Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Definitions
2.2. Data Collection and Statistical Analysis
3. Results
3.1. General Subject Characteristics and Incidence of Acute Kidney Injury
3.2. Pre-Transplant Risk Factors for Developing AKI
3.3. Post-Transplant Risk Factors for Developing AKI
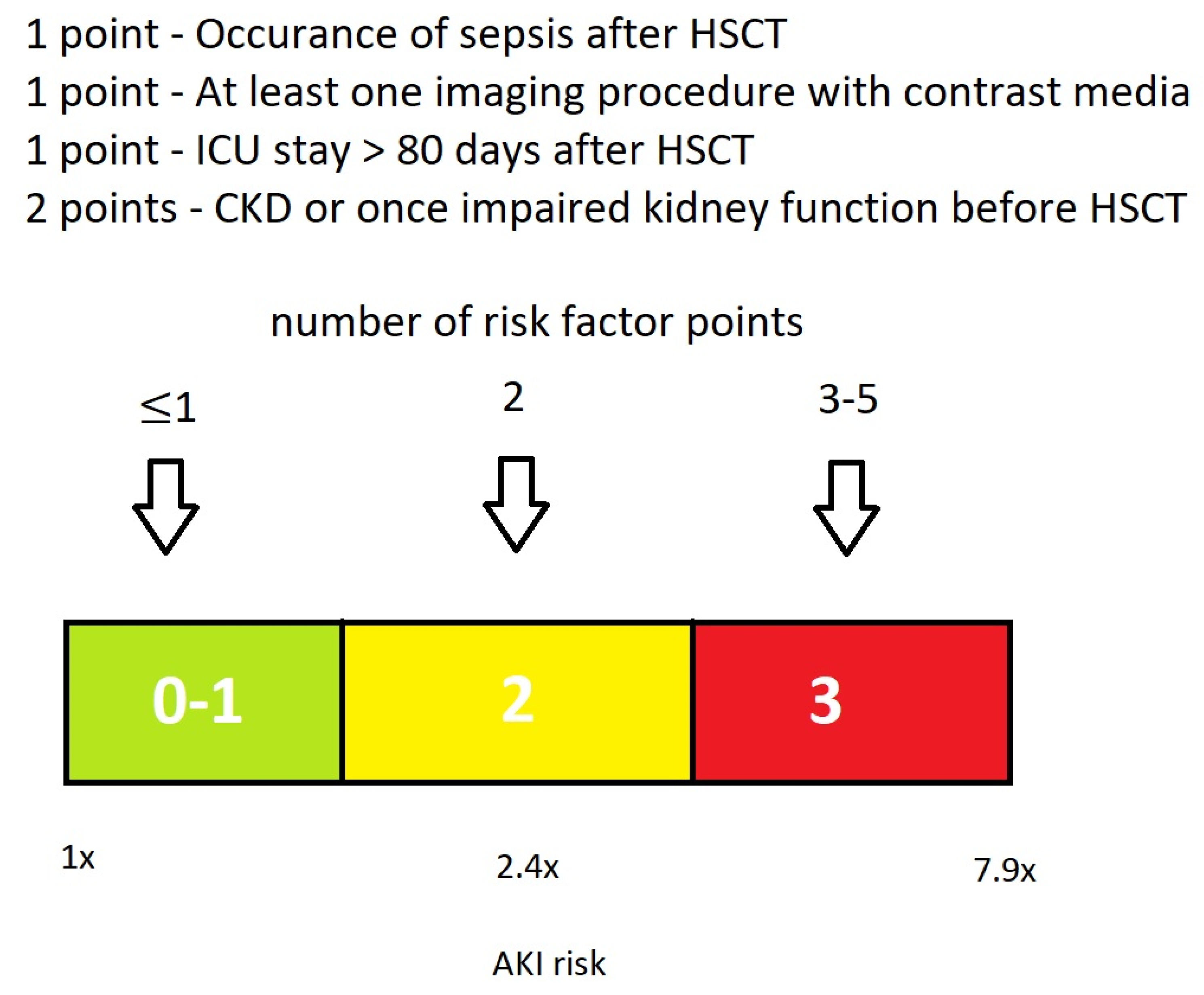
3.4. Proposal of a Basic EHRs Dataset and Score for the Calculation of HCT-AKIR
4. Discussion
4.1. Incidence of AKI
4.2. Multivariate Significant Risk Factors for AKI
4.2.1. Pre-Transplant CKD or Once-Impaired Kidney Function
4.2.2. Post-Transplant Sepsis
4.2.3. Post-Transplant ICU Stay and Imaging Procedures with Contrast Media
4.2.4. Proposal of a Basic Dataset for the Calculation of HCT-AKIR Score
4.2.5. Weaknesses and Strength of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majumdar, A. Sepsis-induced acute kidney injury. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2010, 14, 14–21. [Google Scholar] [CrossRef]
- Müller, L.P.; Müller-Tidow, C. The Indications for Allogeneic Stem Cell Transplantation in Myeloid Malignancies. Dtsch. Ärzteblatt Int. 2015, 112, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Henig, I.; Zuckerman, T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med. J. 2014, 5, e0028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caliskan, Y.; Besisik, S.K.; Sargin, D.; Ecder, T. Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2006, 38, 141–147. [Google Scholar] [CrossRef]
- Canet, E.; Zafrani, L.; Lambert, J.; Thieblemont, C.; Galicier, L.; Schnell, D.; Raffoux, E.; Lengline, E.; Chevret, S.; Darmon, M.; et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS ONE 2013, 8, e55870. [Google Scholar] [CrossRef] [PubMed]
- Kogon, A.; Hingorani, S. Acute kidney injury in hematopoietic cell transplantation. Semin. Nephrol. 2010, 30, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S.; Neves, M. Acute kidney injury in HCT: An update. Bone Marrow Transplant. 2016, 51, 755–762. [Google Scholar] [CrossRef]
- Hingorani, S. Renal complications of hematopoietic-cell transplantation. N. Engl. J. Med. 2016, 374, 2256–2267. [Google Scholar] [CrossRef]
- Kersting, S.; Dorp, S.V.; Theobald, M.; Verdonck, L.F. Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol. Blood Marrow Transplant. 2008, 14, 125–131. [Google Scholar] [CrossRef]
