Retrospective Analysis of the Effect of Postmenopausal Women Medications on SARS-CoV-2 Infection Progression
Abstract
:1. Introduction
2. Materials and Methods
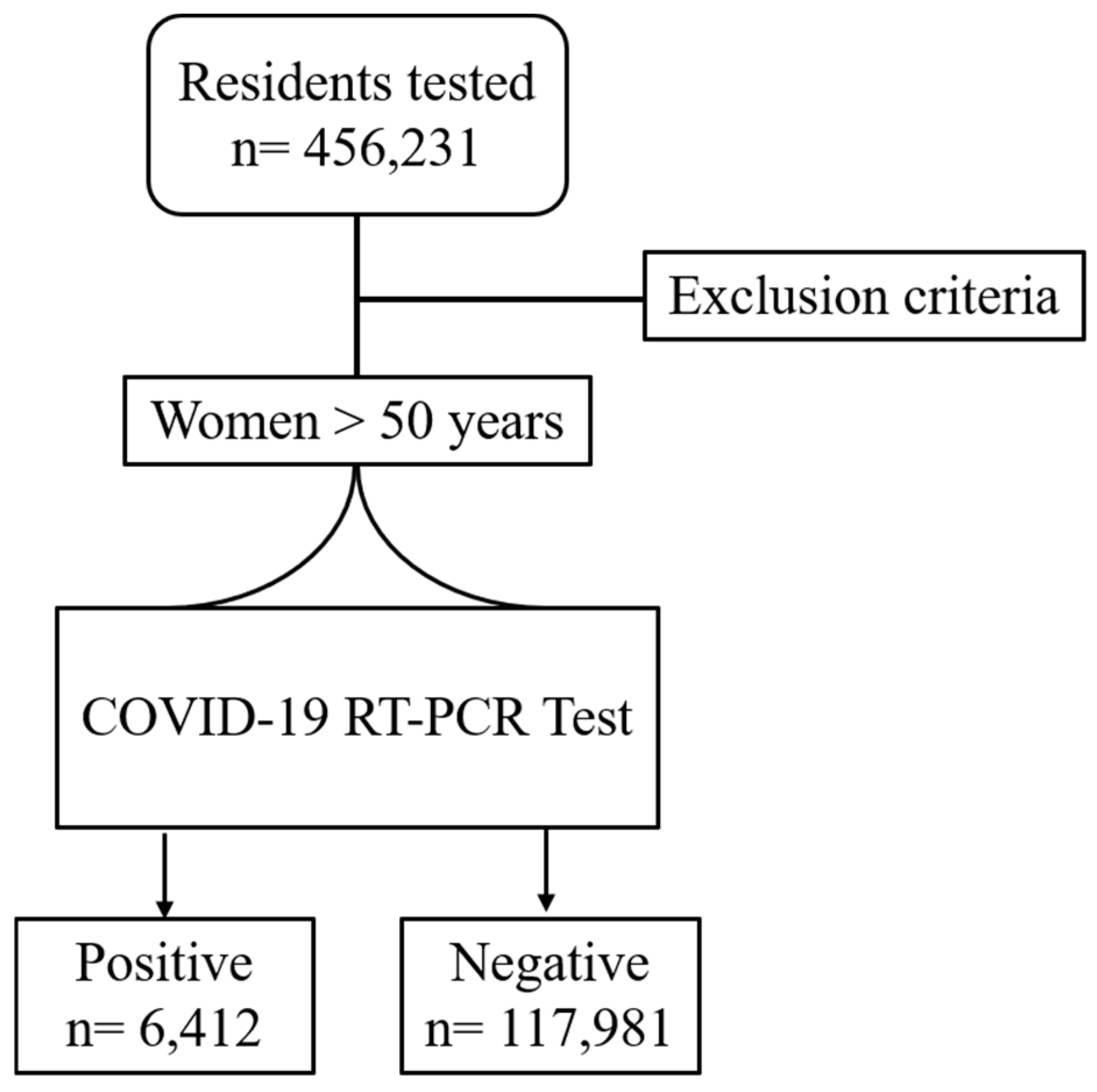
2.1. Study Population
- Bisphosphonates (BI): Alendronic Acid, Ibandronic Acid, Zolendronic Acid, and combinations with cholecalciferol.
