Cutaneous Sarcoidosis Induced by Laser Therapy: Case Report and Review of the Literature
Abstract
:1. Introduction
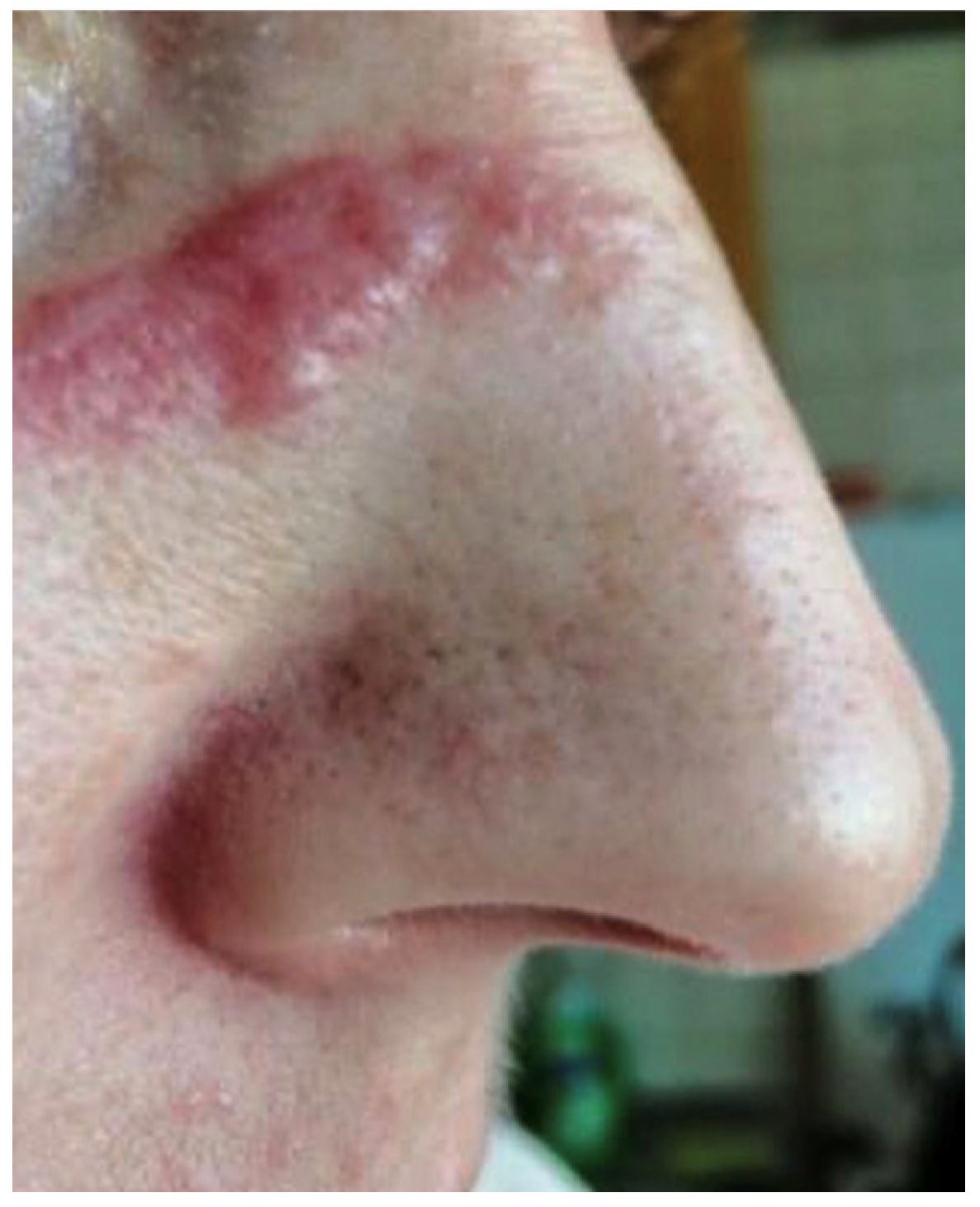
2. Clinical Case Presentation
3. Discussion and Review of the Literature
3.1. Symptoms of Cutaneous Sarcoidosis
3.2. Diagnosis of Cutaneous Sarcoidosis
3.3. Treatment of Cutaneous Sarcoidosis
3.4. Cosmetic Procedure and Cutaneous Sarcoidosis
3.5. Laser Therapy and Cutaneous Sarcoidosis
3.5.1. Pulsed Dye Laser
3.5.2. Carbon Dioxide Laser
3.5.3. Intense Pulse Light
3.5.4. Combined Laser Therapies
3.6. Laser as a Factor Inducing Cutaneous Sarcoidosis
3.7. Isotretinoin and Cutaneous Sarcoidosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.-Y.; Müller-Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Haimovic, A.; Sanchez, M.; Judson, M.A.; Prystowsky, S. Sarcoidosis: A comprehensive review and update for the dermatologist: Part II. Extracutaneous disease. J. Am. Acad. Dermatol. 2012, 66, 719.e1–719.e10. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J. Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- Mañá, J.; Marcoval, J. Skin manifestations of sarcoidosis. Presse Med. 2012, 41 Pt 2, e355–e374. [Google Scholar] [CrossRef] [PubMed]
- Haimovic, A.; Sanchez, M.; Judson, M.A.; Prystowsky, S. Sarcoidosis: A comprehensive review and update for the dermatologist: Part I. Cutaneous disease. J. Am. Acad. Dermatol. 2012, 66, 699.e1–699.e18. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Yadav, D.; Puranik, N.; Guleria, R.; Jin, J.-O. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J. Clin. Med. 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Cozier, Y.C. Epidemiology of sarcoidosis: Current findings and future directions. Ther. Adv. Chronic Dis. 2018, 9, 227–240. [Google Scholar] [CrossRef]
- Newman, K.L.; Newman, L.S. Occupational causes of sarcoidosis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 145–150. [Google Scholar] [CrossRef]
- Vihlborg, P.; Bryngelsson, I.-L.; Andersson, L.; Graff, P. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: A retrospective cohort study. BMJ Open 2017, 7, e016839. [Google Scholar] [CrossRef]
- Newman, L. Aetiologies of sarcoidosis. Eur. Respir. Monogr. 2005, 32, 23–48. [Google Scholar]
- Vidal, S.; de la Horra, C.; Martín, J.; Montes-Cano, M.A.; Rodríguez, E.; Respaldiza, N.; Rodríguez, F.; Varela, J.M.; Medrano, F.J.; Calderón, E.J. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin. Microbiol. Infect. 