.C.; Jones, S.A.; de Lusignan, S. Incidence, Demographics, and Clinical Characteristics of Diabetes of the Exocrine Pancreas (Type 3c): A Retrospective Cohort Study. Diabetes Care 2017, 40, 1486–1493. [Google Scholar] [CrossRef]
- Cho, J.; Scragg, R.; Petrov, M.S. Postpancreatitis Diabetes Confers Higher Risk for Pancreatic Cancer Than Type 2 Diabetes: Results from a Nationwide Cancer Registry. Diabetes Care 2020, 43, 2106–2112. [Google Scholar] [CrossRef]
- Tramacere, I.; Scotti, L.; Jenab, M.; Bagnardi, V.; Bellocco, R.; Rota, M.; Corrao, G.; Bravi, F.; Boffetta, P.; La Vecchia, C. Alcohol drinking and pancreatic cancer risk: A meta-analysis of the dose-risk relation. Int. J. Cancer 2010, 126, 1474–1486. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Gou, Y.-W.; Jin, W.-W.; Xiao, M.; Fang, H.-Y. Association between alcohol intake and the risk of pancreatic cancer: A dose–response meta-analysis of cohort studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Jacobs, E.J.; Deka, A.; McCullough, M.L.; Patel, A.V.; Thun, M.J. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch. Intern. Med. 2011, 171, 444–451. [Google Scholar] [CrossRef]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.C.; Rebours, V.; Védié, A.L.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2018, 143, 801–812. [Google Scholar] [CrossRef]
- Shan, Y.-S.; Chen, L.-T.; Wu, C.-H.; Chang, Y.-F.; Lee, C.-T.; Chiang, N.-J.; Chao, Y.-J.; Yen, C.-J.; Tsai, H.-J.; Huang, H.-E.; et al. No association between alcohol consumption and pancreatic cancer even among individuals genetically susceptible to the carcinogenicity of alcohol. Sci. Rep. 2021, 11, 14567. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, H.; English, D.R.; Hodge, A.M.; Room, R.; Hopper, J.L.; Milne, R.L.; Giles, G.G.; MacInnis, R.J. Lifetime alcohol intake and pancreatic cancer incidence and survival: Findings from the Melbourne Collaborative Cohort Study. Cancer Causes Control 2019, 30, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ma, Y.; Shankar, S.; Srivastava, R.K. Chronic ethanol exposure of human pancreatic normal ductal epithelial cells induces cancer stem cell phenotype through SATB2. J. Cell. Mol. Med. 2018, 22, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
- Serio, R.N.; Gudas, L.J. Modification of stem cell states by alcohol and acetaldehyde. Chem. Biol. Interact. 2020, 316, 108919. [Google Scholar] [CrossRef]
- Li, T.-D.; Yang, H.-W.; Wang, P.; Song, C.-H.; Wang, K.-J.; Dai, L.-P.; Shi, J.-X.; Zhang, J.-Y.; Ye, H. Coffee consumption and risk of pancreatic cancer: A systematic review and dose–response meta-analysis. Int. J. Food Sci. Nutr. 2019, 70, 519–529. [Google Scholar] [CrossRef]
- Nie, K.; Xing, Z.; Huang, W.; Wang, W.; Liu, W. Coffee intake and risk of pancreatic cancer: An updated meta-analysis of prospective studies. Minerva Med. 2016, 107, 270–278. [Google Scholar]
- Bae, J.-M.; Shim, S.R. Coffee Consumption and Pancreatic Cancer Risk: A Meta-Epidemiological Study of Population-based Cohort Studies. Asian Pac. J. Cancer Prev. 2020, 21, 2793–2798. [Google Scholar] [CrossRef]
- Turati, F.; Galeone, C.; Edefonti, V.; Ferraroni, M.; Lagiou, P.; La Vecchia, C.; Tavani, A. A meta-analysis of coffee consumption and pancreatic cancer. Ann. Oncol. 2012, 23, 311–318. [Google Scholar] [CrossRef]
- Zhou, C.D.; Kuan, A.S.; Reeves, G.K.; Green, J.; Floud, S.; Beral, V.; Yang, T.O.; Million Women Study Collaborators. Coffee and pancreatic cancer risk among never-smokers in the UK prospective Million Women Study. Int. J. Cancer 2019, 145, 1484–1492. [Google Scholar] [CrossRef]
