Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Definitions
2.2. Data Collection and Statistical Analysis
3. Results
3.1. General Subject Characteristics and Incidence of Acute Kidney Injury
3.2. Pre-Transplant Risk Factors for Developing AKI
3.3. Post-Transplant Risk Factors for Developing AKI
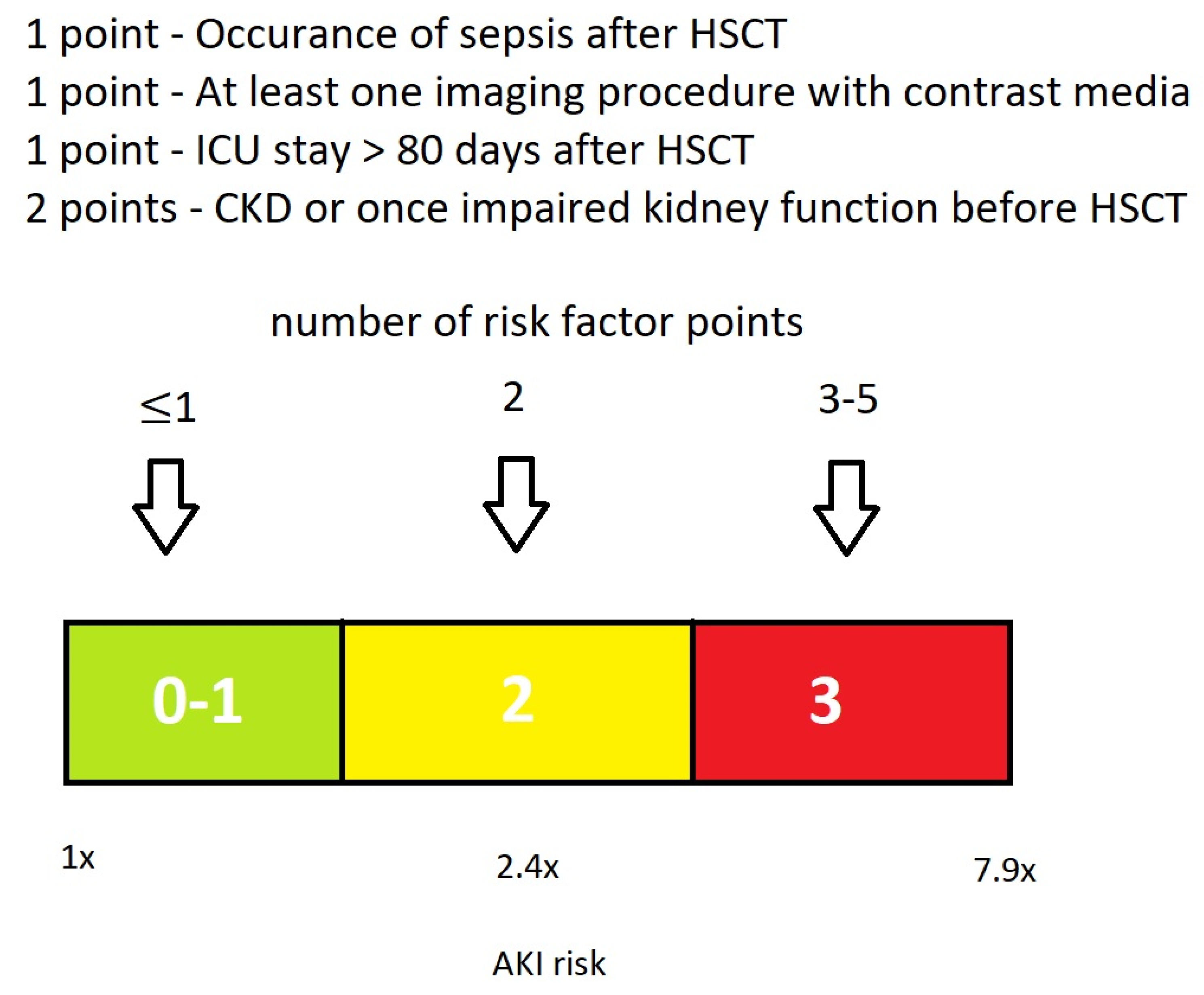
3.4. Proposal of a Basic EHRs Dataset and Score for the Calculation of HCT-AKIR
4. Discussion
4.1. Incidence of AKI
4.2. Multivariate Significant Risk Factors for AKI
4.2.1. Pre-Transplant CKD or Once-Impaired Kidney Function
4.2.2. Post-Transplant Sepsis
4.2.3. Post-Transplant ICU Stay and Imaging Procedures with Contrast Media
4.2.4. Proposal of a Basic Dataset for the Calculation of HCT-AKIR Score
4.2.5. Weaknesses and Strength of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majumdar, A. Sepsis-induced acute kidney injury. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2010, 14, 14–21. [Google Scholar] [CrossRef]
- Müller, L.P.; Müller-Tidow, C. The Indications for Allogeneic Stem Cell Transplantation in Myeloid Malignancies. Dtsch. Ärzteblatt Int. 2015, 112, 262–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henig, I.; Zuckerman, T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med. J. 2014, 5, e0028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caliskan, Y.; Besisik, S.K.; Sargin, D.; Ecder, T. Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2006, 38, 141–147. [Google Scholar] [CrossRef]
- Canet, E.; Zafrani, L.; Lambert, J.; Thieblemont, C.; Galicier, L.; Schnell, D.; Raffoux, E.; Lengline, E.; Chevret, S.; Darmon, M.; et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS ONE 2013, 8, e55870. [Google Scholar] [CrossRef] [PubMed]
- Kogon, A.; Hingorani, S. Acute kidney injury in hematopoietic cell transplantation. Semin. Nephrol. 2010, 30, 615–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes, J.A.; Jorge, S.; Neves, M. Acute kidney injury in HCT: An update. Bone Marrow Transplant. 2016, 51, 755–762. [Google Scholar] [CrossRef]
- Hingorani, S. Renal complications of hematopoietic-cell transplantation. N. Engl. J. Med. 2016, 374, 2256–2267. [Google Scholar] [CrossRef]
- Kersting, S.; Dorp, S.V.; Theobald, M.; Verdonck, L.F. Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol. Blood Marrow Transplant. 2008, 14, 125–131. [Google Scholar] [CrossRef][Green Version]
