Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep
Abstract
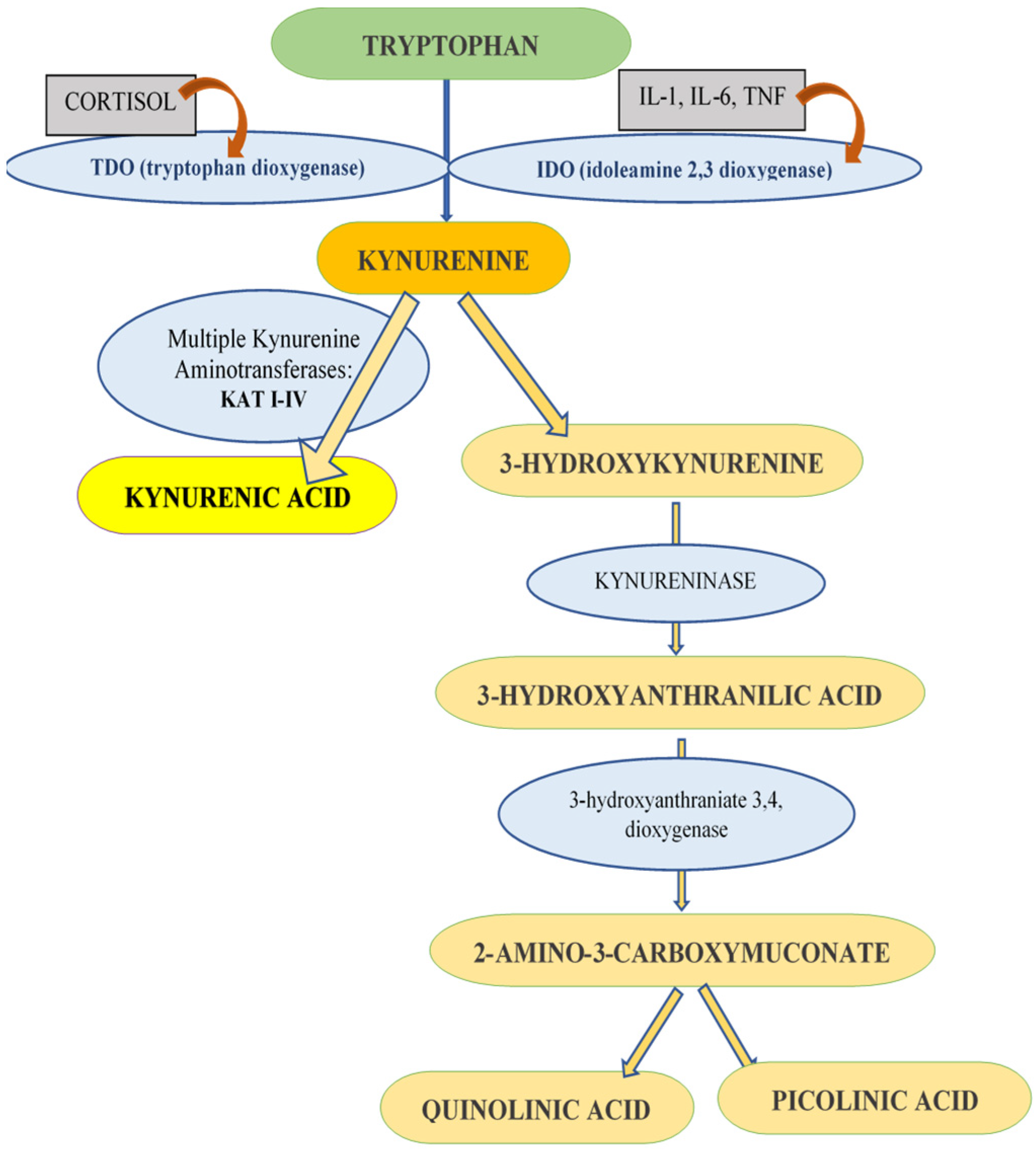
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. The Fluorescence of Blood Plasma Samples
2.3. Computed Tomography (CT)
2.4. Statistical Analysis
3. Results
3.1. Fluorescence Spectroscopy Plasma Analysis
3.2. CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varcasia, A.; Tamponi, C.; Ahmed, F.; Cappai, M.G.; Porcu, F.; Mehmood, N.; Dessì, G.; Scala, A. Taenia multiceps coenurosis: A review. Parasites Vectors 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Zakian, A.; Farjanikish, G.; Yeganeh, F.F.; Raisi, A.; Samadipoor, M. Clinical report of Coenurosis cerebralis outbreak in Lori sheep. Comp. Clin. Pathol. 2021, 30, 729–733. [Google Scholar] [CrossRef]
- Scala, A.; Cancedda, G.M.; Varcasia, A.; Ligios, C.; Garippa, G.; Genchi, C. A survey of Taenia multiceps coenurosis in Sardinian sheep. Vet. Parasitol. 2007, 143, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.R. Diagnosis and treatment of coenurosis in sheep. Vet. Parasitol. 2012, 189, 75–78. [Google Scholar] [CrossRef]
- Evangelisti, M.A.; Varcasia, A.; Deiana, R.; Zobba, R.; Passino, E.S.; Scala, A.; Melosu, V.; Pipia, A.P.; Tamponi, C.; Manunta, M.L. Clinical evolution of cerebral coenurosis from invasive to chronic infec tion in sheep and a goat. J. Infect. Dev. Ctries. 2016, 31, 10. [Google Scholar] [CrossRef][Green Version]
- Shahzad, A.; Edetsberger, M.; Koehler, G. Fluorescence Spectroscopy: An Emerging Excellent Diagnostic Tool in Medical Sciences. Appl. Spectrosc. Rev. 2010, 45, 1–11. [Google Scholar] [CrossRef]
- Musteata, M.; Nicolescu, A.; Solcan, G.; Deleanu, C. The 1H NMR Profile of Healthy Dog Cerebrospinal Fluid. PLoS ONE 2013, 8, e81192. [Google Scholar] [CrossRef]