- Kersting, S.; Koomans, H.A.; Hené, R.J.; Verdonck, L.F. Acute renal failure after allogeneic myeloablative stem cell transplantation: Retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007, 39, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, V.; Gupta, M.; Manu, G.; Kwatra, S.; Owusu, O.-T.; Raina, R. Acute Kidney Injury in Hematopoietic Stem Cell Transplantation: A Review. Int. J. Nephrol. 2016, 2016, 5163789. [Google Scholar] [CrossRef] [PubMed]
- Mae, H.; Ooi, J.; Takahashi, S.; Tomonari, A.; Tsukada, N.; Konuma, T. Early renal injury after myeloablative cord blood transplantation in adults. Leuk. Lymphoma 2008, 49, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Yarlagadda, S.G.; Storer, B.; Sorror, M.; Storb, R.; Sandmaier, B. Impact of Acute Kidney Injury on Long Term Mortality after Nonmyeloablative Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2008, 14, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Saddadi, F.; Najafi, I.; Hakemi, M.S.; Falaknazi, K.; Attari, F.; Bahar, B. Frequency, risk factors, and outcome of acute kidney injury following bone marrow transplantation at Dr Shariati Hospital in Tehran. Iran. J. Kidney Dis. 2010, 4, 20–26. [Google Scholar] [PubMed]
- Fujii, T.; Uchino, S.; Takinami, M.; Bellomo, R. Validation of the Kidney Disease Improving Global Outcomes Criteria for AKI and Comparison of Three Criteria in Hospitalized Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S. Acute kidney injury following HCT: Incidence, risk factors and outcome. Bone Marrow Transplant. 2011, 46, 1399–1408. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Lopes, J.A.; Gonçalves, S.; Jorge, S.; Raimundo, M.; Resende, L.; Lourenço, F.; Lacerda, J.F.; Martins, C.; do Carmo, J.A.; Lacerda, J.M.F.; et al. Contemporary analysis of the influence of acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone Marrow Transplant. 2008, 42, 619–626. [Google Scholar] [CrossRef]
- Sun, J.; McNaughton, C.D.; Zhang, P.; Perer, A.; Gkoulalas-Divanis, A.; Denny, J.C.; Kirby, J.; Lasko, T.; Saip, A.; Malin, B.A. Predicting changes in hypertension control using electronic health records from a chronic disease management program. J. Am. Med. Inform. Assoc. 2013, 21, 337–344. [Google Scholar] [CrossRef]
- Khalilia, M.; Choi, M.; Henderson, A.; Iyengar, S.; Braunstein, M.; Sun, J. Clinical Predictive Modeling Development and Deployment through FHIR Web Services. AMIA Annu. Symp. Proc. 2015, 2015, 717–726. [Google Scholar] [PubMed] [PubMed Central]