- Kersting, S.; Koomans, H.A.; Hené, R.J.; Verdonck, L.F. Acute renal failure after allogeneic myeloablative stem cell transplantation: Retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007, 39, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, V.; Gupta, M.; Manu, G.; Kwatra, S.; Owusu, O.-T.; Raina, R. Acute Kidney Injury in Hematopoietic Stem Cell Transplantation: A Review. Int. J. Nephrol. 2016, 2016, 5163789. [Google Scholar] [CrossRef] [PubMed]
- Mae, H.; Ooi, J.; Takahashi, S.; Tomonari, A.; Tsukada, N.; Konuma, T. Early renal injury after myeloablative cord blood transplantation in adults. Leuk. Lymphoma 2008, 49, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Yarlagadda, S.G.; Storer, B.; Sorror, M.; Storb, R.; Sandmaier, B. Impact of Acute Kidney Injury on Long Term Mortality after Nonmyeloablative Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2008, 14, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Saddadi, F.; Najafi, I.; Hakemi, M.S.; Falaknazi, K.; Attari, F.; Bahar, B. Frequency, risk factors, and outcome of acute kidney injury following bone marrow transplantation at Dr Shariati Hospital in Tehran. Iran. J. Kidney Dis. 2010, 4, 20–26. [Google Scholar] [PubMed]
- Fujii, T.; Uchino, S.; Takinami, M.; Bellomo, R. Validation of the Kidney Disease Improving Global Outcomes Criteria for AKI and Comparison of Three Criteria in Hospitalized Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S. Acute kidney injury following HCT: Incidence, risk factors and outcome. Bone Marrow Transplant. 2011, 46, 1399–1408. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Lopes, J.A.; Gonçalves, S.; Jorge, S.; Raimundo, M.; Resende, L.; Lourenço, F.; Lacerda, J.F.; Martins, C.; do Carmo, J.A.; Lacerda, J.M.F.; et al. Contemporary analysis of the influence of acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone Marrow Transplant. 2008, 42, 619–626. [Google Scholar] [CrossRef]
- Sun, J.; McNaughton, C.D.; Zhang, P.; Perer, A.; Gkoulalas-Divanis, A.; Denny, J.C.; Kirby, J.; Lasko, T.; Saip, A.; Malin, B.A. Predicting changes in hypertension control using electronic health records from a chronic disease management program. J. Am. Med. Inform. Assoc. 2013, 21, 337–344. [Google Scholar] [CrossRef]
- Khalilia, M.; Choi, M.; Henderson, A.; Iyengar, S.; Braunstein, M.; Sun, J. Clinical Predictive Modeling Development and Deployment through FHIR Web Services. AMIA Annu. Symp. Proc. 2015, 2015, 717–726. [Google Scholar] [PubMed] [PubMed Central]
