Abstract
Oncologic back pain, infection, inflammation, and trauma are the only specific etiologies of chronic low back pain (CLBP) in contrast to most patients who have non-specific CLBP. In oncologic patients developing CLBP, it is critically important to perform further investigation to exclude spinal metastases (SM).The incidence of cancer is increasing, with 15.7–30% developing SM. In the case of symptomatic SM, we can distinguish three main categories: tumor pain; mechanical pain due to instability, with or without pathologic fractures; and metastatic epidural spinal cord compression (MESCC) or radicular compression. Treatment of SM-related pain is dependent on these categories and consists of symptomatic treatment, target therapy to the bone, radiotherapy, systemic oncologic treatment, and surgery. The care for SM is a multidisciplinary concern, with rapid evolutions in all specialties involved. It is of primordial importance to incorporate the knowledge of specialists in all participating disciplines, such as oncology, radiotherapy, and spinal surgery, to determine the adequate treatment to preserve ambulatory function and quality of life while limiting the burden of treatment if possible. Awareness of potential SM is the first and most important step in the treatment of SM-related pain. Early diagnosis and timely treatment could prevent further deterioration. In this review, we explore the pathophysiology and symptomatology of SM and the treatment options for SM-related pain: tumor pain; mechanical pain due to instability, with or without pathologic fractures; and MESCC or radicular compression.
1. Introduction
In 2020, approximately 1 out of 13 people suffered from chronic low back pain (CLBP), equating to 619 million people worldwide. This prevalence is rising swiftly; a 60% increase compared to 1990 is documented, and a further rise to an estimated 843 million by 2050 is expected [1]. In high-income countries, CLBP is even more prevalent compared to middle- or low-income countries (32.9 ± 10.0 vs. 25.4 ± 18.3 or 16.7 ± 15.7); this difference is speculated to be attributable to less access to health insurance/healthcare, higher levels of exercise, shorter height, and higher pain thresholds [2]. The pathophysiology causing this CLBP is most often non-malignant. However, the incidence, survival, and prevalence of oncologic disease are increasing, resulting in more patients at risk for developing back pain caused by spinal metastases (SM). SM were often associated with a poor outcome, but due to the rapidly evolving systemic, radiotherapeutic, and surgical treatment modalities, the survival of oncologic patients with or without SM is improving [3,4,5]. Oncologic back pain, infection, inflammation, and trauma are the only specific etiologies of CLBP in contrast to most patients who have non-specific CLBP. In oncologic patients developing CLBP, it is critically important to perform further investigation to exclude SM.
SM can be asymptomatic; nonetheless, in most cases, they are a cause of CLBP. Moreover, SM can lead to spinal instability, with mechanical pain with or without pathologic vertebral compression fractures (pVCF) (12.6% of SM) or metastatic spinal cord compression (MESCC) (9.6% of SM). Both pVCF and MESCC are associated with significant morbidity and are highly impactful on patients’ quality of life (QoL). Despite this high symptomatic burden, the delay between first symptoms and diagnosis is often large (mean duration 66 days from the point at which the patient reported their first relevant symptom to a health professional, interquartile range 37–205 days) [6].
The purpose of this narrative review is to highlight the importance of prompt diagnosis of SM in this overview of the epidemiology, symptomatology, pathophysiology, and treatment modalities of back pain resulting from SM. We describe SM-related symptomatology in three main categories: tumor pain, mechanical instability, and MESCC.
2. Epidemiology
The World Health Organization (WHO) reports the worldwide incidence of cancer has increased by 50% over the last decade to an estimated 18 million new cases in 2018: 11.7% of all cancers being breast carcinoma (2.26 million), 7.3% prostate carcinoma (1.41 million), and 11.4% lung carcinoma (2.21 million). The WHO predicts an exponential growth of cancer, with nearly 30 million new cases in 2040 and an estimated prevalence of 80 million patients with cancer [7]. One out of six oncologic patients suffers from SM, according to clinical data; in contrast, in the end-of-life setting, even 30% of these patients have SM [5]. Combining these data, we estimate that there will be between 13.3 and 27 million patients with SM in 2040. Compared to the global incidence of CLBP of 619 million patients, there are 2–4 patients with SM for every 100 patients with CLBP. Almost two-thirds of these SM occur in patients with breast, prostate, or lung cancer [8,9,10]. Regional differences exist in the frequency of these primary tumors [7].
SM can be asymptomatic, cause biological pain, or result in complicated SM with either MESCC or pVCF (or a combination of these). Overall, 1 out of 8 SM causes pVCF and nearly 10% result in MESCC. In 2040, an estimated 1.33–2.7 million will have MESCC and 1.66–3.37 million will experience pVCF. Differences between primary tumor types are observed. In breast carcinoma patients, the incidence of pVCF is significantly larger compared to prostate carcinoma patients (16.6% vs. 7.7%) (Figure 1) [5]. This can be explained by the type of bone metastases; in breast cancer patients, they are typically osteolytic; in contrast, prostate carcinoma tends to lead to osteoblastic lesions [11]. In MESCC, no significant difference between primary tumor types is observed.