- Amar, F.; April, A.; Abran, A. Electronic Health Record and Semantic Issues Using Fast Healthcare Interoperability Resources: Systematic Mapping Review. J. Med. Internet Res. 2024, 26, e45209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Canet, E.; Lengline, E.; Zafrani, L.; Peraldi, M.N.; Socié, G.; Azoulay, E. Acute kidney injury in critically ill allo-HSCT recipients. Bone Marrow Transplant. 2014, 49, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; Perez-Pitarch, A.; Garcia-Cadenas, I.; Barba, P.; Hernandez-Boluda, J.C.; Esquirol, A.; Fox, M.L.; Terol, M.J.; Queraltó, J.M.; Vima, J.; et al. A Time-to-Event Model for Acute Kidney Injury after Reduced-Intensity Conditioning Stem Cell Transplantation Using a Tacrolimus- and Sirolimus-based Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2017, 23, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Mori, J.; Ohashi, K.; Akiyama, H.; Morito, T.; Tsuchiya, K.; Nitta, K.; Sakamaki, H. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant. 2010, 45, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.S.; Xie, R.J.; Wang, M.; Feng, S.Z.; Han, M.Z. An evaluation of the RIFLE criteria for acute kidney injury after myeloablative allogeneic haematopoietic stem cell transplantation. Swiss Med. Wkly. 2011, 141, w13225. [Google Scholar] [CrossRef]
- Parikh, C.R.; McSweeney, P.A.; Korular, D.; Ecder, T.; Merouani, A.; Taylor, J.; Slat-Vasquez, V.; Shpall, E.J.; Jones, R.B.; Bearman, S.I.; et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002, 62, 566–573. [Google Scholar] [CrossRef]
- Piñana, J.L.; Valcárcel, D.; Martino, R.; Barba, P.; Moreno, E.; Sureda, A.; Vega, M.; Delgado, J.; Briones, J.; Brunet, S.; et al. Study of kidney function impairment after reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. A single-center experience. Biol. Blood Marrow Transplant. 2009, 15, 21–29. [Google Scholar] [CrossRef]
- Yegenaga, I.; Hoste, E.; Van Biesen, W.; Vanholder, R.; Benoit, D.; Kantarci, G.; Dhondt, A.; Colardyn, F.; Lameire, N. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: Results of a prospective study. Am. J. Kidney Dis. 2004, 43, 817–824. [Google Scholar] [CrossRef]