- Amar, F.; April, A.; Abran, A. Electronic Health Record and Semantic Issues Using Fast Healthcare Interoperability Resources: Systematic Mapping Review. J. Med. Internet Res. 2024, 26, e45209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Canet, E.; Lengline, E.; Zafrani, L.; Peraldi, M.N.; Socié, G.; Azoulay, E. Acute kidney injury in critically ill allo-HSCT recipients. Bone Marrow Transplant. 2014, 49, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; Perez-Pitarch, A.; Garcia-Cadenas, I.; Barba, P.; Hernandez-Boluda, J.C.; Esquirol, A.; Fox, M.L.; Terol, M.J.; Queraltó, J.M.; Vima, J.; et al. A Time-to-Event Model for Acute Kidney Injury after Reduced-Intensity Conditioning Stem Cell Transplantation Using a Tacrolimus- and Sirolimus-based Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2017, 23, 1177–1185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ando, M.; Mori, J.; Ohashi, K.; Akiyama, H.; Morito, T.; Tsuchiya, K.; Nitta, K.; Sakamaki, H. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant. 2010, 45, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.S.; Xie, R.J.; Wang, M.; Feng, S.Z.; Han, M.Z. An evaluation of the RIFLE criteria for acute kidney injury after myeloablative allogeneic haematopoietic stem cell transplantation. Swiss Med. Wkly. 2011, 141, w13225. [Google Scholar] [CrossRef]
- Parikh, C.R.; McSweeney, P.A.; Korular, D.; Ecder, T.; Merouani, A.; Taylor, J.; Slat-Vasquez, V.; Shpall, E.J.; Jones, R.B.; Bearman, S.I.; et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002, 62, 566–573. [Google Scholar] [CrossRef]
- Piñana, J.L.; Valcárcel, D.; Martino, R.; Barba, P.; Moreno, E.; Sureda, A.; Vega, M.; Delgado, J.; Briones, J.; Brunet, S.; et al. Study of kidney function impairment after reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. A single-center experience. Biol. Blood Marrow Transplant. 2009, 15, 21–29. [Google Scholar] [CrossRef]
- Yegenaga, I.; Hoste, E.; Van Biesen, W.; Vanholder, R.; Benoit, D.; Kantarci, G.; Dhondt, A.; Colardyn, F.; Lameire, N. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: Results of a prospective study. Am. J. Kidney Dis. 2004, 43, 817–824. [Google Scholar] [CrossRef]