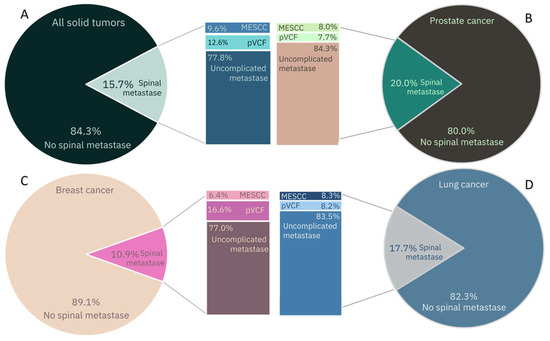
Figure 1.
Epidemiology of spinal metastases, metastatic epidural spinal cord compression (MESCC), pathologic vertebral compression fractures (pVCF) in solid tumors. (A) All solid tumors. (B) Lung cancer. (C) Breast cancer. (D) Prostate cancer [5].
3. Symptomatology and Pathophysiology
SM as diagnosed on medical imaging can be asymptomatic in approximately 25% [12]. In the case of symptomatic SM, we can distinguish three main categories: tumor pain; mechanical pain due to instability, with or without pathologic fractures; and MESCC or radicular compression.
3.1. Tumor Pain
The pathophysiology of biological pain resulting from SM is a complex and multifaceted process in which the pain is not proportional to the number or size of the SM [13,14].
The bone is innervated by thin myelinated, A-δ, or unmyelinated C sensory nerves. The periosteum is the most densely innervated, by a factor 50 times denser compared to the bone marrow and 1000 times denser compared to calcified bone. Tumor cells and the inflammatory response release nerve growth factor (NGF), which promotes pathological sprouting of the sensory nerves leading to a 10- to 70-fold increase in the density of the nerve fibers and the formation of neuroma-like structures within them [12,15].
The tumor cells release a multitude of cytokines and mediators that recruit and activate inflammatory cells (T-cells, mast cells, macrophage, stromal cells); disrupt the equilibrium of osteoclasts (receptor activator of nuclear factor kappa-Β ligand (RANKL)) and osteoblasts (endothelin (ET)); and activate the nociceptive fibers of the sensory nervous system (prostaglandin E2 (PGE2), adenosine triphosphate (ATP), lactic acid and H+, ET, tumor necrosis factor (TNF) alfa, interleukin (IL) 6, -8, -15,-16, NGF, bradykinin, …), causing the pain sensation.
The activation of the inflammatory cells promotes additional release of these cytokines and mediators, resulting in secondary activation of the nociceptive fibers, leading to an increase in pain. Due to the natural circadian cortisol fluctuations, the low cortisol levels at night result in increased activity of the inflammatory cells and increased swelling surrounding the SM, causing an increase in pain with the typical nightly back pain in SM [12].
The release of RANKL, and to a lesser extent H+, activates osteoclast activity, causing a decrease in bone mineralization and impaired bone strength. The increased osteoclast activity leads to a local acidotic effect by the Warburg mechanism, protecting the osteoclasts from intracellular acidosis by releasing H+ and lactate. This leads to further stimulation of the nociceptive fibers [12,16].
The combination of these mechanisms leads to an increase in sensory nerve density, and activation of these results in the pain sensation.
3.2. Mechanical Pain Due to Instability with or without Pathologic Fractures
Pathologic vertebral compression fractures (pVCF) and spinal instability (without fracture) are strongly associated with pain and have a large, negative influence on the quality of life (QoL) and activity of patients [17].
The spinal instability neoplastic score (SINS) (Table 1) was developed to facilitate the estimation of fracture or instability risk. The SINS ameliorates the communication between different care providers and has great interrater reliability [18,19]. This scoring system uses six parameters to determine the risk of instability: two regarding the localization, the level of the lesion and the posterolateral involvement; one on vertebral body collapse or large involvement; one on the alignment; one on the presence of mechanical pain or occasional pain; and the last one on the type of the lesion. Lytic lesions are associated with a higher score and, thus, a higher risk of instability compared to mixed or blastic lesions [19].

Table 1.
Spinal instability neoplastic score [19].
SM disturb the equilibrium in the complex and interconnected process of bone formation; this can lead to an excessive proliferation and maturation of osteoclasts, leading to a loss of mineralization and the development of osteolytic lesions. These processes form a complex vicious cycle, with tumor growth and bone resorption stimulating each other [11]. Lytic lesions, zones with reduced bone mineral density (BMD), are associated with reduced bone strength and increased risk for fracture [20,21,22].
The SINS is well correlated with pain and mobility. Out of all parameters measured by SINS, mechanical pain has the highest correlation with pain and QoL [23].
3.3. Metastatic Epidural Spinal Cord Compression or Radicular Compression
MESCC occurs when cancer metastases in the spine grow into the epidural space and cause compression of the spinal cord. The degree of MESCC is described by the Bilsky grade 0 to 3, with 2 and 3 defined as high-grade and 1a, 1b, and 1c as low-grade. The intra- and interrater variability of this scale is excellent on T2-weighted magnetic resonance images [24].
Back pain is the most common initial symptom of SM and may be observed in as many as 88–94% of patients at the time of diagnosis. MESCC can lead to radicular pain due to the radicular compression (50% as the initial symptom and close to 80% at diagnosis) or more specific symptoms due to spinal cord compression, often with an insidious onset; ataxia (67%) is a more frequently presenting symptom compared to motor weakness, which is only the initial symptom in 40%, but close to 90% at the time of diagnosis. Sensory disturbances (30% to 75%) and/or bladder dysfunction (5 to 60% at diagnosis) may be other presenting symptoms of MESCC. Often, symptoms are slowly progressive, and patients may only seek medical assistance or get diagnosed when their mobility is affected despite experiencing symptoms for several weeks or even months [6,25,26,27].