- Parikh, C.R.; Sandmaier, B.M.; Storb, R.F.; Blume, K.G.; Sahebi, F.; Maloney, D.G.; Maris, M.B.; Nieto, Y.; Edelstein, C.L.; Schrier, R.W.; et al. Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J. Am. Soc. Nephrol. 2004, 15, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Kagoya, Y.; Kataoka, K.; Nannya, Y.; Kurokawa, M. Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ordoñez, J.D.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Go, A.S. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rifkin, D.E.; Blantz, R.C. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin. J. Am. Soc. Nephrol. 2010, 5, 1690–1695. [Google Scholar] [CrossRef]
- Waikar, S.S.; Liu, K.D.; Chertow, G.M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 2008, 3, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.-F.; Liu, B.-C.; Ding, J.-H.; Chen, B.-A.; Xu, W.-L.; Qian, J. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant. 2010, 45, 153–158. [Google Scholar] [CrossRef]
- Mori, J.; Ohashi, K.; Yamaguchi, T.; Ando, M.; Hirashima, Y.; Kobayashi, T.; Kakihana, K.; Sakamaki, H. Risk assessment for acute kidney injury after allogeneic hematopoietic stem cell transplantation based on Acute Kidney Injury Network criteria. Intern. Med. 2012, 51, 2105–2110. [Google Scholar] [CrossRef]
- Sehgal, B.; George, P.; John, M.J.; Samuel, C. Acute kidney injury and mortality in hematopoietic stem cell transplantation: A single-center experience. Indian J. Nephrol. 2017, 27, 13–19. [Google Scholar] [CrossRef]
- Didsbury, M.S.; Mackie, F.E.; Kennedy, S.E. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatr. Transplant. 2015, 19, 460–470. [Google Scholar] [CrossRef]
- Yu, Z.P.; Ding, J.H.; Chen, B.A.; Liu, B.C.; Liu, H.; Li, Y.F.; Ding, B.H.; Qian, J. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin. J. Cancer 2010, 29, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Druml, W. Kontrastmittel-induzierte Nephropathie: Gibt es die überhaupt? Forum für Nephrologie und Hypertensiologie. Nephro-news (2):2-7. DtschArzteblInt 2015, 112, 262–270. 2017. Available online: https://medicom.cc/de/publikationen/nephro-news/201702/entries/01-Kontrastmittel-induzierte-Nephropathie.php (accessed on 12 June 2024).
- Gan, Z.; Chen, L.; Wu, M.; Liu, L.; Shi, L.; Li, Q.; Zhang, Z.; Lai, Y. Predicting the risk of acute kidney injury after hematopoietic stem cell transplantation: Development of a new predictive nomogram. Sci. Rep. 2022, 12, 15316. