Disseminate Cutaneous Vasculitis Associated with Durvalumab Treatment—Case Report, Mini-Review on Cutaneous Side Effects of Immune Checkpoint Inhibitor Therapies with Machine Learning Perspectives
Abstract
1. Introduction
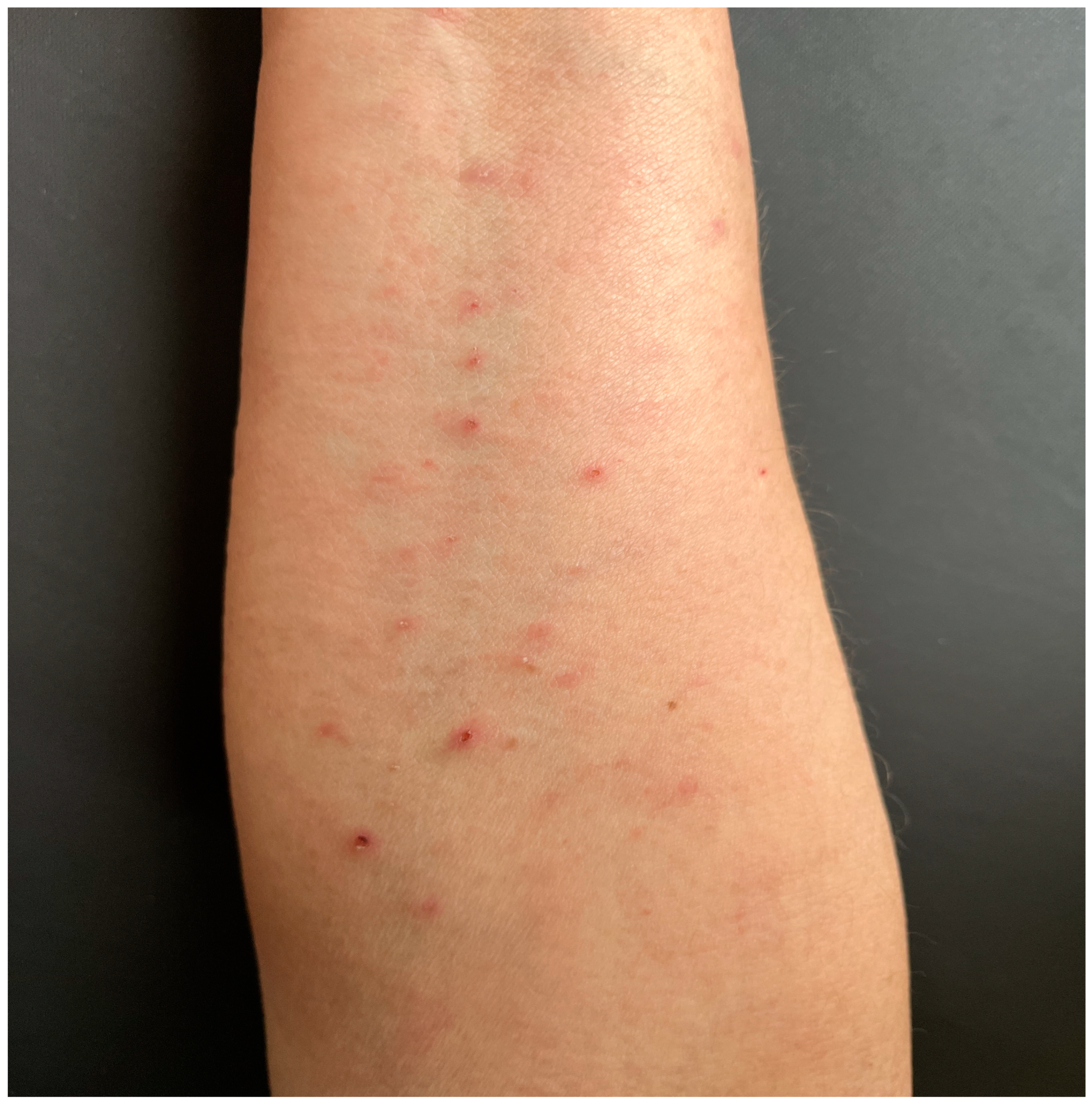
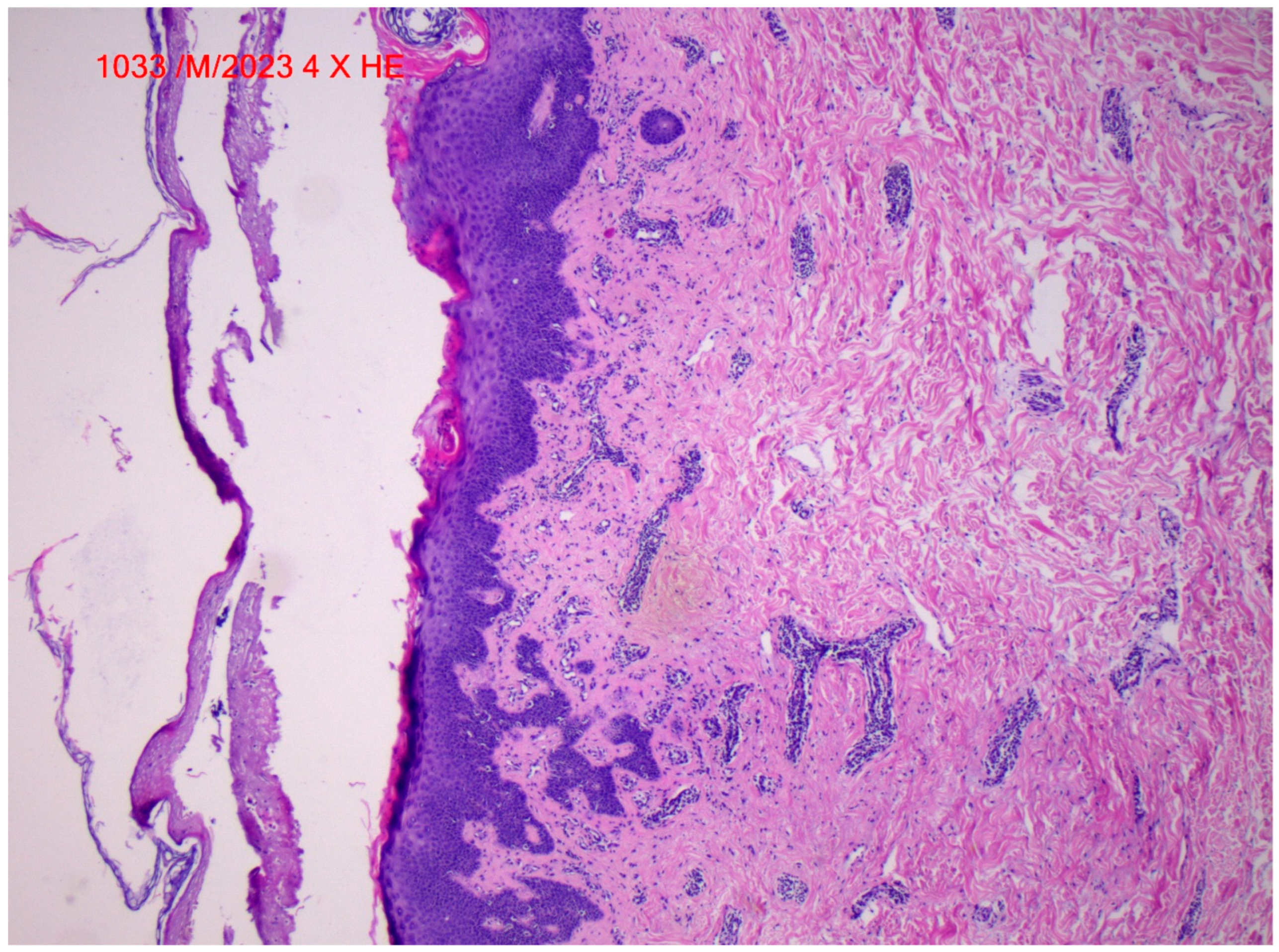
2. Case Report
3. Discussion
Challenges and Prospective Future Application of Machine Learning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elia, G.; Ferrari, S.M.; Galdiero, M.R.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Varricchi, G.; Fallahi, P.; Antonelli, A. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101370. [Google Scholar] [CrossRef]
- Michot, J.M.; Lazarovici, J.; Tieu, A.; Champiat, S.; Voisin, A.L.; Ebbo, M.; Godeau, B.; Michel, M.; Ribrag, V.; Lambotte, O. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur. J. Cancer 2019, 122, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Z.; Lu, X. Impact of immunosenescence and inflammaging on the effects of immune checkpoint inhibitors. Cancer Pathog. Ther. 2023, 2, 24–30. [Google Scholar] [CrossRef]
- Duma, N.; Mansfield, A. Is durvalumab the solution for unresectable stage III non-small cell lung cancer? Transl. Cancer Res. 2018, 7, S89–S93. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Alvarez-Argote, J.; Dasanu, C.A. Durvalumab in cancer medicine: A comprehensive review. Expert Opin. Biol. Ther. 2019, 19, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; D’Alessandro, R.; Rizzo, A.; Schirizzi, A.; Vallarelli, S.; Ostuni, C.; Troiani, L.; Lolli, I.R.; Lotesoriere, C.; Giannelli, G. Durvalumab in advanced cholangiocarcinoma: Is someone knocking down the door? Immunotherapy 2023, 15, 477–486. [Google Scholar] [CrossRef]
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open- label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Solis, S.L.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S.; et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol. 2022, 23, 1156–1166. [Google Scholar] [CrossRef]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.A.; Park, Y.H.; Delord, J.P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of Recombinant DNA Technology to Improve Life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, S.C.; Pisetsky, D.S. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 2019, 58 (Suppl. S7), vii59–vii67. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Liudahl, S.M.; Coussens, L.M. B cells as biomarkers: Predicting immune checkpoint therapy adverse events. J. Clin. Investig. 2018, 128, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Grabie, N.; Lichtman, A.H.; Padera, R. T cell checkpoint regulators in the heart. Cardiovasc. Res. 2019, 115, 869–877. [Google Scholar] [CrossRef]
- Lleo, A.; Rimassa, L.; Colombo, M. Hepatotoxicity of immune check point inhibitors: Approach and management. Dig. Liver Dis. 2019, 51, 1074–1078. [Google Scholar] [CrossRef]
- Jayakrishnan, B.; Al-Moundhri, M.; Burney, I.; Al-Hashami, Z.; Al-Bimani, K. Pulmonary toxicities of immune check point inhibitors in the management of cancer: Mini review. Adv. Respir. Med. 2022, 90, 219–229. [Google Scholar] [CrossRef]
- Alonso, F.; Martín de Francisco, Á.L.M.; Auñón, P.; García-Carro, C.; García, P.; Gutiérrez, E.; Mcía, M.; Quintana, L.F.; Quiroga, B.; Soler, M.J.; et al. Onconephrology Group of the Spanish Society of Nephrology. Adverse renal effects of check-point inhibitors (ICI) in cancer patients: Recommendations of the Onco-nephrology Working Group of the Spanish Society of Nephrology. Nefrologia (Engl. Ed.) 2023, 43, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor-related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. [Google Scholar] [CrossRef]
- Plachouri, K.M.; Vryzaki, E.; Georgiou, S. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Summarized Overview. Curr. Drug Saf. 2019, 14, 14–20. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Boulinguez, S.; Pagès, C.; Riffaud, L.; Lamant, L.; Chira, C.; Boyrie, S.; Vigarios, E.; Tournier, E.; Meyer, N. Toxicités dermatologiques des inhibiteurs de checkpoint immunologiques [Dermatologic toxicities of immune checkpoint inhibitors]. Ann. Dermatol. Venereol. 2018, 145, 313–330. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Chiu, M.N.; Pilkhwal, S.S. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: Pathogenesis, treatment, and surveillance. Cutan. Ocul. Toxicol. 2022, 41, 73–90. [Google Scholar] [CrossRef]