- Estrogen and Progestinic (EP) combination: Estradiol, Estriol, Medroxyprogesterone, Tibolone, Dienogest, Drospirenone, Norethisterone, and combinations.
- Estrogen Modulators (EMs): Raloxifene and Bazedoxifene.
- Cholecalciferol (vitamin D3) and combinations of vitamin D3 and calcium (CC).
2.2. Sources of Study Data
2.3. Statistical Analysis
2.4. Ethics
2.5. Data Availability
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Falahi, S.; Kenarkoohi, A. Sex and Gender Differences in the Outcome of Patients with COVID-19. J. Med. Virol. 2021, 93, 151–152. [Google Scholar] [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones with Inflammation and Disease Severity in Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef]
- Ghare Naz, M.S.; Banaei, M.; Dashti, S.; Tehrani, F.R. An Overview of Sex Hormones in Relation to SARS-CoV-2 Infection. Future Virol. 2021, 16, 555–564. [Google Scholar] [CrossRef]
- Lipsa, A.; Prabhu, J.S. Gender Disparity in COVID-19: Role of Sex Steroid Hormones. Asian Pac. J. Trop. Med. 2021, 14, 5–9. [Google Scholar] [CrossRef]
- Lott, N.; Gebhard, C.E.; Bengs, S.; Haider, A.; Kuster, G.M.; Regitz-Zagrosek, V.; Gebhard, C. Sex Hormones in SARS-CoV-2 Susceptibility: Key Players or Confounders? Nat. Rev. Endocrinol. 2023, 19, 217–231. [Google Scholar] [CrossRef]
- Foresta, C.; Rocca, M.S.; Di Nisio, A. Gender Susceptibility to COVID-19: A Review of the Putative Role of Sex Hormones and X Chromosome. J. Endocrinol. Investig. 2021, 44, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Solis, O.; Beccari, A.R.; Iaconis, D.; Talarico, C.; Ruiz-Bedoya, C.A.; Nwachukwu, J.C.; Cimini, A.; Castelli, V.; Bertini, R.; Montopoli, M.; et al. The SARS-CoV-2 Spike Protein Binds and Modulates Estrogen Receptors. Sci. Adv. 2022, 8, eadd4150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, H.; Wang, M.; Wang, L.; Zou, H.; Li, S.; Liu, R. Estrogen Ameliorates Allergic Airway Inflammation by Regulating Activation of NLRP3 in Mice. Biosci. Rep. 2019, 39, BSR20181117. [Google Scholar] [CrossRef]
- Lahm, T.; Crisostomo, P.R.; Markel, T.A.; Wang, M.; Weil, B.R.; Novotny, N.M.; Meldrum, D.R. The Effects of Estrogen on Pulmonary Artery Vasoreactivity and Hypoxic Pulmonary Vasoconstriction: Potential New Clinical Implications for an Old Hormone. Crit. Care Med. 2008, 36, 2174–2183. [Google Scholar] [CrossRef]
- Fantozzi, E.T.; Breithaupt-Faloppa, A.C.; Ricardo-da-Silva, F.Y.; Rodrigues-Garbin, S.; Romero, D.C.; da Silva Rodrigues, A.; Riffo-Vasquez, Y.; Tavares-de-Lima, W. Estradiol Mediates the Long-Lasting Lung Inflammation Induced by Intestinal Ischemia and Reperfusion. J. Surg. Res. 2018, 221, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Zorzi, M.; Cocetta, V.; Prayer-Galetti, T.; Guzzinati, S.; Bovo, E.; Rugge, M.; Calcinotto, A. Clinical Outcome of SARS-CoV-2 Infection in Breast and Ovarian Cancer Patients Who Underwent Antiestrogenic Therapy. Ann. Oncol. 2021, 32, 676–677. [Google Scholar] [CrossRef]
- Lasso, G.; Mayer, S.V.; Winkelmann, E.R.; Chu, T.; Elliot, O.; Patino-Galindo, J.A.; Park, K.; Rabadan, R.; Honig, B.; Shapira, S.D. A Structure-Informed Atlas of Human-Virus Interactions. Cell 2019, 178, 1526–1541.e16. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.M.; Brannan, J.M.; Delos, S.E.; Shoemaker, C.J.; Stossel, A.; Lear, C.; Hoffstrom, B.G.; Dewald, L.E.; Schornberg, K.L.; Scully, C.; et al. FDA-Approved Selective Estrogen Receptor Modulators Inhibit Ebola Virus Infection. Sci. Transl. Med. 2013, 5, 190ra79. [Google Scholar] [CrossRef]