- Ran, H.-Q.; Wang, J.-Z.; Sun, C.-Q. Coffee Consumption and Pancreatic Cancer Risk: An Update Meta-analysis of Cohort Studies. Pak. J. Med. Sci. 2016, 32, 253–259. [Google Scholar] [CrossRef]
- Lukic, M.; Nilsson, L.M.; Skeie, G.; Lindahl, B.; Braaten, T. Coffee consumption and risk of rare cancers in Scandinavian countries. Eur. J. Epidemiol. 2018, 33, 287–302. [Google Scholar] [CrossRef]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-156545-5. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Charlotte, F.; Goodman, Z.; McHutchison, J.; Albrecht, J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J. Hepatol. 2001, 34, 730–739. [Google Scholar] [CrossRef]
- Dandri, M.; Locarnini, S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012, 61 (Suppl. 1), i6–i17. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sakurai, I.; Shimoda, T.; Abe, K.; Okano, T.; Shikata, T. Detection of HBsAg in the pancreas. Acta Pathol. Jpn. 1981, 31, 711–717. [Google Scholar] [CrossRef]
- Montalbano, M.; Neff, G.W. Management of recurrent viral hepatitis B and C after liver transplantation. Curr. Gastroenterol. Rep. 2006, 8, 60–66. [Google Scholar] [CrossRef]
- Huang, J.; Magnusson, M.; Törner, A.; Ye, W.; Duberg, A.-S. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: A nationwide study in Sweden. Br. J. Cancer 2013, 109, 2917–2923. [Google Scholar] [CrossRef]
- Hassan, M.M.; Li, D.; El-Deeb, A.S.; Wolff, R.A.; Bondy, M.L.; Davila, M.; Abbruzzese, J.L. Association between Hepatitis B Virus and Pancreatic Cancer. J. Clin. Oncol. 2008, 26, 4557–4562. [Google Scholar] [CrossRef]
- Xu, J.-H.; Fu, J.-J.; Wang, X.-L.; Zhu, J.-Y.; Ye, X.-H.; Chen, S.-D. Hepatitis B or C viral infection and risk of pancreatic cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013, 19, 4234–4241. [Google Scholar] [CrossRef]
- Parsa, I.; Longnecker, D.S.; Scarpelli, D.G.; Pour, P.; Reddy, J.K.; Lefkowitz, M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985, 45, 1285–1290. [Google Scholar]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol. 2016, 65, 146–181. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Feng, B.; Wang, W.; Yao, G.; Zhai, J.; Li, X. Increased Risk of Pancreatic Cancer Related to Gallstones and Cholecystectomy: A Systematic Review and Meta-Analysis. Pancreas 2016, 45, 503–509. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Joshi, A.D.; Wu, K.; Simon, T.G.; Yuan, C.; Jin, L.; Long, L.; Kim, M.N.; Lo, C.-H.; et al. Gallstones and risk of cancers of the liver, biliary tract and pancreas: A prospective study within two U.S. cohorts. Br. J. Cancer 2022, 127, 1069–1075. [Google Scholar] [CrossRef]
- Rosato, V.; Gómez-Rubio, P.; Molina-Montes, E.; Márquez, M.; Löhr, M.; O’Rorke, M.; Michalski, C.W.; Molero, X.; Farré, A.; Perea, J.; et al. Gallbladder disease and pancreatic cancer risk: A multicentric case-control European study. Eur. J. Cancer Prev. 2021, 30, 423–430. [Google Scholar] [CrossRef]
- Rosato, V.; Negri, E.; Bosetti, C.; Malats, N.; Gomez-Rubio, P.; Consortium, P.; Maisonneuve, P.; Miller, A.B.; Bueno-de-Mesquita, H.B.; Baghurst, P.A.; et al. Gallbladder disease, cholecystectomy, and pancreatic cancer risk in the International Pancreatic Cancer Case-Control Consortium (PanC4). Eur. J. Cancer Prev. 2020, 29, 408–415. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Martinussen, T.; Sørensen, L.T. Development of upper gastrointestinal cancer in patients with symptomatic gallstones, cholecystectomy, and sphincterotomy: A nationwide cohort study. Scand. J. Surg. 2022, 111, 39–47. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Sørensen, L.T.; Jørgensen, T. Association between Screen-Detected Gallstone Disease and Cancer in a Cohort Study. Gastroenterology 2017, 152, 1965–1974.e1. [Google Scholar] [CrossRef]