2006, 12, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Drake, W.P.; Newman, L.S. Mycobacterial antigens may be important in sarcoidosis pathogenesis. Curr. Opin. Pulm. Med. 2006, 12, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Salameh, H.; Trien, R.; Cooper, C.J.; Paez, D.; Colon, E.; Ajmal, S. Interferon-alpha-induced sarcoidosis in a patient being treated for hepatitis C. Am. J. Case Rep. 2014, 15, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Cohen Aubart, F.; Lhote, R.; Amoura, A.; Valeyre, D.; Haroche, J.; Amoura, Z.; Lebrun-Vignes, B. Drug-induced sarcoidosis: An overview of the WHO pharmacovigilance database. J. Intern. Med. 2020, 288, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Marchell, R.M.; Judson, M. Cutaneous Sarcoidosis. Semin. Respir. Crit. Care Med. 2010, 31, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Marchell, R.M.; Judson, M.A. Chronic cutaneous lesions of sarcoidosis. Clin. Dermatol. 2007, 25, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Garza, D.M.; Chavez-Alvarez, S.; Ocampo-Candiani, J.; Gomez-Flores, M. Erythema Nodosum: A Practical Approach and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2021, 22, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Franzen, D.P.; Brutsche, M.; Nilsson, J.; Böni, C.; Daccord, C.; Distler, O.; Elsener, D.; Funke-Chambour, M.; Gruner, C.; Hayward-Könnecke, H.; et al. Sarcoidosis—A multisystem disease. Swiss Med. Wkly. 2022, 152, w30049. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45, Correction in Nat. Rev. Dis. Primers 2019, 5, 49. [Google Scholar] [CrossRef]
- Kwon, S.H.; Jeong, K.M.; Baek, Y.S.; Jeon, J. Linear scar sarcoidosis on thin blepharoplasty line mimicking a hypertrophic scar: A case report. SAGE Open Med. Case Rep. 2018, 6, 2050313X18803991. [Google Scholar] [CrossRef]
- Kanakamedala, A.D.; Maamari, R.N.; Couch, S.M. Tattoo-associated lacrimal gland enlargement and sarcoidosis. Am. J. Ophthalmol. Case Rep. 2023, 32, 101889. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.-N.; Shin, K.; Kim, H.-S.; Ko, H.-C.; Kim, B.; Kim, M.-B. Scar Sarcoidosis: A Retrospective Investigation into Its Peculiar Clinicopathologic Presentation. Ann. Dermatol. 2022, 34, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Cliff, S.; Felix, R.H.; Singh, L.; Harland, C.C. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J. Cutan. Laser Ther. 1999, 1, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Raulin, C.; Ockenfels, H.-M.; Karsai, S. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: A case report. Dermatol. Surg. 2009, 35, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, C.; Shi, Y.; Zhang, W. Combined pulsed-dye laser and medical therapy for treatment of cutaneous sarcoidosis lesions: A case report. J. Int. Med. Res. 2021, 49, 300060521997745. [Google Scholar] [CrossRef]
- Holzmann, R.D.; Astner, S.; Forschner, T.; Sterry, G. Scar sarcoidosis in a child: Case report of successful treatment with the pulsed dye laser. Dermatol. Surg. 2008, 34, 393–396. [Google Scholar] [CrossRef]