- Shoemaker, C.J.; Schornberg, K.L.; Delos, S.E.; Scully, C.; Pajouhesh, H.; Olinger, G.G.; Johansen, L.M.; White, J.M. Multiple Cationic Amphiphiles Induce a Niemann-Pick C Phenotype and Inhibit Ebola Virus Entry and Infection. PLoS ONE 2013, 8, e56265. [Google Scholar] [CrossRef]
- Madrid, P.B.; Panchal, R.G.; Warren, T.K.; Shurtleff, A.C.; Endsley, A.N.; Green, C.E.; Kolokoltsov, A.; Davey, R.; Manger, I.D.; Gilfillan, L.; et al. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Infect. Dis. 2015, 1, 317–326. [Google Scholar] [CrossRef]
- Gambacciani, M.; Levancini, M. Hormone Replacement Therapy and the Prevention of Postmenopausal Osteoporosis. Prz. Menopauzalny 2014, 13, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L. Effects of Estrogens and SERMs on Bone Metabolism: Clinical Aspects. In Osteoporosis: Pathophysiology and Clinical Management; Leder, B.Z., Wein, M.N., Eds.; Contemporary Endocrinology; Springer International Publishing: Cham, Switzerland, 2020; pp. 239–257. ISBN 978-3-319-69287-6. [Google Scholar]
- Armeni, E.; Paschou, S.A.; Goulis, D.G.; Lambrinoudaki, I. Hormone Therapy Regimens for Managing the Menopause and Premature Ovarian Insufficiency. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101561. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Hampson, G. The Pathogenesis, Diagnosis, Investigation and Management of Osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-Y.; Yang, Y.; Jung, H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Thomas, S. Use of SERMs for Treatment in Postmenopausal Women. J. Steroid Biochem. Mol. Biol. 2014, 142, 142–154. [Google Scholar] [CrossRef]
- Cauley, J.A.; Seeley, D.G.; Ensrud, K.; Ettinger, B.; Black, D.; Cummings, S.R. Estrogen Replacement Therapy and Fractures in Older Women. Ann. Intern. Med. 1995, 122, 9–16. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 Outbreak in the Italian Municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chinn, J.; De Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male Gender Is a Predictor of Higher Mortality in Hospitalized Adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef]
- Ambrosino, I.; Barbagelata, E.; Ortona, E.; Ruggieri, A.; Massiah, G.; Giannico, O.V.; Politi, C.; Moretti, A.M. Gender Differences in Patients with COVID-19: A Narrative Review. Monaldi Arch. Chest Dis. 2020, 90. [Google Scholar] [CrossRef]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of Sex and Gender on COVID-19 Outcomes in Europe. Biol. Sex. Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Rahimi, B.; Panahi, M.; Abkhiz, S.; Saraygord-Afshari, N.; Milani, M.; Alizadeh, E. An Overview of Betacoronaviruses-Associated Severe Respiratory Syndromes, Focusing on Sex-Type-Specific Immune Responses. Int. Immunopharmacol. 2021, 92, 107365. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Poon, L.L.M.; Lee, K.C.; Ng, W.F.; Lai, S.T.; Leung, C.Y.; Chu, C.M.; Hui, P.K.; Mak, K.L.; Lim, W.; et al. Lung Pathology of Fatal Severe Acute Respiratory Syndrome. Lancet 2003, 361, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Chong, D.S.Y.; Lai, W.Y.Y. Do Men Have a Higher Case Fatality Rate of Severe Acute Respiratory Syndrome than Women Do? Am. J. Epidemiol. 