- Wang, C.-C.; Tseng, M.-H.; Wu, S.-W.; Yang, T.-W.; Chen, H.-Y.; Sung, W.-W.; Su, C.-C.; Wang, Y.-T.; Chen, W.-L.; Lai, H.-C.; et al. Symptomatic cholelithiasis patients have an increased risk of pancreatic cancer: A population-based study. J. Gastroenterol. Hepatol. 2021, 36, 1187–1196. [Google Scholar] [CrossRef]
- Yu, J.; Ploner, A.; Chen, M.S.; Zhang, J.; Sandborgh-Englund, G.; Ye, W. Poor dental health and risk of pancreatic cancer: A nationwide registry-based cohort study in Sweden, 2009–2016. Br. J. Cancer 2022, 127, 2133–2140. [Google Scholar] [CrossRef]
- Chang, J.S.; Tsai, C.-R.; Chen, L.-T.; Shan, Y.-S. Investigating the Association between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas 2016, 45, 134–141. [Google Scholar] [CrossRef]
- Michaud, D.S.; Joshipura, K.; Giovannucci, E.; Fuchs, C.S. A Prospective Study of Periodontal Disease and Pancreatic Cancer in US Male Health Professionals. JNCI J. Natl. Cancer Inst. 2007, 99, 171–175. [Google Scholar] [CrossRef]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and are Highly Subject Specific but Differ between Pancreatic Cancer and Non-Cancer Subjects. Cancer Epidemiol. Biomark. Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Huang, J.; Zagai, U.; Hallmans, G.; Nyrén, O.; Engstrand, L.; Stolzenberg-Solomon, R.; Duell, E.J.; Overvad, K.; Katzke, V.A.; Kaaks, R.; et al. Helicobacter pylori infection, chronic corpus atrophic gastritis and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort: A nested case-control study. Int. J. Cancer 2017, 140, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Bulajic, M.; Panic, N.; Löhr, J.M. Helicobacter pylori and pancreatic diseases. World J. Gastrointest. Pathophysiol. 2014, 5, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Cullin, N.; Antunes, C.A.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rubio, P.; Piñero, J.; Molina-Montes, E.; Gutiérrez-Sacristán, A.; Marquez, M.; Rava, M.; Michalski, C.W.; Farré, A.; Molero, X.; Löhr, M.; et al. Pancreatic cancer and autoimmune diseases: An association sustained by computational and epidemiological case–control approaches. Int. J. Cancer 2019, 144, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Westermann, R.; Zobbe, K.; Cordtz, R.; Haugaard, J.H.; Dreyer, L. Increased cancer risk in patients with cutaneous lupus erythematosus and systemic lupus erythematosus compared with the general population: A Danish nationwide cohort study. Lupus 2021, 30, 752–761. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Wang, Y.; Bai, Y.; Gu, D. Association between Systemic Lupus Erythematosus and Cancer Morbidity and Mortality: Findings from Cohort Studies. Front. Oncol. 2022, 12, 860794. [Google Scholar] [CrossRef]
- Osman, M.A.; Alkhouly, M.; Elmohaseb, G.F.; Nassef, E.M.; Mohamed, I.G.R.; Mancy, I.M.E.; Sabry, S.; Abdulrehim, M.M.; Eliwa, A.; Eisa, Y.H.; et al. Relation between Non-Alcoholic Fatty Pancreas and Clinical and Biochemical Parameters in Women with Polycystic Ovary Syndrome: A Multi-Centric Study. Int. J. Gen. Med. 2022, 15, 8225–8233. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Falconer, H.; Yin, L.; Xu, L.; Ye, W. Association between Polycystic Ovary Syndrome and Cancer Risk. JAMA Oncol. 2019, 5, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Peeri, N.C.; Landicino, M.V.; Saldia, C.A.; Kurtz, R.C.; Rolston, V.S.; Du, M. Association between Polycystic Ovary Syndrome and Risk of Pancreatic Cancer. JAMA Oncol. 