The interval from the first symptoms reported to a health professional to the diagnosis of MESCC often seems too long (mean duration 66 days) [6]. Multiple studies report that approximately one-third of MESCC patients lose their ambulatory function [5]. The proportion of neurologically intact patients with MESCC decreased from 94% at three months after radiographic diagnosis to 43% at two years [28]. One may conclude that if untreated, virtually all patients with MESCC will eventually develop a neurological deficit. This loss of independence has a substantial reduction in quality of life (QoL) both in patients who are non-ambulators or with assistance, 88% and 22%, respectively. Independent ambulatory patients with SM have a QoL similar to those without SM [29].
Spinal cord injury (SCI) from MESCC is characterized by a (slow) developing chronic compression resulting in several pathophysiological processes, in contrast to an acute SCI caused by a sudden trauma. Direct cord compression and epidural venous plexus obstruction lead to compressive vasogenic edema in the spinal cord. Chronic spinal cord compression disrupts the blood–spinal cord barrier [30] (the functional equivalent of the blood–brain barrier), leading to a change in the osmotic gradient that results in increased ion and protein transport, leading to perivascular swelling and edema. The compression also induces an inflammatory response. These processes can lead to acute vascular events with ischemia and infarction, resulting in more rapidly progressive loss of function [31,32].
4. Treatment of Spinal Metastases-Related Pain
As a consequence of the rapid evolution of medical advances in oncologic therapies, treatment algorithms for SM become obsolete within a few years of their creation, even before widespread implementation is reached. To overcome these shortcomings, the NOMS (neurologic, oncologic, mechanical, and systemic) framework was created. In contrast to algorithms, this framework is a dynamic decision framework that incorporates a neurologic, oncologic, mechanical, and systemic assessment of patients to guide us to optimal treatment approaches. New techniques and treatments are easily incorporated into this framework [33,34,35]. Elaborating on all aspects of this multidisciplinary framework will go beyond the scope of this review. We will focus on pain-related treatments in light of the three main categories of pain in SM (Figure 2).
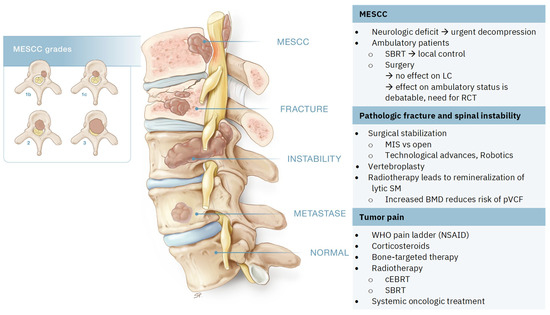
Figure 2.
Spinal metastases (SM) and the three main categories of pain. Metastatic epidural spinal cord compression (MESCC) grades described by Bilsky et al. [24]: grades 2 and 3 are considered high grade, grade 1b and 1c are low grade. SBRT: stereotactic body radiation therapy, LC: local control, RCT: randomized controlled trial, MIS: minimal invasive surgery, BMD: bone mineral density, pVCF: pathologic vertebral compression fractures, WHO: World Health Organization, NSAID: non-steroidal anti-inflammatory drugs, cEBRT: conventional external beam radiation therapy.
4.1. Tumor Pain
The treatment of tumor pain in SM is multimodal, with analgesics, anti-inflammatory medication, and bone-targeted therapy to increase bone quality or reduce the pathologic increase in the density of nerve fibers. Furthermore, this symptomatic treatment, an anticancer treatment that includes local treatment such as radiotherapy or surgery as well as systemic treatment, has an important role in the treatment of tumor pain.
The WHO pain ladder should be used to reduce the pain quickly at rest as well as during movement. The use of analgesics does not reduce the risk for skeletal-related events (SREs). Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit PGE2 synthesis via cyclooxygenases (COX), resulting in a reduction in PG-induced sensitization. Therefore, they are considered more useful because local inflammation is an important factor in the pathogenesis of SM-related pain. However, the evidence supporting this clinical experience is limited [31,36,37,38,39].
On the other hand, opioids are efficacious against neuropathic, nociceptive, and mixed pain. Various opioids with different pharmacological properties exist; there is not one superior to another in terms of efficacy [38,39]. With the use of opioids comes the risk of tachyphylaxis. This results in an increasing need for opioids to achieve the same analgesic effect. Long-term use of opioids can lead to addiction [40]. Therefore, we must be careful to preserve these medications for patients with severe pain refractory to other analgesics. It is important to be cognizant of the potential neuropsychiatric effects that opioids can have on an individual, especially for those under palliative care. By having these understandings, patient quality of life can be improved, healthcare system costs can be decreased, and patient outcomes can be met and exceeded [40].
Corticosteroids exert potent anti-swelling and anti-inflammatory effects. On one hand, the reduction in perilesional edema leads to an improvement in analgesia. On the other hand, they influence the nociceptor activation by reducing the level of pro-inflammatory cytokines and directly decreasing the pathological electrical activity of damaged peripheral nociceptive fibers; these mechanisms result in a decrease in pain intensity. The WHO strongly recommends the use of adjuvant corticosteroids in adults and adolescents with cancer-related pain to achieve pain control based on moderate-quality evidence [39]. A Cochrane database systematic review concluded that the evidence for the efficacy of corticosteroids to achieve pain control in oncologic patients is weak, despite significant pain relief that has been described in multiple studies [41]. Long-term use of corticosteroids can induce several important side effects. Therefore, the use of corticosteroids should be limited to a short period, sufficient to achieve pain control before further treatment, such as radiotherapy, can be initiated.