2004, 159, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fett, C.; Mack, M.; Ten Eyck, P.P.; Meyerholz, D.K.; Perlman, S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017, 198, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, R.; Nishiura, H.; Kutsuna, S.; Hayakawa, K.; Ohmagari, N. Clinical Determinants of the Severity of Middle East Respiratory Syndrome (MERS): A Systematic Review and Meta-Analysis. BMC Public Health 2016, 16, 1203. [Google Scholar] [CrossRef]
- Levenson, A.S.; Kliakhandler, I.L.; Svoboda, K.M.; Pease, K.M.; Kaiser, S.A.; Ward, J.E., III; Jordan, V.C. Molecular Classification of Selective Oestrogen Receptor Modulators on the Basis of Gene Expression Profiles of Breast Cancer Cells Expressing Oestrogen Receptor α. Br J. Cancer 2002, 87, 449–456. [Google Scholar] [CrossRef]
- Levenson, A.S.; Wolf, D.M.; Catherino, W.H.; Takei, H.; Jordan, V.C. Understanding the Antiestrogenic Actions of Raloxifene and a Mechanism of Drug Resistance to Tamoxifen. Breast Cancer 1998, 5, 99–106. [Google Scholar] [CrossRef]
- Lippman, S.M.; Brown, P.H. Tamoxifen Prevention of Breast Cancer: An Instance of the Fingerpost. J. Natl. Cancer Inst. 1999, 91, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.C.; Cervino, E.V.; Rentero, M.L.; Crespo, E.C.; Álvaro, A.O.; Casillas, M. Raloxifene: Mechanism of Action, Effects on Bone Tissue, and Applicability in Clinical Traumatology Practice. Open Orthop. J. 2009, 3, 14–21. [Google Scholar] [CrossRef]
- Peretz, J.; Pekosz, A.; Lane, A.P.; Klein, S.L. Estrogenic Compounds Reduce Influenza A Virus Replication in Primary Human Nasal Epithelial Cells Derived from Female, but Not Male, Donors. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L415–L425. [Google Scholar] [CrossRef]
- Hong, S.; Chang, J.; Jeong, K.; Lee, W. Raloxifene as a Treatment Option for Viral Infections. J. Microbiol. 2021, 59, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K.; Rosel, D.; BrÁbek, J. Raloxifene and Bazedoxifene Could Be Promising Candidates for Preventing the COVID-19 Related Cytokine Storm, ARDS and Mortality. In Vivo 2020, 34, 3027–3028. [Google Scholar] [CrossRef]
- Allegretti, M.; Cesta, M.C.; Zippoli, M.; Beccari, A.; Talarico, C.; Mantelli, F.; Bucci, E.M.; Scorzolini, L.; Nicastri, E. Repurposing the Estrogen Receptor Modulator Raloxifene to Treat SARS-CoV-2 Infection. Cell Death Differ. 2021, 29, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Chang, J.; Park, S.; Kim, J.; Jeon, S.; Kim, D.H.; Kim, Y.-E.; Lee, J.C.; Im, S.; Jo, Y.; et al. Rapid Discovery and Classification of Inhibitors of Coronavirus Infection by Pseudovirus Screen and Amplified Luminescence Proximity Homogeneous Assay. Antivir. Res. 2023, 209, 105473. [Google Scholar] [CrossRef]
- Dompé Farmaceutici, S.P.A. Multicenter, Adaptive, Randomized, Placebo-Controlled, Double Blind, Parallel-Group Phase 2/3 Trial, to Study Efficacy and Safety of Two Doses of Raloxifene in Adult Paucisymptomatic COVID-19 Patients; clinicaltrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