- Nikolaou, V.A.; Apalla, Z.; Carrera, C.; Fattore, D.; Sollena, P.; Riganti, J.; Segura, S.; Freites-Martinez, A.; Lallas, K.; Romano, M.C.; et al. Clinical associations and classification of immune checkpoint inhibitor-induced cutaneous toxicities: A multicentre study from the European Academy of Dermatology and Venereology Task Force of Dermatology for Cancer Patients. Br. J. Dermatol. 2022, 187, 962–969. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Pahalyants, V.; Kalinich, M.; Murphy, W.; Yu, K.H.; Wang, F.; Chen, S.T.; Reynolds, K.; Kwatra, S.G.; Semenov, Y.R. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A United States population-level analysis. J. Am. Acad. Dermatol. 2022, 86, 563–572. [Google Scholar] [CrossRef]
- Gupta, S.; Xu, D.; Hadfield, J.; Prentice, D. Durvalumab-associated vasculitis presenting as ‘the blue toe syndrome’. BMJ Case Rep. 2020, 13, e235886. [Google Scholar] [CrossRef]
- Panebianco, M.; Ragazzi, M.; Asensio, N.M.; Pagano, M.; Gnoni, R.; Boni, C. A case of necrotizing vasculitis with panniculitis, during sorafenib treatment for hepatocellular carcinoma, appeared in disease progression. J. Gastrointest. Oncol. 2014, 5, E121–E124. [Google Scholar]
- Brandi, G.; Venturi, M.; Dika, E.; Maibach, H.; Patrizi, A.; Biasco, G. Cutaneous leukocytoclastic vasculitis due to erlotinib: Just an adverse event or also a putative marker of drug efficacy? Cutan. Ocul. Toxicol. 2013, 32, 336–338. [Google Scholar] [CrossRef]
- Fekete, G.L.; Fekete, L. Cutaneous leukocytoclastic vasculitis associated with erlotinib treatment: A case report and review of the literature. Exp. Ther. Med. 2019, 17, 1128–1131. [Google Scholar] [CrossRef]
- Joly-Chevrier, M.; Nguyen, A.X.L.; Liang, L.R.C.; Lesko-Krleza, M.; Lefrançois, P. The State of Artificial Intelligence in Skin Cancer Publications. J. Cutan. Med. Surg. 2024, 28, 146–152. [Google Scholar] [CrossRef]
- Wei, M.L.; Tada, M.; So, A.; Torres, R. Artificial intelligence and skin cancer. Front. Med. 2024, 11, 1331895. [Google Scholar] [CrossRef]
- Jutzi, T.B.; Krieghoff-Henning, E.I.; Holland-Letz, T.; Utikal, J.S.; Hauschild, A.; Schadendorf, D.; Sondermann, W.; Fröhling, S.; Hekler, A.; Schmitt, M.; et al. Artificial Intelligence in Skin Cancer Diagnostics: The Patients’ Perspective. Front. Med. 2020, 7, 233. [Google Scholar] [CrossRef]
- Sanchez, K.; Kamal, K.; Manjaly, P.; Ly, S.; Mostaghimi, A. Clinical Application of Artificial Intelligence for Non-melanoma Skin Cancer. Curr. Treat. Options Oncol. 2023, 24, 373–379. [Google Scholar] [CrossRef]