4.1.1. Bone-Targeted Therapy
The benefit of bisphosphonates (BPs) is well proven, with significant pain prevention and reduced risk of skeletal-related events (SREs) [42,43]. BPs exert a direct apoptotic effect on osteoclasts and lead to inhibition of the development of inflammation surrounding the tumor. The WHO recommends the use of BPs to prevent and treat bone pain in adults with cancer [39].
Denosumab is a human, monoclonal synthetic antibody that inhibits the tumor-induced proliferation and maturation of osteoclasts by binding to RANKL to prevent its interaction with RANK. It delays SREs and prevents the recurrence of bone pain. Denosumab reduces the risk of SREs and improves functional outcomes more than BPs but comes with an increased risk of osteonecrosis of the jaw [44,45].
The analgesic role of Denosumab and BPs is debatable; nonetheless, these medications help to prevent pain by reducing the risk of SREs and delaying the onset of bone pain [46].
Pre-clinical studies demonstrated the ability of Tanezumab, a recombinant humanized monoclonal antibody, to bind NGF to prevent the increased synthesis of pronociceptive substances and tumor-induced sprouting and/or formation of neuroma-like structures; by these processes, it reduces the pain sensation [47]. Clinical data on the efficacy in cancer-induced pain are sparse, with only one randomized-controlled trial (RCT) comparing Tanezumab with a placebo. This study failed to show a significant difference in pain relief between these groups. In the subgroup of patients with higher baseline pain and lower total opioid use, there was a significant improvement in pain at 8 weeks [48,49]. Nonetheless, because of the limited evidence, the WHO experts have not yet been able to recommend or not recommend Tanezumab; to this date, there is no FDA (Food and Drug Administration) or EMA (European Medicines Agency) approval for Tanezumab.
4.1.2. Radiotherapy
Radiotherapy is the most important local oncologic treatment modality in the treatment of tumor pain in SM. Surgery should be reserved for spinal instability, pathologic fractures, and symptomatic spinal cord or radicular compression.
Conventional external beam radiation therapy (cEBRT) is the most widespread, easily available local treatment for SM. Mostly a single dose of 8 Gy is delivered. During the last decades, the use of stereotactic body radiotherapy (SBRT) for spinal lesions has emerged. SBRT allows higher doses to the lesion while sparing the surrounding tissues and organs at risk; thus, a higher effective dose can be delivered without increased toxicity to healthy tissue. SBRT results in an increase in local control (LC) [50,51]. The results of RCTs on the pain response of cEBRT and SBRT are conflicting. Bindels et al. recently published a systematic review and meta-analysis comparing SBRT and cEBRT for painful SM. cEBRT leads to a pain response (decline of at least 2 points on an 11-point scale without an increase in opioid use) in 52% (41–64%) compared to 62% (55–68%), RR 1.22 (95% CI 0.96–1.54). There was a significant benefit for SBRT over cEBRT in complete pain response (pain score = 0) (RR 2.47 (95% CI 1.24–4.91). Further research is needed to study the associations of specific dose regimens and could be used to help identify what subgroups benefit from SBRT [52].
4.2. Mechanical Instability with or without Pathologic Fractures
The benefit of surgery in patients with proven instability (SINS > 13 and SINS 7–12 with mechanical pain) is well established. Surgical stabilization of lesions with SINS ≤ 6 is not indicated since there is no benefit regarding pain, QoL, or activity [17].
During the last decades, there has been a tendency towards minimal invasive surgery (MIS). Technological advances, such as computed tomography (CT)-navigated screw placement and more recently robot-assisted screw placement [53,54], are extremely helpful in the evolution of safe and MIS techniques. The use of robotic systems leads to safer placement of thoracic pedicle screws [55]. If a posterior fusion is performed, this most often includes two levels above and two below. In non-junctional levels, short constructions (cement augmented one level above and one below) show a similarly low rate of material failure compared to the more conventional two above and two below [56]. These short constructs can lead to less invasive surgery, reducing the risk of wound complications. After MIS, wound complication rates are reduced significantly compared to open procedures (6.6% vs. 11.5%; p < 0.05) [57].
Percutaneous vertebroplasty is an even less invasive technique. In selected cases, vertebroplasty is associated with rapid pain relief, starting immediately after the procedure, which is sustained in follow-up [58].
Multiple studies compared open and MIS techniques. Regarding pain relief, there is no significant difference between open and MIS. MIS is associated with reduced blood loss, lower rate of wound complications, and shorter length of stay [59]. In oncologic patients, these benefits are important, since this facilitates a swift start of systemic oncologic treatment and/or post-operative radiotherapy, leading to improved local control [60].
Despite all of these technical improvements. The result of surgery is strongly dependent on the indication. In the case of mechanical pain or proven instability, surgical stabilization leads to improvement in pain, QoL, and activity. In the absence of mechanical pain or instability, surgery has no benefit regarding these outcomes.
The risk of surgery is increased in oncologic patients due to a nutritionally depleted status, comorbidities, and/or potential side effects of systemic therapy. Recently, two systematic reviews examined the surgical complications in degenerative spine surgery [61] and surgery for SM [62]. In surgery for SM, the prevalence of surgical site infections (SSI) is doubled compared to degenerative spine surgery (6.5% (135/2088) vs. 3.1% (603/22475)). These increased risks and consequences must be taken into account when deciding if the burden of surgery would be beneficial for the individual patient.