- Ciobanu, D.M.; Olar, L.E.; Ștefan, R.; Veresiu, I.A.; Bala, C.G.; Mircea, P.A.; Roman, G. Fluorophores advanced glycation end products (AGEs)-to-NADH ratio is predictor for diabetic chronic kidney and cardiovascular disease. J. Diabetes Its Complicat. 2015, 29, 893–897. [Google Scholar] [CrossRef]
- Olar, L.E.; Ciobanu, D.M.; Matei, F.; Papuc, I. The assessment of fluorophores advanced glycation end products-to-kynurenine ratio in healthy and diabetic rats and humans. Stud. UBB Chem. 2018, LXIII, 37–53. [Google Scholar] [CrossRef]
- Lim, C.; Bilgin, A.; Lovejoy, D.; Tan, V.; Bustamante, S.; Taylor, B.T.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.M.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef]
- Vamos, E.; Pardutz, A.; Klivenyi, P.; Toldi, J.; Vecsei, L. The role of kynurenines in disorders of the central nervous system: Possibilities for neuroprotection. J. Neurol. Sci. 2009, 283, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Eftekhar, S.P. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int. J. Tryptophan Res. 2022, 28, 11786469221096643. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Whetsell, W.O., Jr.; Mangano, R.M. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science 1983, 219, 316–318. [Google Scholar] [CrossRef]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef]
- Muntean, C.M.; Stefan, R.; Tabaran, A.; Tripon, C.; Bende, A.; Falamas, A.; Colobatiu, L.M.; Olar, L.E. The influence of UV femtosecond laser pulses on bacterial DNA structure, as proved by Fourier transform infrared (FT-IR) spectroscopy. ChemistrySelect 2021, 6, 6957–6972. [Google Scholar] [CrossRef]
- Muntean, C.M.; Ştefan, R.; Tǎbǎran, A.; Bende, A.; Fǎlǎmaş, A.; Olar, L.E. Characterization of the Structural Changes of the Genomic DNA of Staphylococcus aureus Due to Femtosecond Laser Irradiation by Fourier Transform Infrared (FT-IR) Spectroscopy. Anal. Lett. 2024, 57, 711–726. [Google Scholar] [CrossRef]