- Goodman, M.M.; Alpern, K. Treatment of lupus pernio with the flashlamp pulsed dye laser. Lasers Surg. Med. 1992, 12, 549–551. [Google Scholar] [CrossRef]
- Zaouak, A.; Koubaa, W.; Hammami, H.; Fenniche, S. Unconventional use of fractional ablative CO2 laser in facial cutaneous sarcoïdosis. Dermatol. Ther. 2017, 30, e12569. [Google Scholar] [CrossRef]
- O’Donoghue, N.B.; Barlow, R.J. Laser remodelling of nodular nasal lupus pernio. Clin. Exp. Dermatol. 2006, 31, 27–29. [Google Scholar] [CrossRef]
- Stack, B.C.; Hall, P.J.; Goodman, A.L.; Perez, I.R. CO2 laser excision of lupus pernio of the face. Am. J. Otolaryngol. 1996, 17, 260–263. [Google Scholar] [CrossRef]
- Piccolo, D.; Di Marcantonio, D.; Crisman, G.; Cannarozzo, G.; Sannino, M.; Chiricozzi, A.; Chimenti, S. Unconventional use of intense pulsed light. BioMed Res. Int. 2014, 2014, 618206. [Google Scholar] [CrossRef] [PubMed]
- Emer, J.; Uslu, U.; Waldorf, H. Improvement in lupus pernio with the successive use of pulsed dye laser and nonablative fractional resurfacing. Dermatol. Surg. 2014, 40, 201–202. [Google Scholar] [CrossRef]
- Grema, H.; Greve, B.; Raulin, C. Scar sarcoidosis—Treatment with the Q-switched ruby laser. Lasers Surg. Med. 2002, 30, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Ekbäck, M.; Molin, L. Effective laser treatment in a case of lupus pernio. Acta Derm. Venereol. 2005, 85, 521–522. [Google Scholar] [CrossRef]
- Momen, S.; Al-Niaimi, F.; Barlow, R.; Mallipeddi, R. The use of lasers in cutaneous sarcoid: Is there a role? J. Cosmet. Laser Ther. 2016, 18, 335–338. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, S.-J.; Im, M.; Lee, Y.; Seo, Y.-J.; Lee, J.-H. Scar Sarcoidosis Induced by Pulsed Dye Laser Treatment. Ann. Dermatol. 2016, 28, 509–510. [Google Scholar] [CrossRef]
- Kormeili, T.; Neel, V.; Moy, R.L. Cutaneous sarcoidosis at sites of previous laser surgery. Cutis 2004, 73, 53–55. [Google Scholar] [PubMed]
- Georgiou, S.; Monastirli, A.; Pasmatzi, E.; Tsambaos, D. Cutaneous sarcoidosis: Complete remission after oral isotretinoin therapy. Acta Derm. Venereol. 1998, 78, 457–459. [Google Scholar] [CrossRef]
- Waldinger, T.P.; Ellis, C.N.; Quint, K.; Voorhees, J.J. Treatment of cutaneous sarcoidosis with isotretinoin. Arch. Dermatol. 1983, 119, 1003–1005. [Google Scholar] [CrossRef]




| Authors and Type of the Study | Clinical Picture | Patient Age/Gender | Laser/Setting | Treatment Number | Outcome | Follow Up |
|---|---|---|---|---|---|---|
| Roos et al. case report [24] | Nodules on back | 63 F | FPDL 585 nm 0.5 ms, 12 mm, 6 J/cm2 | 1 | After four weeks, lesions had completely resolved | Prednisolone added at 4 weeks for systemic disease; at 13 months no recurrence of lesions |