2022, 8, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.A.; Lidegaardi, Ø.; Skovlund, C.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Mørch, L.S. Hormonal contraceptive use and risk of pancreatic cancer—A cohort study among premenopausal women. PLoS ONE 2018, 13, e0206358. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lv, J.; Li, Y.; Yuan, S.; Wang, Z.; He, S. Relationship between female hormonal and menstrual factors and pancreatic cancer: A meta-analysis of observational studies. Medicine 2015, 94, e177. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Dossus, L.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Tumino, R.; Masala, G.; Krogh, V.; Panico, S.; et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2013, 132, 2164–2175. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fuhler, G.M.; BN, N.; Jose, T.; Bruno, M.J.; Peppelenbosch, M.P.; Konstantinov, S.R. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 2017, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jin, M.; Liu, Y.; Jin, L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 572492. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Sharif, R.; Dawra, R.; Wasiluk, K.; Phillips, P.; Dudeja, V.; Kurt-Jones, E.; Finberg, R.; Saluja, A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut 2009, 58, 813–819. [Google Scholar] [CrossRef]
- Yin, H.; Pu, N.; Chen, Q.; Zhang, J.; Zhao, G.; Xu, X.; Wang, D.; Kuang, T.; Jin, D.; Lou, W.; et al. Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-κB pathway in pancreatic cancer. Cell Death Dis. 2021, 12, 1033. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Wang, Q.; Zheng, D.; Jiang, X.; Xu, L. LPS induced miR-181a promotes pancreatic cancer cell migration via targeting PTEN and MAP2K4. Dig. Dis. Sci. 2014, 59, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Andrikou, K.; Sotte, V.; Bittoni, A.; Lanese, A.; Pellei, C.; Piva, F.; Conti, A.; Nabissi, M.; Santoni, G.; et al. Toll like receptors and pancreatic diseases: From a pathogenetic mechanism to a therapeutic target. Cancer Treat. Rev. 2015, 41, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The fecal microbiota of patients with pancreatic ductal adenocarcinoma and autoimmune pancreatitis characterized by metagenomic sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Sammallahti, H.; Sarhadi, V.K.; Kokkola, A.; Ghanbari, R.; Rezasoltani, S.; Asadzadeh Aghdaei, H.; Puolakkainen, P.; Knuutila, S. Oncogenomic Changes in Pancreatic Cancer and Their Detection in Stool. Biomolecules 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Hank, T.; Qadan, M.; Ciprani, D.; Michelakos, T.; Niess, H.; Heiliger, C.; Ilmer, M.; D’Haese, J.G.; Ferrone, C.R.; et al. Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. Br. J. Surg. 2021, 108, 709–716. [Google Scholar] [CrossRef]
- Ross, K. Mapping Pathways from Stress to Cancer Progression. JNCI J. Natl. Cancer Inst. 2008, 100, 914–917. [Google Scholar] [CrossRef]
- Kennedy, B.; Valdimarsdóttir, U.; Sundström, K.; Sparén, P.; Lambe, M.; Fall, K.; Fang, F. Loss of a parent and the risk of cancer in early life: A nationwide cohort study. Cancer Causes Control 2014, 25, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Valdimarsdóttir, U.; Fall, K.; Ye, W.; Fang, F. Pancreatic Cancer Risk after Loss of a Child: A Register-based Study in Sweden During 1991–2009. Am. J. Epidemiol. 2013, 178, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Weddle, D.L.; Tithoff, P.; Williams, M.; Schuller, H.M. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 2001, 22, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Luo, K.; Lv, Z.; Huang, J. Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology 2012, 59, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 2009, 20, 477–482. [Google Scholar] [CrossRef]