- Parikh, C.R.; Sandmaier, B.M.; Storb, R.F.; Blume, K.G.; Sahebi, F.; Maloney, D.G.; Maris, M.B.; Nieto, Y.; Edelstein, C.L.; Schrier, R.W.; et al. Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J. Am. Soc. Nephrol. 2004, 15, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Kagoya, Y.; Kataoka, K.; Nannya, Y.; Kurokawa, M. Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 394–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, C.Y.; Ordoñez, J.D.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Go, A.S. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rifkin, D.E.; Blantz, R.C. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin. J. Am. Soc. Nephrol. 2010, 5, 1690–1695. [Google Scholar] [CrossRef]
- Waikar, S.S.; Liu, K.D.; Chertow, G.M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 2008, 3, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.-F.; Liu, B.-C.; Ding, J.-H.; Chen, B.-A.; Xu, W.-L.; Qian, J. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant. 2010, 45, 153–158. [Google Scholar] [CrossRef]
- Mori, J.; Ohashi, K.; Yamaguchi, T.; Ando, M.; Hirashima, Y.; Kobayashi, T.; Kakihana, K.; Sakamaki, H. Risk assessment for acute kidney injury after allogeneic hematopoietic stem cell transplantation based on Acute Kidney Injury Network criteria. Intern. Med. 2012, 51, 2105–2110. [Google Scholar] [CrossRef]
- Sehgal, B.; George, P.; John, M.J.; Samuel, C. Acute kidney injury and mortality in hematopoietic stem cell transplantation: A single-center experience. Indian J. Nephrol. 2017, 27, 13–19. [Google Scholar] [CrossRef]
- Didsbury, M.S.; Mackie, F.E.; Kennedy, S.E. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatr. Transplant. 2015, 19, 460–470. [Google Scholar] [CrossRef]
- Yu, Z.P.; Ding, J.H.; Chen, B.A.; Liu, B.C.; Liu, H.; Li, Y.F.; Ding, B.H.; Qian, J. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin. J. Cancer 2010, 29, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Druml, W. Kontrastmittel-induzierte Nephropathie: Gibt es die überhaupt? Forum für Nephrologie und Hypertensiologie. Nephro-news (2):2-7. DtschArzteblInt 2015, 112, 262–270. 2017. Available online: https://medicom.cc/de/publikationen/nephro-news/201702/entries/01-Kontrastmittel-induzierte-Nephropathie.php (accessed on 12 June 2024).
- Gan, Z.; Chen, L.; Wu, M.; Liu, L.; Shi, L.; Li, Q.; Zhang, Z.; Lai, Y. Predicting the risk of acute kidney injury after hematopoietic stem cell transplantation: Development of a new predictive nomogram. Sci. Rep. 2022, 12, 15316. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Fragão-Marques, M.; Costa, C.; Branco, C.; Marques, F.; Vasconcelos, P.; Martins, C.; Leite-Moreira, A.; Lopes, J.A. Predictive Risk Score for Acute Kidney Injury in Hematopoietic Stem Cell Transplant. Cancers 2023, 15, 3720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sauer, C.M.; Chen, L.C.; Hyland, S.L.; Girbes, A.; Elbers, P.; Celi, L.A. Leveraging electronic health records for data science: Common pitfalls and how to avoid them. Lancet Digit. Health 2022, 4, e893–e898. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients (N) | Percentage (N = 312) | |