In the absence of proven instability or mechanical pain, there is no indication for surgical treatment. In the case of lytic lesions, radiotherapy can improve bone mineral density (BMD), resulting in improved bone strength. In contrast to the benefit of SBRT over cEBRT in local control and maybe also pain response, there is no benefit of SBRT over cEBRT in this process of remineralization. The remineralization is the largest in lytic SM in breast cancer. The only exceptions are lytic SM of renal cell carcinoma; there is no remineralization of renal lytic SM after radiation therapy. These findings should be taken into account when defining a surgical strategy in the potentially unstable group, as defined by the SINS [63].
4.3. Metastatic Epidural Spinal Cord Compression or Radicular Compression
If MESCC leads to a neurologic deficit, the benefit of surgery is well proven. Multiple studies have demonstrated that early intervention improves the odds of functional recovery and that being ambulatory before treatment is the best prognostic factor for retaining ambulatory function [64]. The landmark paper by Patchell et al. concluded that surgery followed by cEBRT was superior compared to cEBRT in maintaining ambulatory function [65]. As the sole RCT on this topic, the impact of this study on the number of surgeries for SM was significant [66].
Especially with the promising results of SBRT, the indication for surgery is debatable in the absence of a neurologic deficit or spinal instability. The effect of surgery can be measured in terms of local control, ambulatory function, quality of life, and complications. A recent meta-analysis demonstrated excellent local control rates of 86% (95% CI 84–88%) for SBRT compared to 60% (95% CI 60–69%) for cEBRT (p < 0.05) in MESCC from solid primary tumors. The effect of surgery on a 1-year LC (0.89 (95% CI 0.66–1.20)) was non-significantly different; therefore, we can conclude that not surgery but radiotherapy, in particular SBRT, provides durable LC [51]. The benefit of surgery to retain ambulatory function was not confirmed by other studies; overall, the effect of surgery on ambulatory function was non-significant (OR 1.51 (95% CI 0.83–274)). Regarding QoL, there seems to be a benefit for surgery; nonetheless, SBRT alone provides significant improvement in pain/discomfort, mobility, and usual activity; this improvement in QoL is preserved for up to 5 years [67]. The risk of complications in surgical treatment exceeds this risk in SBRT (and cEBRT) [51].
Recently, Patel et al. demonstrated excellent local control and functional outcomes in 143 patients with MESCC treated by SBRT alone, suggesting that SBRT is a reasonable approach in inoperable patients or cases unable to be successfully surgically downgraded [68]. Surgery has an important role but does not improve local control or survival in the absence of instability or neurologic deficit. In the case of high-grade MESCC in the absence of a neurologic deficit, the role of surgery is debatable, as some studies demonstrate good LC for SBRT without preceding surgery. A randomized study comparing surgery followed by SBRT and SBRT alone is needed to determine if the burden of surgery provides additional benefits for patients with MESCC [51].
5. Discussion
Quality of life (QoL) has emerged as a primordial outcome parameter in the evaluation of treatment effects. Quality of life (QOL) and length of life (LOL) are intertwined outcome parameters, as QOL has been demonstrated as a prognostic factor for survival [69,70]. Oncologic patients and their caregivers face difficult decisions regarding different treatment options and their respective impact on QOL and LOL. In elderly patients, QOL is often preferred above LOL, whereas in younger patients, aggressive treatment protocols tend to be chosen to increase LOL [71]. Since curative treatment is not possible in metastatic disease with SM, QoL is the driving force in deciding on treatment plans. The burden of treatment should be weighted to the benefit.
The care for SM is a multidisciplinary concern. It is of primordial importance to incorporate the knowledge of specialists in all participating disciplines, such as oncology, radiotherapy, and spinal surgery, to determine the adequate treatment to preserve ambulatory function and QoL while limiting the burden of treatment if possible. Timely diagnosis and subsequent adequate treatment can prevent further deterioration, improve pain, and retain ambulatory function in these patients.
Awareness of potential SM and defining the type of pain (biological, mechanical, and/or radicular (MESCC)) for each involved vertebra is the first and most important step in the treatment of SM-related pain. Early diagnosis and timely treatment could prevent further deterioration, for example, Denosumab or bisphosphonates preventing the progression of lytic SM and pathologic fractures; timely radiotherapy providing local control, preventing symptomatic spinal cord compression and leading to remineralization of lytic SM; or timely surgical stabilization preventing significant pathologic fractures with invalidating pain or neurologic deficit or urgent surgery to decompress the spinal cord in cases of neurologic deficit or gait disturbances.
Awareness of SM in patients with back pain prevents a delay in diagnosis and subsequent treatment. A swift start in adequate treatment prevents further deterioration and ameliorates the QoL.
6. Conclusions
SM-related pain can be described in three main categories: tumor pain, mechanical pain, and MESCC. Awareness of SM in oncologic patients with back pain is of primordial importance. This prevents a delay in diagnosis and subsequent treatment. A swift diagnosis and start of adequate treatment can prevent further deterioration and ameliorate the QoL. Systemic therapy and bone-modifying agents are important in the prevention of symptomatic SM or SRE. Radiotherapy leads to local control, pain control, and remineralization of lytic lesions. Surgery has an important place in treating instability and in urgent decompression in cases of neurologic deficit in MESCC. The care for patients with SM is a multidisciplinary concern due to the rapid evolutions in all disciplines, which need to be incorporated in defining the best treatment strategy.