- Bennouar, S.; Cherif, A.B.; Kessira, A.; Bennouar, D.-E.; Abdi, S. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2021, 40, 104–110. [Google Scholar] [CrossRef]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does Vitamin D Deficiency Increase the Severity of COVID-19? Clin. Med. 2020, 20, e107–e108. [Google Scholar] [CrossRef]
- Wang, M.-K.; Yu, X.-L.; Zhou, L.-Y.; Si, H.-M.; Hui, J.-F.; Yang, J.-S. Potential Role of Vitamin D in Patients with Diabetes, Dyslipidaemia, and COVID-19. World J. Crit. Care Med. 2022, 11, 112–114. [Google Scholar] [CrossRef]
- Thacher, T.D. Evaluating the Evidence in Clinical Studies of Vitamin D in COVID-19. Nutrients 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 Ng/ML 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Sulli, A.; Smith, V.; Pizzorni, C.; Seriolo, B. Vitamin D, Steroid Hormones, and Autoimmunity. Ann. N. Y Acad. Sci. 2014, 1317, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data from Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Chen, J.; Mei, K.; Xie, L.; Yuan, P.; Ma, J.; Yu, P.; Zhu, W.; Zheng, C.; Liu, X. Low Vitamin D Levels Do Not Aggravate COVID-19 Risk or Death, and Vitamin D Supplementation Does Not Improve Outcomes in Hospitalized Patients with COVID-19: A Meta-Analysis and GRADE Assessment of Cohort Studies and RCTs. Nutr. J. 2021, 20, 89. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low Vitamin D Levels Are Associated with Long COVID Syndrome in COVID-19 Survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does Vitamin D Supplementation Reduce COVID-19 Severity?—A Systematic Review. QJM 2022, 115, hcac040. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of Combined Calcium and Vitamin D Supplementation on Osteoporosis in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Sun, J.-K.; Zhang, W.-H.; Zou, L.; Liu, Y.; Li, J.-J.; Kan, X.-H.; Dai, L.; Shi, Q.-K.; Yuan, S.-T.; Yu, W.-K.; et al. Serum Calcium as a Biomarker of Clinical Severity and Prognosis in Patients with Coronavirus Disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Elham, A.S.; Azam, K.; Azam, J.; Mostafa, L.; Nasrin, B.; Marzieh, N. Serum Vitamin D, Calcium, and Zinc Levels in Patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Berlansky, S.; Sallinger, M.; Grabmayr, H.; Humer, C.; Bernhard, A.; Fahrner, M.; Frischauf, I. Calcium Signals during SARS-CoV-2 Infection: Assessing the Potential of Emerging Therapies. Cells 2022, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; Al Fahdi, F.; Al Salmi, I.; Al Khalili, H.; Gokhale, A.; Khamis, F. Serum Calcium and Vitamin D Levels: Correlation with Severity of COVID-19 in Hospitalized Patients in Royal Hospital, Oman. Int. J. Infect. Dis. 2021, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Jackson, C.A.; Rowlands, J.V.; Mishra, G.D. Socioeconomic Position, Lifestyle Factors and Age at Natural Menopause: A Systematic Review and Meta-Analyses of Studies across Six Continents. Int. J. Epidemiol. 2014, 43, 1542–1562. [Google Scholar] [CrossRef]
- Available online: www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency (accessed on 1 February 2021).
| Cohort of Women | SARS-CoV-2 Positive | SARS-CoV-2 Negative | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Total | 124,393 | 100 | 6412 | 100 | 117,981 | 100 | - |
| Age (years) | |||||||
| (median, IQR) | 64 | 55–82 | 75 | 57–87 | 63 | 55–81 | <0.0001 |
| Type of comorbidity 1 | |||||||