|---|---|---|---|
| Gender | male/female | 200/112 | 64.1%/35.9% |
| Mean age | 55.42 years (range 19–78) | ||
| Previous illnesses | aHT | 117 | 37.5% |
| DM | 51 | 16.3% | |
| Comorbidity score | 0 | 151 | 48.4% |
| 1–2 | 99 | 31.7% | |
| ≥3 | 62 | 19.9% | |
| Kidney function | Mean eGFR | 82.68 ± 12.53 mL/min/1.73 m2 | |
| CKD | 49 | 15.7% | |
| CKD stage 1 | 1 | 0.3% | |
| CKD stage 2 | 39 | 12.5% | |
| CKD stage 3 | 9 | 2.9% | |
| CKD stage 4 and 5 | 0 | 0% | |
| Ø CKD | 263 | 84.3% | |
| normal eGFR | 231 | 74% | |
| once-impaired kidney function or proteinuria | 32 | 10.3% | |
| Hematologic diseases | AML | 160 | 51.3% |
| MDS | 48 | 15.4% | |
| HL | 38 | 12.2% | |
| ALL | 25 | 8.0% | |
| CLL | 15 | 4.8% | |
| other hematologic diseases | 26 | 8.3% | |
| Conditioning | myeloablative | 116 | 37.2% |
| reduced intensity | 196 | 62.8% | |
| Conditioning therapy | only chemotherapy | 163 | 52.2% |
| ATG | 93 | 29.8% | |
| TBI 2 Gy | 31 | 9.9% | |
| TBI 8 Gy | 8 | 2.6% | |
| TBI 12 Gy | 12 | 3.8% | |
| RIT | 5 | 1.6% | |
| Stem cell source | PBSCT | 271 | 86.9% |
| Bone marrow | 41 | 13.1% | |
| HLA compatibility | 10/10 HLA matched | 227 | 72.8% |
| 9/10 HLA matched | 64 | 20.5% | |
| haploidentical | 21 | 6.7% | |
| Relation to donor | MSIB | 23 | 7.4% |
| MMSIB | 2 | 0.6% | |
| MUD | 204 | 65.4% | |
| MMUD | 62 | 19.9% | |
| Chemotherapy | All Subjects (%) | With AKI (%) | Without AKI (%) | p-Value |
|---|---|---|---|---|
| Cytarabin | 201 (64.4) | 129 (65.2) | 72 (63.2) | 0.799 |
| Daunorubicin | 126 (40.3) | 80 (40.4) | 46 (40.4) | 0.958 |
| Azacitidin | 81 (26) | 48 (24.2) | 33 (29) | 0.338 |
| Mitoxantron | 53 (17) | 34 (17.2) | 19 (16.7) | 0.936 |
| Vincristin | 52 (16.7) | 32 (16.2) | 20 (17.5) | 0.727 |
| Hydroxydaunorubicin | 39 (12.5) | 25 (12.7) | 19 (16.7) | 0.952 |
| Melphalan | 35 (11.2) | 22 (11.1) | 13 (11.4) | 0.928 |
| Bendamustin | 32 (10.3) | 17 (8.6) | 15 (13.2) | 0.191 |
| Cisplatin | 32 (10.3) | 21 (10.6) | 11 (9.6) | 0.808 |
| AKI Stages | Patients (N) | Percent of All 312 Patients | Percent of 198 Patients with AKI |
|---|---|---|---|