- Tena, L.; De Miguel, R.; Castells, E.; Escudero, A.; Lacasta, D. Chronic coenurosis in sheep: Spontaneous remission of clinical signs and role of CT and MRI in the diagnosis and follow-up. Vet. Rec. Case Rep. 2020, 8, e001092. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches; Springer: Berlin/Heidelberg, Germany, 2017; pp. 117–138. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- Saito, K.; Markey, S.P.; Heyes, M.P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience 1992, 51, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Smythe, G.A.; Poljak, A.; Bustamante, S.; Braga, O.; Maxwell, A.; Grant, R.; Sachdev, P. ECNI GC-MS analysis of picolinic and quinolinic acids and their amides in human plasma, CSF, and brain tissue. Adv. Exp. Med. Biol. 2003, 527, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Campanale, A.; Inserra, A.; Comai, S. Therapeutic modulation of the kynurenine pathway in severe mental illness and comorbidities: A potential role for serotonergic psychedelics. Prog. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 134, 111058. [Google Scholar] [CrossRef] [PubMed]
- Mudry, J.M.; Alm, P.S.; Erhardt, S.; Goiny, M.; Fritz, T.; Caidahl, K.; Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 754–761. [Google Scholar] [CrossRef]
- Baumgartner, R.; Berg, M.; Matic, L.; Polyzos, K.P.; Forteza, M.J.; Hjorth, S.A.; Schwartz, T.W.; Paulsson-Berne, G.; Hansson, G.K.; Hedin, U.; et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J. Intern. Med. 2021, 289, 53–68. [Google Scholar] [CrossRef]
- Skorobogatov, K.; Autier, V.; Foiselle, M.; Richard, J.R.; Boukouaci, W.; Wu, C.L.; Raynal, S.; Carbonne, C.; Laukens, K.; Meysman, P.; et al. Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav. Immun.-Health 2023, 27, 100584. [Google Scholar] [CrossRef]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