Author Contributions
Conceptualization, R.V.d.B. and E.V.d.K.; methodology, R.V.d.B.; formal analysis, R.V.d.B.; investigation, R.V.d.B.; data curation, R.V.d.B.; writing—original draft preparation, R.V.d.B.; writing—review and editing, R.V.d.B., C.B., M.P. and E.V.d.K.; visualization, R.V.d.B.; supervision, E.V.d.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Stephanie Philippaerts—https://www.spmedical-illustration.com/ accessed on 1 July 2024—for the graphic illustration of Figure 2.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organisation. WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults in Primary and Community Care Settings; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- WHO Report on Cancer Setting Priorities, Investing Wisely and Providing Care for All 2020 WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. 2020. Available online: http://apps.who.int/bookorders (accessed on 1 June 2024).
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M. Global surveillance of trends in cancer survival: Analysis of individual records for 37,513,025 patients di-agnosed with one of 18 cncers during 2000–2014 from 322 populatio-based registries in 71 countries (CONCORD-3). Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Brande, R.V.D.; Cornips, E.M.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef] [PubMed]
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. “Don’t wait for a sensory level-Listen to the symptoms: A prospective audit of the delays in diagnosis of malignant cord compression. Clin. Oncol. 2002, 14, 472–480. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Svensson, E.; Christiansen, C.F.; Ulrichsen, S.P.; Rørth, M.R.; Sørensen, H.T. Survival after bone metastasis by primary cancer type: A Danish population-based cohort study. BMJ Open 2017, 7, e016022. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef]
- Phanphaisarn, A.; Patumanond, J.; Settakorn, J.; Chaiyawat, P.; Klangjorhor, J.; Pruksakorn, D. Prevalence and survival patterns of patients with bone metastasis from common cancers in thailand. Asian Pac. J. Cancer 2016, 17, 4335–4340. [Google Scholar]
- Schmid-Alliana, A.; Schmid-Antomarchi, H.; Al-Sahlanee, R.; Lagadec, P.; Scimeca, J.-C.; Verron, E. Understanding the Progression of Bone Metastases to Identify Novel Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wordliczek, J. Bone Pain in Cancer Patients: Mechanisms and Current Treatment. Int. J. Mol. Sci. 2019, 20, 6047. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Pellegrino, C.; Mangini, M.; Fontana, F.; Piacentino, F.; Recaldini, C.; Rovera, F.; Dionigi, G.; Boni, L.; et al. Ablation of painful metastatic bone tumors: A systematic review. Int. J. Surg. 2008, 6, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Pawinski, A.; Dalhaug, A. Continuous controversy about radiation oncologists’ choice of treatment regimens for bone metastases: Should we blame doctors, cancer-related features, or design of previous clinical studies? Radiat. Oncol. 2013, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017, 36, 108. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F. Acidic microenvironment and bone pain in cancer-colonized bone. BoneKEy Rep. 2015, 4, 690. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Barzilai, O.; Reiner, A.S.; DiStefano, N.; McLaughlin, L.; Ogilvie, S.; Bilsky, M.; Laufer, I. Patient-reported outcomes after surgical stabilization of spinal tumors: Symptom-based validation of the Spinal Instability Neoplastic Score (SINS) and surgery. Spine J. 2018, 18, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ahmed, A.K.; Cottrill, E.; Westbroek, E.M.; Goodwin, M.L.; Sciubba, D.M. Intra- and interobserver reliability of the Spinal Instability Neoplastic Score system for instability in spine metastases: A systematic review and meta-analysis. Ann. Transl. Med. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the spine oncology study group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T.; Roos, B.; Nachemson, A. The Bone Mineral Content and Ultimate Compressive Strength of Lumbar Vertebrae. Spine 1980, 5, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kraxenberger, M.; Schröder, C.; Geith, T.; Büttner, A.; von Schulze-Pellengahr, C.; Birkenmaier, C.; Müller, P.E.; Jansson, V.; Wegener, B. Fracture generation in human vertebrae under compression loading: The influence of pedicle preservation and bone mineral density on in vitro fracture behavior. Technol. Health Care 2018, 26, 155–163. [Google Scholar] [CrossRef]
- Cummings, S.R.; Karpf, D.B.; Harris, F.; Genant, H.K.; Ensrud, K.; LaCroix, A.Z.; Black, D.M. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am. J. Med. 2002, 112, 281–289. [Google Scholar] [CrossRef]
- Versteeg, A.L.; Sahgal, A.; Laufer, I.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; Boriani, S.; Bettegowda, C.; et al. Correlation between the Spinal Instability Neoplastic Score (SINS) and Patient Reported Outcomes. Glob. Spine J. 2021, 13, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Kutka, M.; Lees, K.; Abson, C.; Hadaki, M.; Cooke, D.; Neill, C.; Sheriff, M.; Karathanasi, A.; Boussios, S. Management of metastatic spinal cord compression in secondary care: A practice reflection from medway maritime hospital, Kent, UK. J. Pers. Med. 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Helweg-Larsen, S.; Sørensen, P.S. Symptoms and signs in metastatic spinal cord compression: A study of progression from first symptom until diagnosis in 153 patients. Eur. J. Cancer 1994, 30, 396–398. [Google Scholar] [CrossRef]