| Max. Stage 1 | 55 | 17.62% | 27.8% |
| Max. Stage 2 | 79 | 25.32% | 39.9% |
| Max. Stage 3 | 64 | 20.51% | 32.3% |
| Total number of patients with AKI | 198 | 63.5% | 100% |
| Total number of patients without AKI | 114 | 36.5% | - |
| Number of AKI | Patients (N) | Percent of All 312 Patients | Percent of 198 Patients with AKI |
| Max. one AKI | 122 | 39.10% | 61.62% |
| Max. two AKI | 47 | 15.06% | 23.74% |
| Max. three AKI | 15 | 4.81% | 7.57% |
| Max. four AKI | 8 | 2.56% | 4.04% |
| Max. five AKI | 6 | 1.92% | 3.03% |
| Total number of patients with AKI | 198 | 63.5% | 100% |
| Total number of AKI | 323 | - | - |
| Factor | All Patients (% of 312) | Patients with AKI (% of 198) | Patients without AKI (% of 114) | Univariate Analysis (p-Value) | Multivariate Analysis p-Value/Odds Ratio (95%CI) | |
|---|---|---|---|---|---|---|
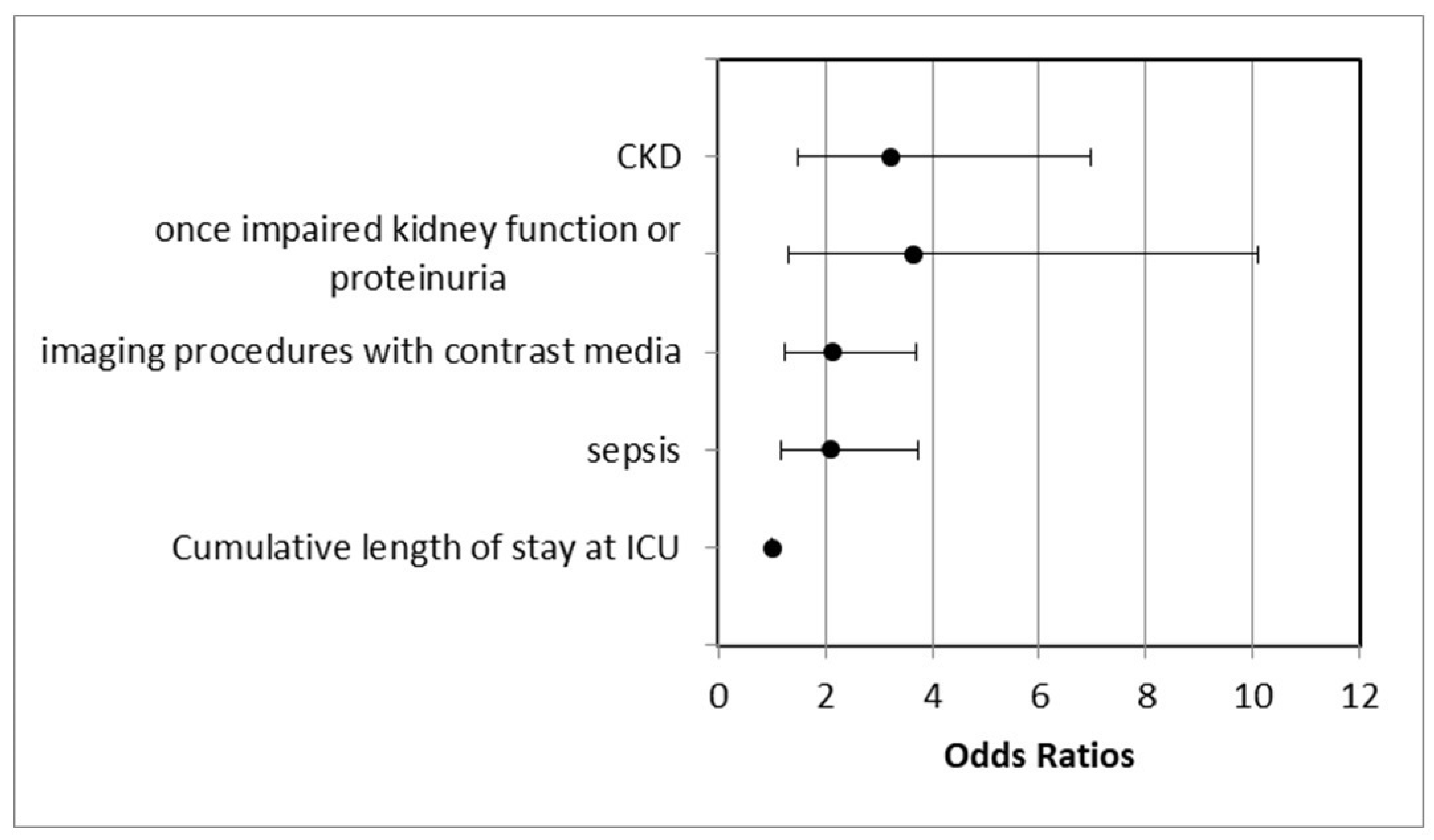
| CKD | 49 (15.7) | 39 (19.7) | 10 (8.8) | 0.000 | 0.003/3.224 (1.488–6.984) | |
| Once-impaired kidney function or proteinuria | 32 (10.3) | 27 (13.6) | 5 (4.4) | 0.013/3.635 (1.308–10.103) | ||
| Normal eGFR | 231 (74%) | 132 (66.6) | 99 (86.8%) | |||
| aHT | 117 (37.5) | 84 (42.4) | 33 (28.9) | 0.021 | 0.072 | |
| Comorbidity score | 0 | 151 (48.4) | 89 (44.9) | 0.035 | 0.209 | |
| 1–2 | 99 (31.7) | 61 (30.8) | ||||
| ≥3 | 62 (19.9) | 48 (24.3) | ||||
| Age | Mean age (range) | 0.074 | - | |||
| 55.42 years (19–75) | 56.47 years (21–75) | 53.59 years (19–74) | ||||
| Gender | male | 200 (64.1) | 134 (67.7) | 66 (57.9) | 0.083 | - |
| female | 112 (35.9) | 64 (32.3) | 48 (42.1) | |||
| DM | 51 (16.3) | 37 (18.7) | 14 (12.3) | 0.141 | - | |
| Stem cell source | PBSCT | 271 (86.9) | 176 (88.9) | 95 (83.3) | 0.162 | - |
| Bone marrow | 41 (13.1) | 22 (11.1) | 19 (16.6) | |||
| Conditioning regimens | myeloablative | 116 (37.2) | 74 (37.4) | 42 (36.8) | 0.925 | - |
| reduced | 196 (62.8) | 124 (62.6) | 72 (63.2) | |||
| HLA compatibility | HLA 10/10 | 227 (72.8) | 143 (72.2) | 84 (73.7) | 0.166 | - |
| HLA 9/10 | 64 (20.5) | 45 (22.7) | 19 (16.7) | |||
| HLA haploidentical | 21 (6.7) | 10 (5.1) | 11 (9.6) | |||
| Relation to donor | MSIB | 23 (7.4) | 13 (6.6) | 10 (8.8) | 0.373 | - |
| MMSIB | 2 (0.6) | 0 (0) | 2 (1.8) | |||
| MUD | 204 (65.4) | 130 (65.7) | 74 (64.9) | |||
| MMUD | 62 (19.9) | 45 (22.7) | 17 (14.9) | |||
| Factor | All Patients (% of 312) | Patients with AKI (% of 198) | Patients without AKI (% of 114) | Univariate Analysis (p-Value) | Multivariate Analysis p-Value/Odds Ratio (95%CI) |