| Dong et al. case report [25] | Erythematous papules on the left part of the face | 46 F | PDL 595 nm 6 ms, 7 mm, 14 J/cm2 | 10 treatments during 15 months | The plaque was thinner, normal skin had appeared; telangiectasia was less obvious | Acitretin (10 mg per day) and hydroxychloroquine (200 mg twice a day) along with PDL therapy |
| Holzmann et al. case report [26] | Erythematous papule on varicella scar site on cheeks | 10 M | PDL 595 mm, 0.5 ms, 7 mm, 7.6–7.8 J/cm2 | 3 treatments at 6-week intervals | Clinical remission after three treatments | No recurrence at 12 months |
| Goodman et al. case report [27] | Lupus pernio granulomatous papules on nasal ala | 39 F | PDL 585 nm, 460 ms, 5 mm, 8 J/cm2 | 2 treatments 7 months apart | Erythema and papular components showed a 75% improvement from baseline after six months | Papules recurred at 2 months, and erythema at 6 months. By 15 months, a significant reappearance of both erythema and papules necessitated a third treatment |
| Zaouak et al. case report [28] | Two infiltrated papulo-nodular plaques on the cheek | 49 F | Ablative fractional CO2 laser 125 mm handpiece, 10 mm spot, 25% density, 10 Watts | 3 sessions with a 2-month interval | Complete resolution of the two facial plaques | No signs of relapse after 6 months |
| O’Donoghue et al. case series [29] | Lupus pernio nose; diffuse violaceous involvement and fleshy nodules on ala rim Violaceous nodule on nasal bridge, nasal swelling and erythema Coalescent fleshy violaceous nodule on nasal tip and left nasal ala | 55F 57 M 58 F | Sarcoid nodules underwent debulking and recontouring with a CO2 laser in “paint mode” (18 W, 6 mm spot size); for cosmetic refinement, patients 1 and 3 received a resurfacing pass (14 W, 6 mm spot size), accompanied by intralesional triamcinolone acetonide (TAC) injection to prevent recurrence | New contour maintained | Six years maintained on 5 mg prednisolone, mild hypopigmentation At 14 months nasal contour was stable At 9 months, partial recurrence with nasal swelling in conjunction with flare of systemic sarcoid | |
| Stack et al. case report [30] | Nodules on nose causing nasal obstruction | 31 M | Excision with CO2 laser and intralesional TAC | 24 months no recurrence | ||
| Piccolo et al. case report [31] | Three nodules located on the anterior and posterior aspects of the pinna | 26 F | IPL 500 nm 2 pulses 5–10 ms, 12–16 J/cm2 | 4 | Reduction of the vascular component and in the consistency of the lesions | Improvement persisted throughout the 6 months |
| Emer et al. case report [32] | Lupus pernio on the nose | Not shown | PDL 595 nm 7 mm, 0.45 ms, 8 J/cm2 NAFR 1550 nm (70 mJ, treatment level 6, 8 passes) | 1 | Reduction of lesions | N.D. |