- Bach, F.; Larsen, B.H.; Rohde, K.; Gjerris, F.; Agerlin, N.; Rasmusson, B.; Stjernholm, P.; Børgesen, S.E.; Bøge-Rasmussen, T.; Sørensen, P.S. Metastatic spinal cord compression-Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir. 1990, 107, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, R.; Sohaib, S.; Barbachano, Y.; Parker, C.; Huddart, R.; Horwich, A.; Dearnaley, D. Frequency of Screening Magnetic Resonance Imaging to Detect Occult Spinal Cord Compromise and to Prevent Neurological Deficit in Metastatic Castration-resistant Prostate Cancer. Clin. Oncol. 2010, 22, 147–152. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.M.; Yeung, C.M.; Tobert, D.G.; Nguyen, L.M.; Passias, P.G.; Shin, J.H.; Kang, J.D.; Ferrone, M.L. Characterizing Health-Related Quality of Life by Ambulatory Status in Patients with Spinal Metastases. Spine 2022, 47, 99–104. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood–spinal cord barrier: Morphology and Clinical Implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef]
- Urch, C. The pathophysiology of cancer-induced bone pain: Current understanding. Palliat. Med. 2004, 18, 267–274. [Google Scholar] [CrossRef]
- Long, H.-Q.; Ren, Z.-X.; Xu, J.-H.; Cheng, X.; Xu, G.-X. Pathophysiological mechanisms of chronic compressive spinal cord injury due to vascular events. Neural Regen. Res. 2023, 18, 790–796. [Google Scholar] [CrossRef]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.C.; Bilsky, M.H. Fifty-year history of the evolution of spinal metastatic disease management. J. Surg. Oncol. 2022, 126, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.C.; Laufer, I.; Bilsky, M.H. Neurologic, Oncologic, Mechanical, and Systemic and Other Decision Frameworks for Spinal Disease. Neurosurg. Clin. N. Am. 2020, 31, 151–166. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) for cancer pain in adults. Cochrane Database Syst. Rev. 2017, 2020, CD012638. [Google Scholar] [CrossRef]
- Isono, M.; Suzuki, T.; Hosono, K.; Hayashi, I.; Sakagami, H.; Uematsu, S.; Akira, S.; DeClerck, Y.A.; Okamoto, H.; Majima, M. Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci. 2011, 88, 693–700. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Kaye, A.D.; Dufrene, K.; Cooley, J.; Walker, M.; Shah, S.; Hollander, A.; Shekoohi, S.; Robinson, C.L. Neuropsychiatric Effects Associated with Opioid-Based Management for Palliative Care Patients. Curr. Pain Headache Rep. 2024, 28, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Haywood, A.; Good, P.; Khan, S.; Leupp, A.; Jenkins-Marsh, S.; Rickett, K.; Hardy, J.R. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database Syst. Rev. 2015, 2021, CD010756. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 2018, CD003474. [Google Scholar] [CrossRef]
- Ross, J.R.; Saunders, Y.; Edmonds, P.M.; Patel, S.; Broadley, K.E.; Johnston, S.R.D. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 2003, 327, 469. [Google Scholar] [CrossRef]
- Lipton, A.; Steger, G.G.; Figueroa, J.; Alvarado, C.; Solal-Celigny, P.; Body, J.J.; de Boer, R.; Berardi, R.; Gascon, P.; Tonkin, K.S.; et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin. Cancer Res. 2008, 14, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cui, X.; Ma, H.; Tang, X. Comparison of denosumab and zoledronic acid for the treatment of solid tumors and multiple myeloma with bone metastasis: A systematic review and meta-analysis based on randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Porta-Sales, J.; Garzón-Rodríguez, C.; Llorens-Torromé, S.; Brunelli, C.; Pigni, A.; Caraceni, A. Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: A systematic review within the European Association for Palliative Care guidelines project. Palliat. Med. 2016, 31, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, W.; Jimenez-Andrade, J.; Stake, J.; Bloom, A.; Kaczmarska, M.; Taylor, R.N.; Freeman, K.; Ghilardi, J.; Kuskowski, M.; Mantyh, P. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010, 171, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Sopata, M.; Katz, N.; Carey, W.; Smith, M.D.; Keller, D.; Verburg, K.M.; West, C.R.; Wolfram, G.; Brown, M.T. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015, 156, 1703–1713. [Google Scholar] [CrossRef]
- Urman, R.; Patel, M.; Kaye, A. Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 111–116. [Google Scholar] [CrossRef]
- Zeng, K.L.; Myrehaug, S.; Soliman, H.; Husain, Z.A.; Tseng, C.-L.; Detsky, J.; Ruschin, M.; Atenafu, E.G.; Witiw, C.D.; Larouche, J.; et al. Mature Local Control and Reirradiation Rates Comparing Spine Stereotactic Body Radiation Therapy With Conventional Palliative External Beam Radiation Therapy. Int. J. Radiat. Oncol. 2022, 114, 293–300. [Google Scholar] [CrossRef]
- Brande, R.V.D.; Thijs, D.; Bilsky, M.; Peeters, M.; Billiet, C.; Van de Kelft, E. Treatment of ambulatory patients with metastatic epidural spinal cord compression: A systematic review and meta-analysis. J. Neurosurg. Spine 2023, 40, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bindels, B.J.J.; Mercier, C.; Gal, R.; Verlaan, J.-J.; Verhoeff, J.J.C.; Dirix, P.; Ost, P.; Kasperts, N.; van der Linden, Y.M.; Verkooijen, H.M.; et al. Stereotactic Body and Conventional Radiotherapy for Painful Bone Metastases. JAMA Netw. Open 2024, 7, e2355409. [Google Scholar] [CrossRef]
- Vardiman, A.B.; Wallace, D.J.; Crawford, N.R.; Riggleman, J.R.; Ahrendtsen, L.A.; Ledonio, C.G. Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J. Robot. Surg. 2020, 14, 409–413. [Google Scholar] [CrossRef]
- Huntsman, K.T.; Ahrendtsen, L.A.; Riggleman, J.R.; Ledonio, C.G. Robotic-assisted navigated minimally invasive pedicle screw placement in the first 100 cases at a single institution. J. Robot. Surg. 2020, 14, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Vardiman, A.B.; Wallace, D.J.; Booher, G.A.; Toossi, N.; Bucklen, B.S. Decreasing the Pedicle Screw Misplacement Rate in the Thoracic Spine with Robot-Guided Navigation. 2023. Available online: www.clinicalspinesurgery.com (accessed on 1 June 2024).