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Fragão-Marques, M.; Costa, C.; Branco, C.; Marques, F.; Vasconcelos, P.; Martins, C.; Leite-Moreira, A.; Lopes, J.A. Predictive Risk Score for Acute Kidney Injury in Hematopoietic Stem Cell Transplant. Cancers 2023, 15, 3720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sauer, C.M.; Chen, L.C.; Hyland, S.L.; Girbes, A.; Elbers, P.; Celi, L.A. Leveraging electronic health records for data science: Common pitfalls and how to avoid them. Lancet Digit. Health 2022, 4, e893–e898. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients (N) | Percentage (N = 312) | |
|---|---|---|---|
| Gender | male/female | 200/112 | 64.1%/35.9% |
| Mean age | 55.42 years (range 19–78) | ||
| Previous illnesses | aHT | 117 | 37.5% |
| DM | 51 | 16.3% | |
| Comorbidity score | 0 | 151 | 48.4% |
| 1–2 | 99 | 31.7% | |
| ≥3 | 62 | 19.9% | |
| Kidney function | Mean eGFR | 82.68 ± 12.53 mL/min/1.73 m2 | |
| CKD | 49 | 15.7% | |
| CKD stage 1 | 1 | 0.3% | |
| CKD stage 2 | 39 | 12.5% | |
| CKD stage 3 | 9 | 2.9% | |
| CKD stage 4 and 5 | 0 | 0% | |
| Ø CKD | 263 | 84.3% | |
| normal eGFR | 231 | 74% | |
| once-impaired kidney function or proteinuria | 32 | 10.3% | |
| Hematologic diseases | AML | 160 | 51.3% |
| MDS | 48 | 15.4% | |
| HL | 38 | 12.2% | |
| ALL | 25 | 8.0% | |
| CLL | 15 | 4.8% | |
| other hematologic diseases | 26 | 8.3% | |
| Conditioning | myeloablative | 116 | 37.2% |
| reduced intensity | 196 | 62.8% | |
| Conditioning therapy | only chemotherapy | 163 | 52.2% |
| ATG | 93 | 29.8% | |
| TBI 2 Gy | 31 | 9.9% | |
| TBI 8 Gy | 8 | 2.6% | |
| TBI 12 Gy | 12 | 3.8% | |
| RIT | 5 | 1.6% | |
| Stem cell source | PBSCT | 271 | 86.9% |
| Bone marrow | 41 | 13.1% | |
| HLA compatibility | 10/10 HLA matched | 227 | 72.8% |
| 9/10 HLA matched | 64 | 20.5% | |
| haploidentical | 21 | 6.7% | |
| Relation to donor | MSIB | 23 | 7.4% |
| MMSIB | 2 | 0.6% | |
| MUD | 204 | 65.4% | |
| MMUD | 62 | 19.9% | |
| Chemotherapy | All Subjects (%) | With AKI (%) | Without AKI (%) | p-Value |
|---|---|---|---|---|
| Cytarabin | 201 (64.4) | 129 (65.2) | 72 (63.2) | 0.799 |
| Daunorubicin | 126 (40.3) | 80 (40.4) | 46 (40.4) | 0.958 |
| Azacitidin | 81 (26) | 48 (24.2) | 33 (29) | 0.338 |
| Mitoxantron | 53 (17) | 34 (17.2) | 19 (16.7) | 0.936 |
| Vincristin | 52 (16.7) | 32 (16.2) | 20 (17.5) | 0.727 |
| Hydroxydaunorubicin | 39 (12.5) | 25 (12.7) | 19 (16.7) | 0.952 |
| Melphalan | 35 (11.2) | 22 (11.1) | 13 (11.4) | 0.928 |
| Bendamustin | 32 (10.3) | 17 (8.6) | 15 (13.2) | 0.191 |
| Cisplatin | 32 (10.3) | 21 (10.6) | 11 (9.6) | 0.808 |