|---|---|---|---|---|---|
| Sepsis | 106 (34) | 81 (40.9) | 25 (21.9) | 0.001 | 0.012/2.097 (1.178–3.733) |
| Imaging procedures with contrast media | 113 (36.2) | 86 (43.4) | 27 (23.7) | 0.001 | 0.007/2.134 (1.234–3.690) |
| Cumulative length of ICU stay | 93.87 days | 103.4 days | 77.14 days | 0.000 | 0.034/1.005 (1.000–1.009) |
| “Toxic“ CsA (>300 ng/mL) and tacrolimus peak plasma level (>20 ng/mL) | 163 (52.3%) | 114 (57.6) | 49 (43%) | 0.013 | 0.071 |
| Duration of the therapy with tacrolimus | 198.29 days | 271.88 days | 78.69 days | 0.009 | Not included |
| Chimerical status on day 14 | 80.91% | 80.71% | 80.23% | 0.866 | - |
| Late chimerical status | 94.34% | 95.39% | 92.45% | 0.246 | - |
| Engraftment day | 20.64 days | 20.88 days | 20.21 days | 0.451 | - |
| CMV-infection | 79 (25.3) | 53 (26.8) | 26 (22.8) | 0.464 | - |
| aGVHD | 165 (52.9) | 107 (54) | 58 (50.9) | 0.590 | - |
| Number of imaging procedures with contrast media | 1.69 | 1.76 | 1.48 | 0.324 | - |
| Therapy with CsA | 283 (90.7) | 181 (91.4) | 102 (89.5) | 0.570 | - |
| Duration of the therapy with CsA | 272.18 days | 257.95 days | 298.04 days | 0.305 | - |
| Therapy withTacrolimus | 43 (13.8) | 26 (13.1) | 17 (14.9) | 0.660 | - |
| Proposed Basic EHRs Dataset | ||
|---|---|---|
| Record | Content | Corresponding FHIRs |
| Procedure | allo-HCT | Procedure |
| Encounter | ICU stay Duration | Encounter |
| Diagnosis | Sepsis CKD AKI | Condition |
| Laboratory Data | Proteinuria Creatinine eGFR | DiagnosticReport Observation |
| Medication | Contrast media | Medication MedicationAdministration |
| Imaging procedure | Imaging procedure using contrast media | DiagnosticReport ImagingStudy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff, E.; Kirilov, N. Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation. Life 2024, 14, 987. https://doi.org/10.3390/life14080987
Bischoff E, Kirilov N. Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation. Life. 2024; 14(8):987. https://doi.org/10.3390/life14080987
Chicago/Turabian StyleBischoff, Elena, and Nikola Kirilov. 2024. "Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation" Life 14, no. 8: 987. https://doi.org/10.3390/life14080987
APA StyleBischoff, E., & Kirilov, N. (2024). Leveraging Electronic Health Records to Predict the Risk of Acute Kidney Injury after Allogeneic Hematopoietic Cell Transplantation. Life, 14(8), 987. https://doi.org/10.3390/life14080987