- Beg, M.S.; Gupta, A.; Sher, D.; Ali, S.; Khan, S.; Gao, A.; Stewart, T.; Ahn, C.; Berry, J.; Mortensen, E.M. Impact of Concurrent Medication Use on Pancreatic Cancer Survival—SEER-Medicare Analysis. Am. J. Clin. Oncol. 2018, 41, 766. [Google Scholar] [CrossRef] [PubMed]
- Daemen, M.J.; Lombardi, D.M.; Bosman, F.T.; Schwartz, S.M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ. Res. 1991, 68, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Shimizu, M.; Sakai, H.; Yasuda, Y.; Ohno, T.; Kochi, T.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 2011, 410, 108–113. [Google Scholar] [CrossRef]
- Fendrich, V.; Chen, N.-M.; Neef, M.; Waldmann, J.; Buchholz, M.; Feldmann, G.; Slater, E.P.; Maitra, A.; Bartsch, D.K. The angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut 2010, 59, 630–637. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, J.; Park, R.W.; Shin, S.J.; Kim, J.; Sung, J.D.; Kim, D.J.; Yang, K. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Cancer: A Population-Based Cohort Study Using a Common Data Model. Diagnostics 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Zhou, J.; Lee, S.; Hung, J.K.F.; Leung, K.S.K.; Liu, Y.; Zhang, Y.; Liu, T.; Wong, W.T.; Wong, I.C.K.; et al. Incidence of pancreatic cancer in angiotensin-converting enzyme inhibitors (ACEIs) versus angiotensin receptor blockers (ARBs): A population-based cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- Mandilaras, V.; Bouganim, N.; Yin, H.; Asselah, J.; Azoulay, L. The use of drugs acting on the renin–angiotensin system and the incidence of pancreatic cancer. Br. J. Cancer 2017, 116, 103–108. [Google Scholar] [CrossRef]
- Liu, H.; D’Alesio, M.; Lebowitz, S.; Hammad, A.; Nassour, I.; Singhi, A.; Bahary, N.; Lee, K.; Zureikat, A.; Paniccia, A. Angiotensin system inhibitor use is associated with longer survival in resected pancreatic cancer. HPB 2021, 23, S466–S467. [Google Scholar] [CrossRef]
- Tseng, K.-Y.; Chou, C.-W. Abstract 6755: The impact of renin-angiotensin system inhibitors on survival outcomes of pancreatic adenocarcinoma patients. Cancer Res. 2023, 83, 6755. [Google Scholar] [CrossRef]
- Gandini, S.; Lowenfels, A.B.; Jaffee, E.M.; Armstrong, T.D.; Maisonneuve, P. Allergies and the risk of pancreatic cancer: A meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1908–1916. [Google Scholar] [CrossRef]
- Cotterchio, M.; Lowcock, E.; Hudson, T.J.; Greenwood, C.; Gallinger, S. Association between allergies and risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 469–480. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Z.; Zhu, J.; Ren, J.; Chen, M.; He, G.; Yu, B. Decreased Risk in the Pancreatic Cancer With History of Hay Fever: A Meta-Analysis. Front. Public Health 2020, 8, 551490. [Google Scholar] [CrossRef]
- Olson, S.H.; Hsu, M.; Satagopan, J.M.; Maisonneuve, P.; Silverman, D.T.; Lucenteforte, E.; Anderson, K.E.; Borgida, A.; Bracci, P.M.; Bueno-de-Mesquita, H.B.; et al. Allergies and Risk of Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Case-Control Consortium. Am. J. Epidemiol. 2013, 178, 691–700. [Google Scholar] [CrossRef]
- Olson, S.H. Selected medical conditions and risk of pancreatic cancer. Mol. Carcinog. 2012, 51, 75–97. [Google Scholar] [CrossRef]
- Santibañez, M.; O’Rorke, M.; O’Leary, E.; Cancela, M.D.C.; Murray, L.; Sharp, L.; on Behalf of the PanCAM Study Group. Allergies, asthma and the risk of pancreatic cancer: A population-based case-control study in Ireland. Eur. Respir. J. 2015, 46, PA3389. [Google Scholar] [CrossRef]