| AKI Stages | Patients (N) | Percent of All 312 Patients | Percent of 198 Patients with AKI |
|---|---|---|---|
| Max. Stage 1 | 55 | 17.62% | 27.8% |
| Max. Stage 2 | 79 | 25.32% | 39.9% |
| Max. Stage 3 | 64 | 20.51% | 32.3% |
| Total number of patients with AKI | 198 | 63.5% | 100% |
| Total number of patients without AKI | 114 | 36.5% | - |
| Number of AKI | Patients (N) | Percent of All 312 Patients | Percent of 198 Patients with AKI |
| Max. one AKI | 122 | 39.10% | 61.62% |
| Max. two AKI | 47 | 15.06% | 23.74% |
| Max. three AKI | 15 | 4.81% | 7.57% |
| Max. four AKI | 8 | 2.56% | 4.04% |
| Max. five AKI | 6 | 1.92% | 3.03% |
| Total number of patients with AKI | 198 | 63.5% | 100% |
| Total number of AKI | 323 | - | - |
| Factor | All Patients (% of 312) | Patients with AKI (% of 198) | Patients without AKI (% of 114) | Univariate Analysis (p-Value) | Multivariate Analysis p-Value/Odds Ratio (95%CI) | |
|---|---|---|---|---|---|---|
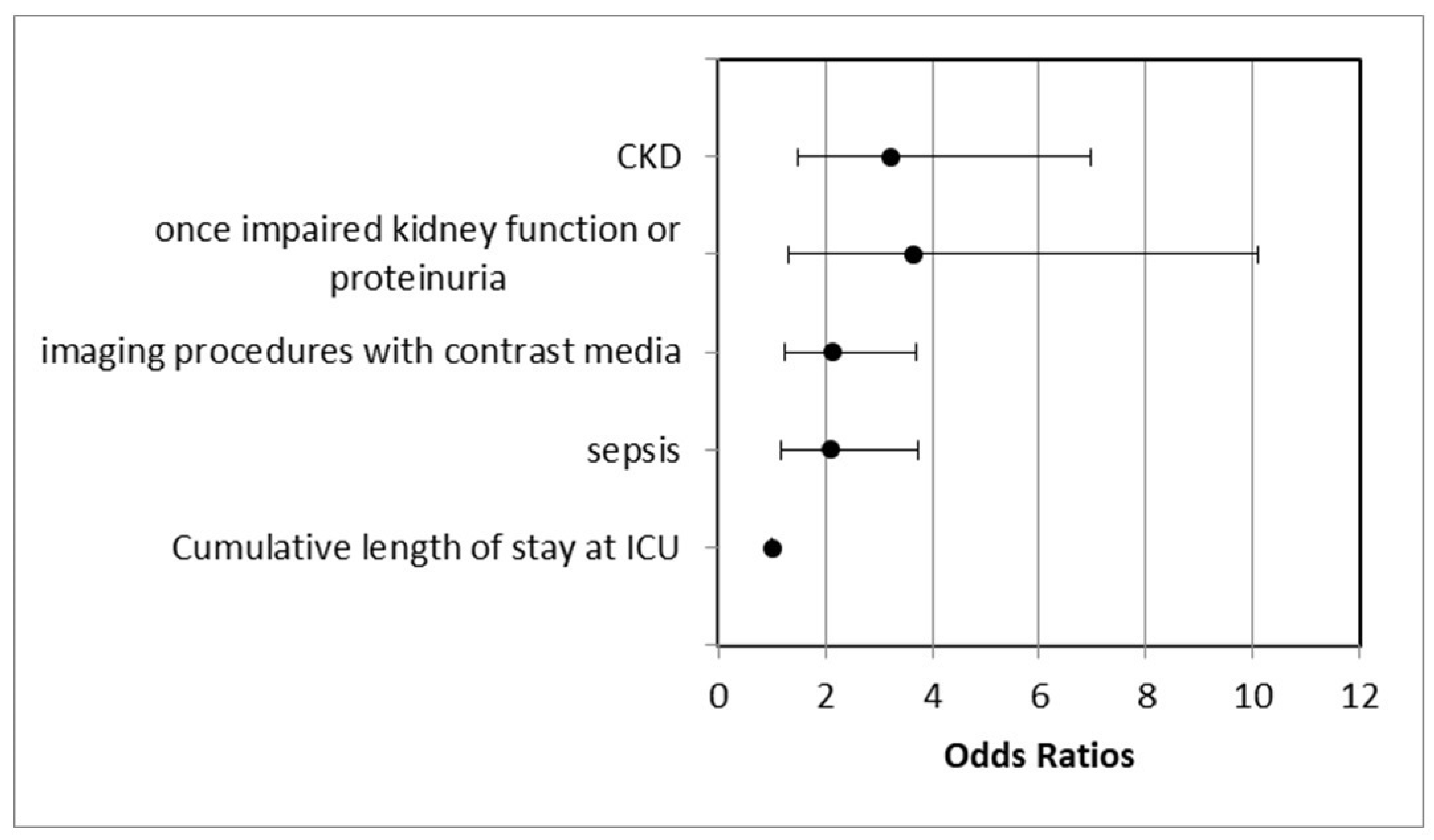
| CKD | 49 (15.7) | 39 (19.7) | 10 (8.8) | 0.000 | 0.003/3.224 (1.488–6.984) | |
| Once-impaired kidney function or proteinuria | 32 (10.3) | 27 (13.6) | 5 (4.4) | 0.013/3.635 (1.308–10.103) | ||
| Normal eGFR | 231 (74%) | 132 (66.6) | 99 (86.8%) | |||
| aHT | 117 (37.5) | 84 (42.4) | 33 (28.9) | 0.021 | 0.072 | |
| Comorbidity score | 0 | 151 (48.4) | 89 (44.9) | 0.035 | 0.209 | |
| 1–2 | 99 (31.7) | 61 (30.8) | ||||
| ≥3 | 62 (19.9) | 48 (24.3) | ||||
| Age | Mean age (range) | 0.074 | - | |||
| 55.42 years (19–75) | 56.47 years (21–75) | 53.59 years (19–74) | ||||
| Gender | male | 200 (64.1) | 134 (67.7) | 66 (57.9) | 0.083 | - |
| female | 112 (35.9) | 64 (32.3) | 48 (42.1) | |||
| DM | 51 (16.3) | 37 (18.7) | 14 (12.3) | 0.141 | - | |
| Stem cell source | PBSCT | 271 (86.9) | 176 (88.9) | 95 (83.3) | 0.162 | - |
| Bone marrow | 41 (13.1) | 22 (11.1) | 19 (16.6) | |||
| Conditioning regimens | myeloablative | 116 (37.2) | 74 (37.4) | 42 (36.8) | 0.925 | - |
| reduced | 196 (62.8) | 124 (62.6) | 72 (63.2) | |||
| HLA compatibility | HLA 10/10 | 227 (72.8) | 143 (72.2) | 84 (73.7) | 0.166 | - |
| HLA 9/10 | 64 (20.5) | 45 (22.7) | 19 (16.7) | |||
| HLA haploidentical | 21 (6.7) | 10 (5.1) | 11 (9.6) | |||
| Relation to donor | MSIB | 23 (7.4) | 13 (6.6) | 10 (8.8) | 0.373 | - |
| MMSIB | 2 (0.6) | 0 (0) | 2 (1.8) | |||
| MUD | 204 (65.4) | 130 (65.7) | 74 (64.9) | |||