| Cardiovascular | 32,369 | 26.0 | 2121 | 33.1 | 30,248 | 25.6 | <0.0001 |
| Neurologic | 11,024 | 8.9 | 814 | 12.7 | 10,210 | 8.7 | <0.0001 |
| Endocrine | 9944 | 8.0 | 572 | 8.9 | 9372 | 7.9 | 0.005 |
| Respiratory | 5484 | 4.4 | 400 | 6.2 | 5084 | 4.3 | <0.0001 |
| Psychiatric | 5274 | 4.2 | 340 | 5.3 | 4934 | 4.2 | <0.0001 |
| Rheumatologic | 4725 | 3.8 | 257 | 4.0 | 4468 | 3.8 | 0.367 |
| Renal | 3217 | 2.6 | 215 | 3.4 | 3002 | 2.5 | <0.0001 |
| Gastroenterological | 2939 | 2.4 | 149 | 2.3 | 2790 | 2.4 | 0.833 |
| Nutrition | 1918 | 1.5 | 107 | 1.7 | 1811 | 1.5 | 0.397 |
| Hematologic | 1639 | 1.3 | 91 | 1.4 | 1548 | 1.3 | 0.464 |
| Toxic | 1322 | 1.1 | 63 | 1.0 | 1259 | 1.1 | 0.520 |
| Others 2 | 2352 | 1.9 | 131 | 2.0 | 2221 | 1.9 | 0.358 |
| Treatment 3 | |||||||
| None | 119,519 | 96.1 | 6215 | 96.9 | 113,304 | 96.0 | 0.004 |
| Bisphosphonates (BIs) | 928 | 0.7 | 44 | 0.7 | 884 | 0.7 | |
| Estrogens and Progestinic (EPs) | 1550 | 1.2 | 53 | 0.8 | 1497 | 1.3 | |
| Estrogen Modulators | 11 | 0.0 | 0 | 0.0 | 11 | 0.0 | |
| Cholecalciferol ± calcium (CC) | 2385 | 1.9 | 100 | 1.6 | 2285 | 1.9 | |
| Infection by SARS-CoV-2 | Hospitalization | Death | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Age | ||||||
| 1-year increase | 1.021 | 1.019–1.023 | 1.018 | 1.013–1.023 | 1.095 | 1.085–1.106 |
| Type of comorbidity | ||||||
| None | 1.00 | - | 1.00 | – | 1.00 | – |
| Neurologic | 1.11 | 1.02–1.21 | 1.24 | 1.00–1.52 | ||
| Psychiatric | 1.11 | 0.98–1.24 | 0.83 | 0.63–1.07 | 1.22 | 0.89–1.66 |
| Cardiovascular | 0.96 | 0.90–1.03 | 0.89 | 0.77–1.04 | 0.89 | 0.74–1.07 |
| Gastroenterological | 0.89 | 0.75–1.05 | 1.22 | 0.84–1.76 | ||
| Hematologic | 0.84 | 0.67–1.03 | ||||
| Toxic | 0.79 | 0.60–1.01 | ||||
| Renal | 1.90 | 1.41–2.57 | 1.75 | 1.24–2.46 | ||
| Rheumatologic | 1.52 | 1.15–2.01 | 1.44 | 0.96–2.12 | ||
| Respiratory | 1.51 | 1.20–1.90 | 1.32 | 1.01–1.73 | ||
| Treatment | ||||||
| None | 1.00 | – | 1.00 | – | 1.00 | – |
| Bisphosphonates (BIs) | 0.80 | 0.58–1.08 | 1.23 | 0.61–2.34 | 1.08 | 0.38–2.61 |
| Estrogens–Progestin (EP) | 0.88 | 0.66–1.15 | 0.83 | 0.36–1.69 | <0.001 | −3.01 |
| Estrogen Modulators (EMs) | <0.001 | 0.00−3.37 | ||||
| Cholecalciferol ± calcium (CC) | 0.74 | 0.60–0.91 | 2.69 | 1.77–4.07 | 0.80 | 0.35–1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocetta, V.; Zorzi, M.; Bejor, S.; Cesta, M.C.; De Pizzol, M.; Theurillat, J.-P.; Allegretti, M.; Alimonti, A.; Montopoli, M.; Rugge, M. Retrospective Analysis of the Effect of Postmenopausal Women Medications on SARS-CoV-2 Infection Progression. Life 2024, 14, 1107. https://doi.org/10.3390/life14091107
Cocetta V, Zorzi M, Bejor S, Cesta MC, De Pizzol M, Theurillat J-P, Allegretti M, Alimonti A, Montopoli M, Rugge M. Retrospective Analysis of the Effect of Postmenopausal Women Medications on SARS-CoV-2 Infection Progression. Life. 2024; 14(9):1107. https://doi.org/10.3390/life14091107
Chicago/Turabian StyleCocetta, Veronica, Manuel Zorzi, Stefano Bejor, Maria Candida Cesta, Maria De Pizzol, Jean-Philippe Theurillat, Marcello Allegretti, Andrea Alimonti, Monica Montopoli, and Massimo Rugge. 2024. "Retrospective Analysis of the Effect of Postmenopausal Women Medications on SARS-CoV-2 Infection Progression" Life 14, no. 9: 1107. https://doi.org/10.3390/life14091107