- Hekler, A.; Utikal, J.S.; Enk, A.H.; Hauschild, A.; Weichenthal, M.; Maron, R.C.; Berking, C.; Haferkamp, S.; Klode, J.; Schadendorf, D.; et al. Superior skin cancer classification by the combination of human and artificial intelligence. Eur. J. Cancer 2019, 120, 114–121. [Google Scholar] [CrossRef]
- Arik, S.; Iantovics, L.B. Next Generation Hybrid Intelligent Medical Diagnosis Systems. In Lecture Notes in Computer Science, Proceedings of the 24th International Conference on Neural Information Processing (ICONIP 2017), Guangzhou, China, 14–18 November 2017; Liu, D., Xie, S., Li, Y., Zhao, D., El-Alfy, E.S., Eds.; Neural Information Processing; Springer: Cham, Switzerland, 2017; Volume 10636, pp. 903–912. [Google Scholar]
- Zamfirescu, C.B.; Duta, L.; Iantovics, B. On Investigating the Cognitive Complexity of Designing the Group Decision Process. Stud. Inform. Control 2010, 19, 263–270. [Google Scholar] [CrossRef]
| Cutaneous Side Effects of Immune Checkpoint Inhibitors | |
|---|---|
| Mild | Severe |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, G.L.; Iantovics, L.B.; Fekete, J.E.; Fekete, L. Disseminate Cutaneous Vasculitis Associated with Durvalumab Treatment—Case Report, Mini-Review on Cutaneous Side Effects of Immune Checkpoint Inhibitor Therapies with Machine Learning Perspectives. Life 2024, 14, 1062. https://doi.org/10.3390/life14091062
Fekete GL, Iantovics LB, Fekete JE, Fekete L. Disseminate Cutaneous Vasculitis Associated with Durvalumab Treatment—Case Report, Mini-Review on Cutaneous Side Effects of Immune Checkpoint Inhibitor Therapies with Machine Learning Perspectives. Life. 2024; 14(9):1062. https://doi.org/10.3390/life14091062
Chicago/Turabian StyleFekete, Gyula Laszlo, Laszlo Barna Iantovics, Júlia Edit Fekete, and Laszlo Fekete. 2024. "Disseminate Cutaneous Vasculitis Associated with Durvalumab Treatment—Case Report, Mini-Review on Cutaneous Side Effects of Immune Checkpoint Inhibitor Therapies with Machine Learning Perspectives" Life 14, no. 9: 1062. https://doi.org/10.3390/life14091062
APA StyleFekete, G. L., Iantovics, L. B., Fekete, J. E., & Fekete, L. (2024). Disseminate Cutaneous Vasculitis Associated with Durvalumab Treatment—Case Report, Mini-Review on Cutaneous Side Effects of Immune Checkpoint Inhibitor Therapies with Machine Learning Perspectives. Life, 14(9), 1062. https://doi.org/10.3390/life14091062