| MMUD | 62 (19.9) | 45 (22.7) | 17 (14.9) | |||
| Factor | All Patients (% of 312) | Patients with AKI (% of 198) | Patients without AKI (% of 114) | Univariate Analysis (p-Value) | Multivariate Analysis p-Value/Odds Ratio (95%CI) |
|---|---|---|---|---|---|
| Sepsis | 106 (34) | 81 (40.9) | 25 (21.9) | 0.001 | 0.012/2.097 (1.178–3.733) |
| Imaging procedures with contrast media | 113 (36.2) | 86 (43.4) | 27 (23.7) | 0.001 | 0.007/2.134 (1.234–3.690) |
| Cumulative length of ICU stay | 93.87 days | 103.4 days | 77.14 days | 0.000 | 0.034/1.005 (1.000–1.009) |
| “Toxic“ CsA (>300 ng/mL) and tacrolimus peak plasma level (>20 ng/mL) | 163 (52.3%) | 114 (57.6) | 49 (43%) | 0.013 | 0.071 |
| Duration of the therapy with tacrolimus | 198.29 days | 271.88 days | 78.69 days | 0.009 | Not included |
| Chimerical status on day 14 | 80.91% | 80.71% | 80.23% | 0.866 | - |
| Late chimerical status | 94.34% | 95.39% | 92.45% | 0.246 | - |
| Engraftment day | 20.64 days | 20.88 days | 20.21 days | 0.451 | - |
| CMV-infection | 79 (25.3) | 53 (26.8) | 26 (22.8) | 0.464 | - |
| aGVHD | 165 (52.9) | 107 (54) | 58 (50.9) | 0.590 | - |
| Number of imaging procedures with contrast media | 1.69 | 1.76 | 1.48 | 0.324 | - |
| Therapy with CsA | 283 (90.7) | 181 (91.4) | 102 (89.5) | 0.570 | - |
| Duration of the therapy with CsA | 272.18 days | 257.95 days | 298.04 days | 0.305 | - |
| Therapy withTacrolimus | 43 (13.8) | 26 (13.1) | 17 (14.9) | 0.660 | - |
| Proposed Basic EHRs Dataset | ||
|---|---|---|
| Record | Content | Corresponding FHIRs |
| Procedure | allo-HCT | Procedure |
| Encounter | ICU stay Duration | Encounter |
| Diagnosis | Sepsis CKD AKI | Condition |
| Laboratory Data | Proteinuria Creatinine eGFR | DiagnosticReport Observation |
| Medication | Contrast media | Medication MedicationAdministration |
| Imaging procedure | Imaging procedure using contrast media | DiagnosticReport ImagingStudy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff, E.; Kirilov, N. Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation. Life 2024, 14, 987. https://doi.org/10.3390/life14080987
Bischoff E, Kirilov N. Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation. Life. 2024; 14(8):987. https://doi.org/10.3390/life14080987
Chicago/Turabian StyleBischoff, Elena, and Nikola Kirilov. 2024. "Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation" Life 14, no. 8: 987. https://doi.org/10.3390/life14080987