- Karim, A.F.; Westenberg, L.E.H.; Eurelings, L.E.M.; Otten, R.; Gerth van Wijk, R. The association between allergic diseases and cancer: A systematic review of the literature. Neth. J. Med. 2019, 77, 42–66. [Google Scholar] [PubMed]
- Huang, B.Z.; Le Marchand, L.; Haiman, C.A.; Monroe, K.R.; Wilkens, L.R.; Zhang, Z.-F.; Setiawan, V.W. Atopic allergic conditions and pancreatic cancer risk: Results from the Multiethnic Cohort Study. Int. J. Cancer 2018, 142, 2019–2027. [Google Scholar] [CrossRef]
- Tirado-Rodríguez, B.; Huerta-Yépez, S. Allergies: Diseases closely related to cancer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 432–445. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.L.; Pierre, J.; Smith-Norowitz, T.A.; Hagler, M.; Bowne, W.; Pincus, M.R.; Mueller, C.M.; Zenilman, M.E.; Bluth, M.H. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin. Exp. Immunol. 2008, 153, 401–409. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B.; Bueno-de-Mesquita, H.B.; Ghadirian, P.; Baghurst, P.A.; Zatonski, W.A.; Miller, A.B.; Duell, E.J.; Boffetta, P.; Boyle, P. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann. Epidemiol. 2010, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.A.; Moss, J.; Karp, D.D.; Atkins, J.T.; Janku, F. The mu opioid receptor: A new target for cancer therapy? Cancer 2015, 121, 2681–2688. [Google Scholar] [CrossRef]
- Wang, F.; Roy, S. Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicol. Pathol. 2017, 45, 150–156. [Google Scholar] [CrossRef]
- Shakeri, R.; Kamangar, F.; Mohamadnejad, M.; Tabrizi, R.; Zamani, F.; Mohamadkhani, A.; Nikfam, S.; Nikmanesh, A.; Sotoudeh, M.; Sotoudehmanesh, R.; et al. Opium use, cigarette smoking, and alcohol consumption in relation to pancreatic cancer. Medicine 2016, 95, e3922. [Google Scholar] [CrossRef]
- Moossavi, S.; Mohamadnejad, M.; Pourshams, A.; Poustchi, H.; Islami, F.; Sharafkhah, M.; Mirminachi, B.; Nasseri-Moghaddam, S.; Semnani, S.; Shakeri, R.; et al. Opium Use and Risk of Pancreatic Cancer: A Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2018, 27, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Barlass, U.; Deshmukh, A.; Beck, T.; Bishehsari, F. Opioid use as a potential risk factor for pancreatic cancer in the United States: An analysis of state and national level databases. PLoS ONE 2021, 16, e0244285. [Google Scholar] [CrossRef]
- Sun, M.; Lin, J.-A.; Chang, C.-L.; Wu, S.-Y.; Zhang, J. Association between long-term opioid use and cancer risk in patients with chronic pain: A propensity score-matched cohort study. Br. J. Anaesth. 2022, 129, 84–91. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Walther, B.A.; Lin, M.-C.; Li, Y.-C.K. Proton Pump Inhibitors Use and the Risk of Pancreatic Cancer: Evidence from Eleven Epidemiological Studies, Comprising 1.5 Million Individuals. Cancers 2022, 14, 5357. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Fan, Y.-X.; Meng, R.; Cai, W.-K.; Yin, S.-J.; Zhou, T.; Huang, Y.-H.; Wang, P.; Jiang, F.-F.; Yang, M.; et al. Proton Pump Inhibitors and Cancer Risk: An Umbrella Review and Meta-analysis of Observational Studies. Am. J. Clin. Oncol. 2022, 45, 475–485. [Google Scholar] [CrossRef]
- Fried, M.; Siegrist, H.; Frei, R.; Froehlich, F.; Duroux, P.; Thorens, J.; Blum, A.; Bille, J.; Gonvers, J.J.; Gyr, K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut 1994, 35, 23–26. [Google Scholar] [CrossRef]