- Newman, W.C.; Amin, A.G.; Villavieja, J.; Laufer, I.; Bilsky, M.H.; Barzilai, O. Short-segment cement-augmented fixation in open separation surgery of metastatic epidural spinal cord compression: Initial experience. Neurosurg. Focus 2021, 50, E11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, N.; Tan, J.H.; Tan, J.H.; Thomas, A.C.; Thomas, A.C.; Tan, J.Y.H.; Tan, J.Y.H.; Madhu, S.; Madhu, S.; et al. The Utility of ‘Minimal Access and Separation Surgery’ in the Management of Metastatic Spine Disease. Glob. Spine J. 2022, 13, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.-S.; Chang, U.-K.; Youn, S.-M. Clinical Outcomes after Percutaneous Vertebroplasty for Pathologic Compression Fractures in Osteolytic Metastatic Spinal Disease. J. Korean Neurosurg. Soc. 2009, 45, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Vachtsevanos, L.; Cattell, A.; Ockendon, M.; Balain, B. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br. J. Neurosurg. 2017, 31, 526–530. [Google Scholar] [CrossRef]
- Blakaj, D.M.; Palmer, J.D.; Dibs, K.; Olausson, A.; Bourekas, E.C.; Boulter, D.; Ayan, A.S.; Cochran, E.; Marras, W.S.; Mageswaran, P.; et al. Postoperative Stereotactic Body Radiotherapy for Spinal Metastasis and Predictors of Local Control. Neurosurgery 2021, 88, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, R.; Huo, X.; Xiong, W.; Kang, L.; Xue, Y. Incidence of Surgical Site Infection after Spine Surgery: A Systematic Review and Meta-analysis. Spine 2020, 45, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, A.M.; Pasku, D.; Quraishi, N.A. Surgical complications and re-operation rates in spinal metastases surgery: A systematic review. Eur. Spine J. 2021, 30, 2791–2799. [Google Scholar] [CrossRef]
- Brande, R.V.D.; Kieboom, M.V.D.; Peeters, M.; Billiet, C.; Van de Kelft, E. Remineralization of lytic spinal metastases after radiation therapy–A retrospective cohort study comparing conventional external beam radiation therapy with stereotactic ablative body radiation. Clin. Transl. Radiat. Oncol. 2024, 48, 100805. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Hu, Y.-C.; Yang, X.-G.; Lun, D.-X.; Wang, F.; Yang, L.; Zhang, H.; Feng, J.-T.; Hua, K.-C. Prognostic Factors of Ambulatory Status for Patients with Metastatic Spinal Cord Compression: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, e278–e290. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.L.; Kshettry, V.R.; Rosenbaum, B.P.; Seicean, A.; Weil, R.J. Effect of a randomized controlled trial on the surgical treatment of spinal metastasis, 2000 through 2010: A population-based cohort study. Cancer 2014, 120, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Mantel, F.; Sweeney, R.A.; Hawkins, M.; Belderbos, J.; Ahmed, M.; Andratschke, N.; Madani, I.; Flentje, M. Long-Term Results of Dose-Intensified Fractionated Stereotactic Body Radiation Therapy (SBRT) for Painful Spinal Metastases. Int. J. Radiat. Oncol. 2021, 110, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.P.; Cao, Y.; Chen, X.; LeCompte, M.C.; Kleinberg, L.; Khan, M.; McNutt, T.; Bydon, A.; Kebaish, K.; Theodore, N.; et al. Oncologic and Functional Outcomes after Stereotactic Body Radiation Therapy for High-Grade Malignant Spinal Cord Compression. Adv. Radiat. Oncol. 2024, 9, 101327. [Google Scholar] [CrossRef] [PubMed]
- Kypriotakis, G.; Vidrine, D.J.; Francis, L.E.; Rose, J.H. The longitudinal relationship between quality of life and survival in advanced stage cancer. Psychooncology 2016, 25, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ediebah, D.E.; Coens, C.; Zikos, E.; Quinten, C.; Ringash, J.; King, M.T.; von Koch, J.S.; Gotay, C.; Greimel, E.; Flechtner, H.; et al. Does change in health-related quality of life score predict survival? Analysis of EORTC 08975 lung cancer trial. Br. J. Cancer 2014, 110, 2427–2433. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).