| Grema et al. case report [33] | Scar sarcoid on right elbow and both knees (red-brown discoloration) | 50 F | QSRL, four treatments Previously treated with PDL with no effect 585 nm, 7 mm, 0.5 ms, 5.5–5.6 J/cm2 | 4 | Lightened after four treatments | No recurrence at three years |
| Ekbäck et al. case report [34] | Lupus pernio of cheek | 57 F | PDL 585 nm, 45 ms, 6.75–7 J/cm2 Frequency-doubled YAG laser 532 nm, 50 ms, 12–16 J/cm2 | 10 treatments over 3 years 2 treatments over 7 months | Limited effect, less redness and thinner lesions Complete healing | N/D |
| Momen et al. case reports [35] | Erythematous plaque on upper lip Nodules and plaques across cheek Erythematous triangular plaque on forehead Lupus pernio | One female, age not shown Two patients, gender and age not shown Three females, age not shown One female, age not shown | Single pass of CO2 laser and two treatments with PDL Two treatments with CO2 laser Singular PDL Seven treatments with PD | Self-reported improvement of 50–60% |
| Author and Type of the Study | Clinical | Patient Age/Gender | Laser/Setting | Treatment | Outcome |
|---|---|---|---|---|---|
| Kim Hr et al. case report [36] | The enlargement of the scar | 71 M | Enlargement of the scar subsequent to three sessions of PDL | Hydroxychloroquine (100 mg b.i.d.) along with topical pimecrolimus and topical steroids | Reduction of the scar |
| Kormeilii T et al. case report [37] | Hypertrophic scars on his left glabellar region and left side of upper lip | 33 M | Progression two years after CO2 laser resurfacing | N/D | N/D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisoń, H.; Simon-Błażewicz, M.; Suseł, J.; Suseł, M.; Woźniak, Z.; Białynicki-Birula, R.; Szepietowski, J.C. Cutaneous Sarcoidosis Induced by Laser Therapy: Case Report and Review of the Literature. Life 2024, 14, 773. https://doi.org/10.3390/life14060773
Cisoń H, Simon-Błażewicz M, Suseł J, Suseł M, Woźniak Z, Białynicki-Birula R, Szepietowski JC. Cutaneous Sarcoidosis Induced by Laser Therapy: Case Report and Review of the Literature. Life. 2024; 14(6):773. https://doi.org/10.3390/life14060773
Chicago/Turabian StyleCisoń, Hanna, Magdalena Simon-Błażewicz, Joanna Suseł, Marianna Suseł, Zdzisław Woźniak, Rafał Białynicki-Birula, and Jacek C. Szepietowski. 2024. "Cutaneous Sarcoidosis Induced by Laser Therapy: Case Report and Review of the Literature" Life 14, no. 6: 773. https://doi.org/10.3390/life14060773
APA StyleCisoń, H., Simon-Błażewicz, M., Suseł, J., Suseł, M., Woźniak, Z., Białynicki-Birula, R., & Szepietowski, J. C. (2024). Cutaneous Sarcoidosis Induced by Laser Therapy: Case Report and Review of the Literature. Life, 14(6), 773. https://doi.org/10.3390/life14060773