- Smith, J.P.; Fonkoua, L.K.; Moody, T.W. The Role of Gastrin and CCK Receptors in Pancreatic Cancer and other Malignancies. Int. J. Biol. Sci. 2016, 12, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Nishijima, S.; Kojima, Y.; Hisada, Y.; Imbe, K.; Miyoshi-Akiyama, T.; Suda, W.; Kimura, M.; Aoki, R.; Sekine, K.; et al. Metagenomic Identification of Microbial Signatures Predicting Pancreatic Cancer from a Multinational Study. Gastroenterology 2022, 163, 222–238. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Kang, J.-H.; Chan, A.T.; Michaud, D.S.; Skinner, H.G.; Giovannucci, E.; Colditz, G.A.; Fuchs, C.S. A prospective study of aspirin use and the risk of pancreatic cancer in women. J. Natl. Cancer Inst. 2004, 96, 22–28. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wan, Y.-D.; Sun, Y.-L.; Li, J.; Zhu, R.-T. Aspirin might reduce the incidence of pancreatic cancer: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 15460. [Google Scholar] [CrossRef]
- Kirkegård, J.; Lund, J.L.; Mortensen, F.V.; Cronin-Fenton, D. Statins and pancreatic cancer risk in patients with chronic pancreatitis: A Danish nationwide population-based cohort study. Int. J. Cancer 2020, 146, 610–616. [Google Scholar] [CrossRef] [PubMed]
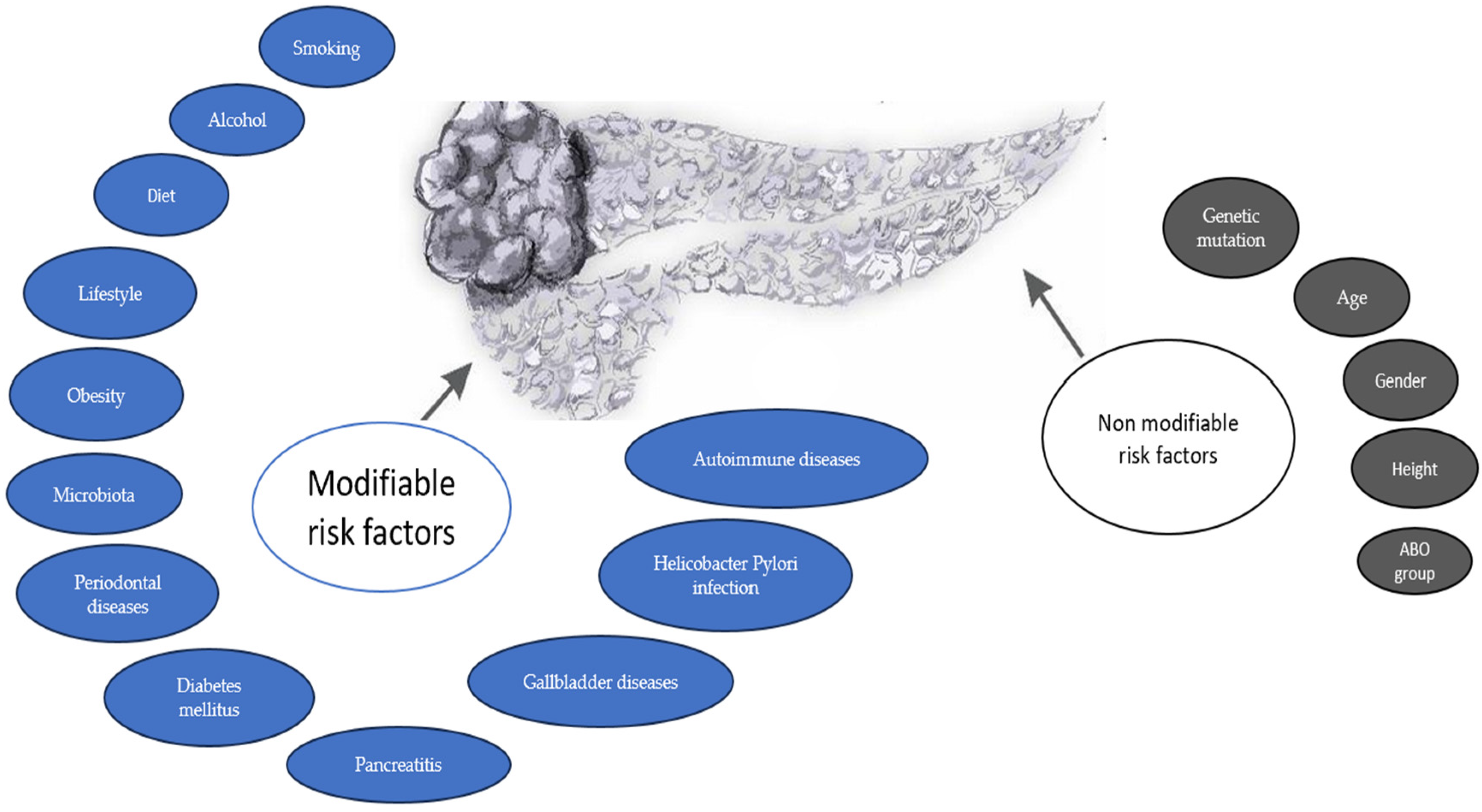
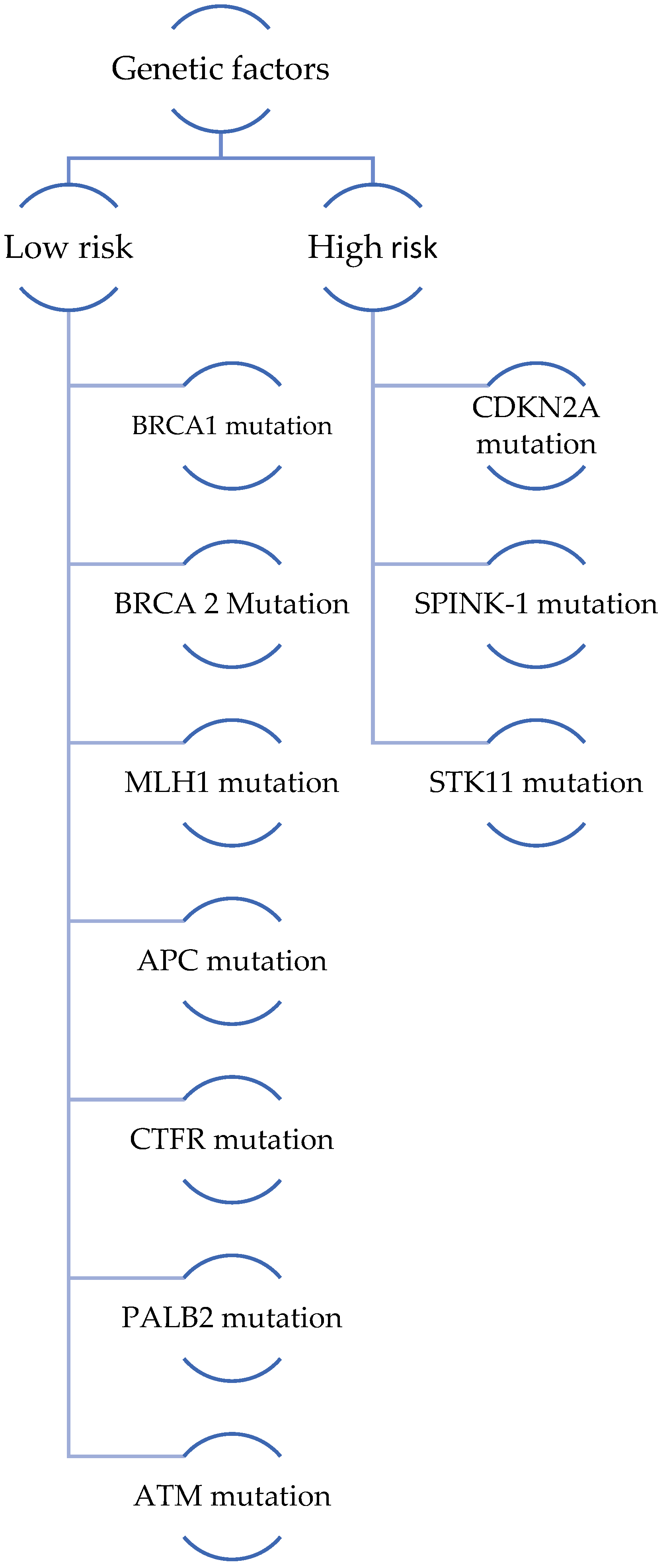
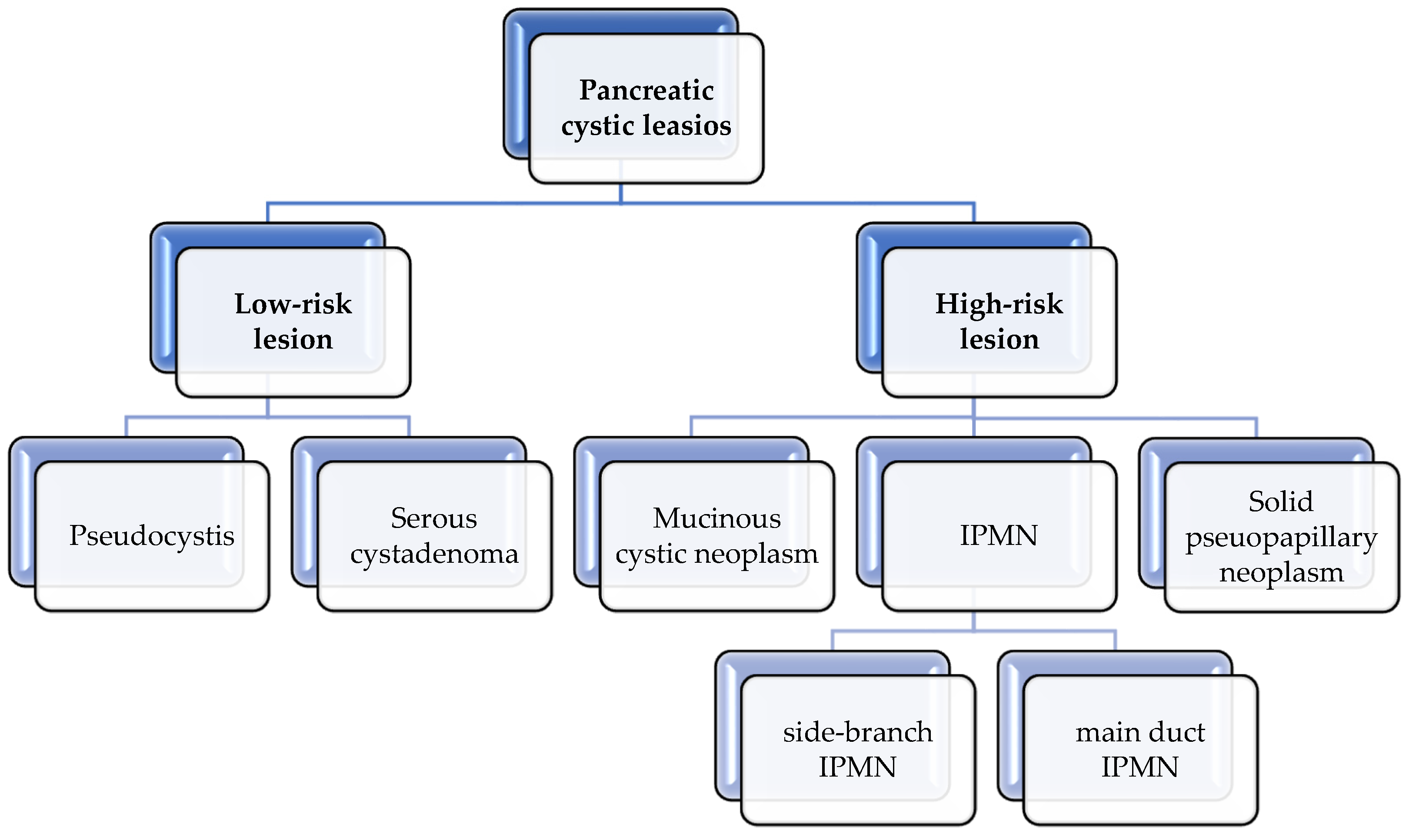
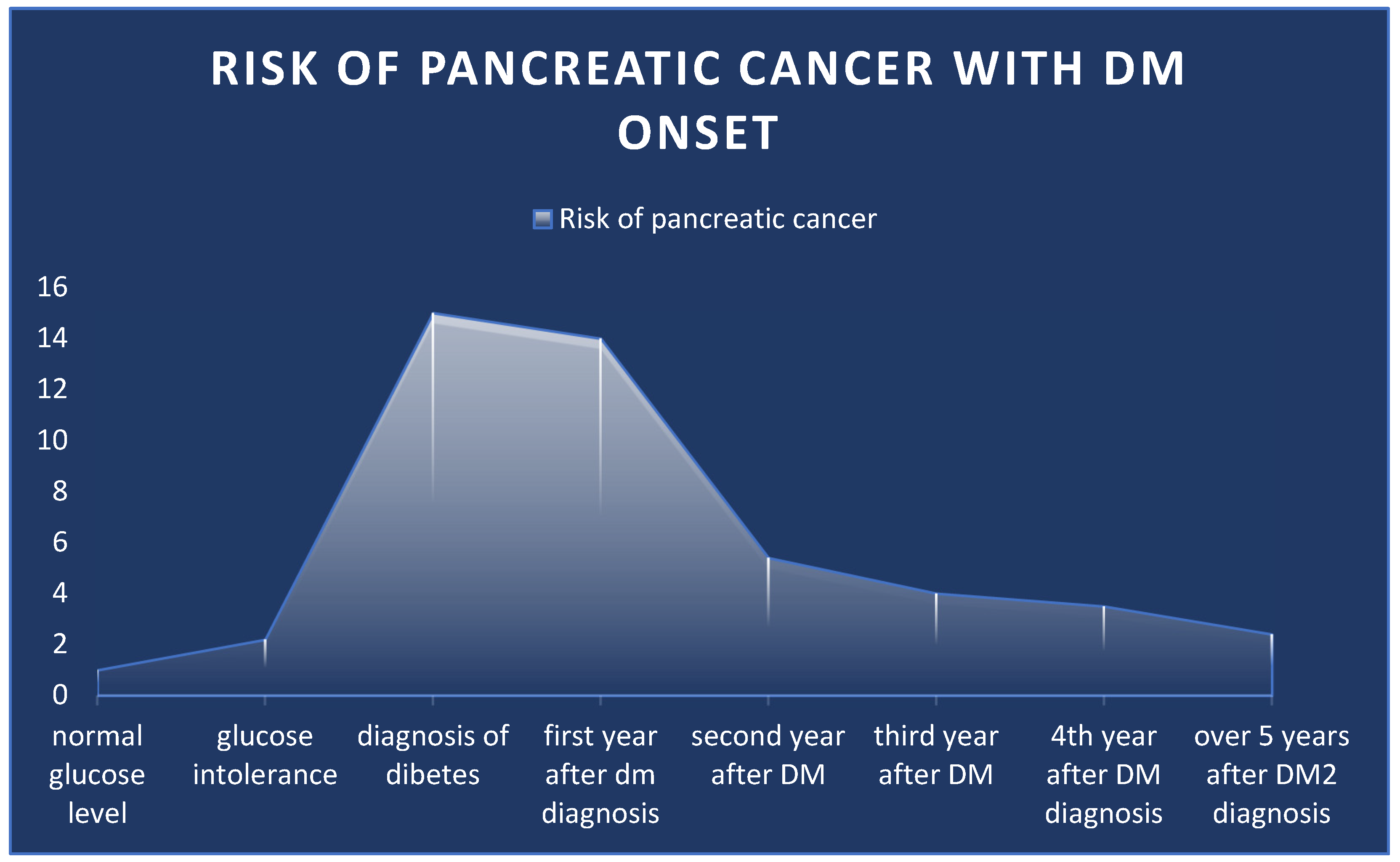
| Gene Mutation | Pancreatic Cancer Risk | CAPS Consensus for Surveillance | At What Age Should Surveillance Begin? According to CAPS |
|---|---|---|---|
| BRCA1 | Inconsistent data about the risk | If at least one affected FDR | |
| BRCA 2 | Inconsistent data about the risk | If at least one affected FDR or ≥two affected blood relatives | 50 years |
| Lynch syndrome—MLH1/MSH2/MSH6 | 8.6-fold increased risk | If at least one affected FDR | |
| FAP—APC mutation | Increased up to 4 times | No data | |
| Peuts–Jeghers syndrome—LKB1/STK11 | 132-increased risk | Regardless of family history | 40 years |
| FAMMM with CDKN2A mutation leading to changes in the p16 protein | 13 to 67-fold increased risk | Regardless of family history | 40 years, and if worrisome features → EUS+/−FNA and close follow-up imaging at 3–6 months |
| Hereditary pancreatitis—SPINK 1 mutation/PRSS1 mutations | 53-fold increased risk | Regardless of family history | 40 years or 20 years after the first pancreatitis attack |
| PALB2 | At least one affected FDR | 50 years | |
| CFTR | 5.3 increased risk | No data | |
| Ataxia-teleangiectasia ATM mutation | At least one affected FDR | 50 years |
| Risk Factor | Impact on Risk | Studies on Risk | What Can Be Done? |
|---|---|---|---|
| Smoking | Up to 2.7-fold increase | Bosetti et al. [115] Anderson et al. [116] Lugo et al. [117] | Smoking cessation campaign Advocating for alternative nicotine-delivering products for those who do not want to quit |
| Diabetes | 14–15-fold increased risk in the first year 2.4–5-fold increased risk in the later years | Dankner et al. [144] Bosetii C et al. [156] | Advocating for a healthy lifestyle and exercise Validating, in prospective studies, the END-PAC model or refined models (adding a family history of pancreatic cancer, a personal history of pancreatitis, COPD, and uric acid) to find the patient at increased risk and including them in screening programs Favoring the use of metformin as the first-line anti-diabetic treatment |
| Obesity, diet and lifestyle | 20–30% increased risk | Berrington de Gonzáles et al. [28] Genkinger et al. [31] Stolzenberg-Solomon RZ et al. [178] Arslan et al. [179] | Advocating for a normal weight, physical activity, and a diet rich in antioxidants such as fruits, nuts, and vegetables, avoiding the use of high amounts of meat and large amounts of soft drinks |
| Pancreatitis | Up to a 24-fold increased risk | Ikeura et al. [225] Hart et al. [226] | Avoiding excessive alcohol consumption Screening for patients with AP aged 57–75 years old, without the two most common ethologic factors of acute pancreatitis Screening for genetic mutations in those with CP and then further screening according to guidelines |
| Alcohol | Up to 1.36-fold increased risk | Tramacere et al. [231] Wang et al. [232] Gapstur et al. [233] | Avoiding excessive alcohol consumption |
| Coffee | Inconsistent data | Li et al. [239] Nie et al. [240] Zhou et al. [243] Ran et al. [244] Lukic et al. [245] | Moderate consumption until further studies are conducted |
| Viral hepatitis | Huang et al. [254] Hassan et al. [255] Xu et al. [256] | Vaccination against Hepatitis B virus, treatment for hepatitis C virus | |
| Gallbladder diseases and cholecystectomy | Inconsistent data | Fan et al. [260] Luo et al. [261] Rosato et al. [263] Shabanzadeh et al. [265] | Promovate |
| Periodontal diseases | Jingru Yu et al. [268] Chang et al. [268] Michaud et al. [269] | Good oral hygiene | |
| Helicobacter Pylori | Controversial data | Risch HA et al. [38] Huang J et al. [277] Bulajic M et al. [278] Cullin N et al. [279] | Promoting a balanced diet |
| Autoimmune diseases | Systemic and cutaneous lupus may be associated with an increased risk, but the data are controversial | Zhang M et al. [282] | A special focus on patients with autoimmune diseases and their treatment |
| POCS | 3.4-fold increased risk | Peeri et al. [285] Yin et al. [284] | Considering the correlation of POCS with obesity and glucose intolerance; perhaps a balanced diet could influence both conditions |
| Microbiota | Inconsistent data | Pushalkar S et al. [289] Liu J et al. [298] Santoni M et al. [299] | Further studies needed |
| Psychological stress | Increased risk | Kennedy B et al. [308] Huang et al. [309] | Psychological and/or psychiatric support for very stressful events |
| Renin-angiotensin inhibitors | May reduce the risk of pancreatic cancer—further data needed | Lee et al. [318] Tse et al. [319] | With a doubtful effect, promoting the use of ACI for those who need antihypertensive treatment |
| Statins | No effect on PC risk | Kirkegård et al. [348] | Not appliable |
| Allergies | May reduce the risk | Gandini et al. [323] Cotterchio M et al. [324] Wang et al. [325] Gandini et al. [323] Karim et al. [329] | Not appliable |
| Opioids | May increase the risk | Shakeri et al. [337] Moossavi et al. [338] Barlass et al. [339] Sun et al. [340] | Limiting the use of opioids; anti-drug campaigns |
| NSAID | Inconsistent data | Schernhammer ES [346] Zhang et al. [347] | Inconsistent data |
| PPI | 63% increased risk | Poly et al. [341] Zhang et al. [342] | Limiting the use; use just for those who need it |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorescu, R.R.; Husar-Sburlan, I.A.; Gheorghe, C. Pancreatic Cancer: A Review of Risk Factors. Life 2024, 14, 980. https://doi.org/10.3390/life14080980
Grigorescu RR, Husar-Sburlan IA, Gheorghe C. Pancreatic Cancer: A Review of Risk Factors. Life. 2024; 14(8):980. https://doi.org/10.3390/life14080980
Chicago/Turabian StyleGrigorescu, Raluca Roxana, Ioana Alexandra Husar-Sburlan, and Cristian Gheorghe. 2024. "Pancreatic Cancer: A Review of Risk Factors" Life 14, no. 8: 980. https://doi.org/10.3390/life14080980
APA StyleGrigorescu, R. R., Husar-Sburlan, I. A., & Gheorghe, C. (2024). Pancreatic Cancer: A Review of Risk Factors. Life, 14(8), 980. https://doi.org/10.3390/life14080980