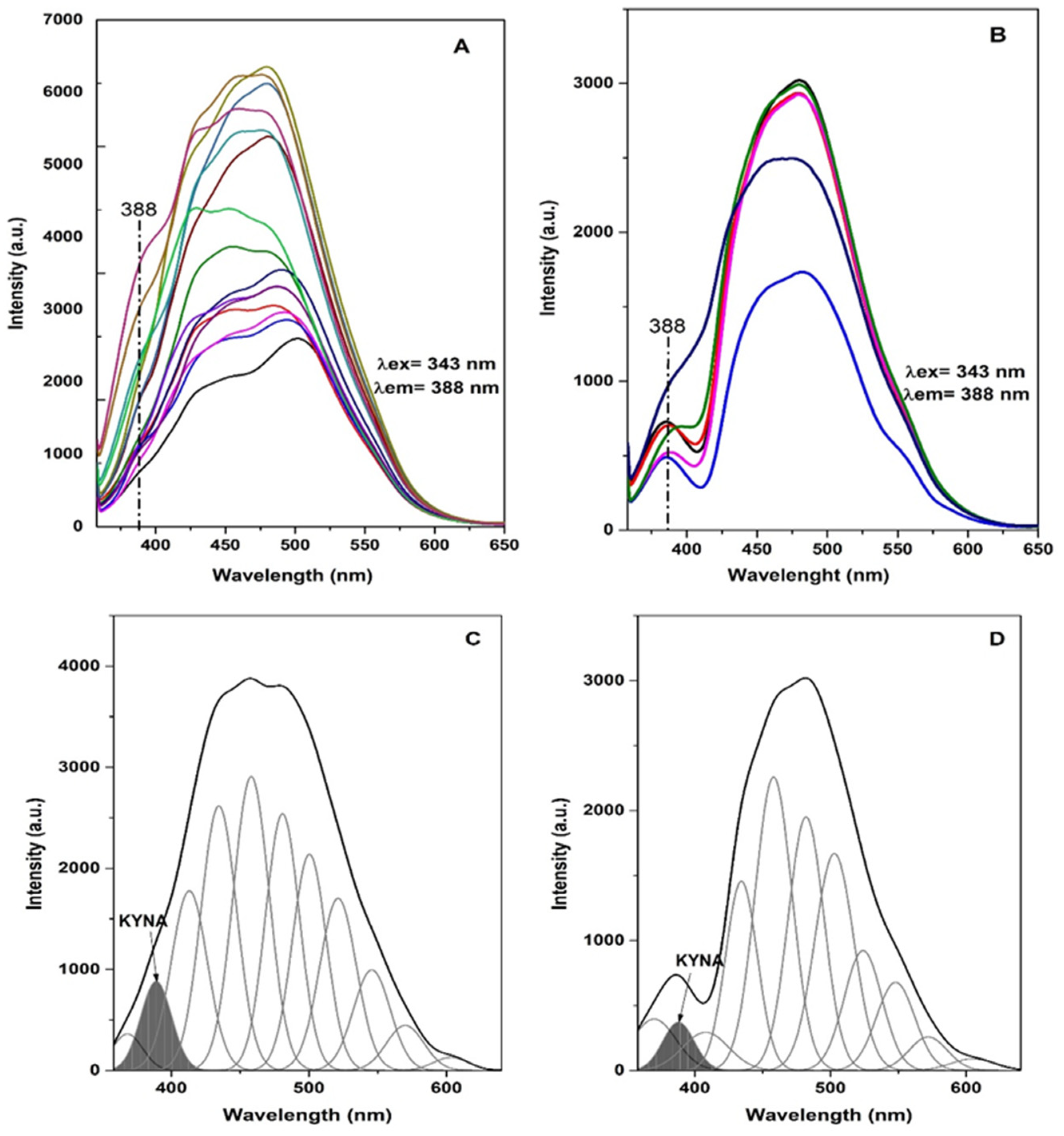

| AREA INT | FWHM | MAX HEIGHT | CENTER GRVTY | AREA INT P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B |
| 18,785 | 10,784 | 32 | 27 | 558 | 371 | 389 | 388 | 5.33 | 3.09 |
| 20,895 | 99,76 | 28.29 | 25 | 697 | 363 | 389 | 389 | 4.75 | 2.89 |
| 22,922 | 84,78 | 26.94 | 25 | 801 | 319 | 389 | 389 | 5.65 | 4.13 |
| 16,693 | 10,530 | 25.55 | 26 | 625 | 381 | 389 | 388 | 4.10 | 3.15 |
| 24,923 | 11,599 | 26 | 25 | 881 | 436 | 389 | 388 | 4.73 | 3.26 |
| 20,239 | 16,976 | 25 | 24 | 761 | 655 | 389 | 389 | 4.13 | 4.96 |
| 29,411 | 30 | 927 | 389 | 6.18 | |||||
| 22,469 | 26 | 814 | 389 | 4.98 | |||||
| 33,046 | 23 | 1312 | 389 | 4.65 | |||||
| 31,583 | 21 | 1380 | 388 | 3.72 | |||||
| 34,364 | 25 | 1301 | 389 | 4.37 | |||||
| 46,428 | 28 | 1565 | 389 | 6.23 | |||||
| 69,379 | 29 | 2260 | 388 | 7.91 | |||||
| 44,726 | 27 | 1565 | 389 | 7.21 | |||||
| 86,257 | 30 | 2671 | 388 | 10.15 | |||||
| Parameter | Group A | Group B |
|---|---|---|
| AREA INT | 28,225.27 ± 15,778.41 | 11,307.17 ± 3270.98 * |
| FWHM | 26.85 ± 3.14 | 25.33 ± 1.37 |
| MAX HEIGHT | 1046.87 ± 501.31 | 419.17 ± 141.94 * |
| AREA INT P | 5.12 ± 1.12 | 3.58 ± 1.11 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olar, L.E.; Tomoiagă, V.D.; Mârza, S.M.; Papuc, I.; Beteg, I.F.; Peștean, P.C.; Musteață, M.; Lăcătuș, C.M.; Marica, R.; Pașca, P.M.; et al. Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep. Life 2024, 14, 1121. https://doi.org/10.3390/life14091121
Olar LE, Tomoiagă VD, Mârza SM, Papuc I, Beteg IF, Peștean PC, Musteață M, Lăcătuș CM, Marica R, Pașca PM, et al. Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep. Life. 2024; 14(9):1121. https://doi.org/10.3390/life14091121
Chicago/Turabian StyleOlar, Loredana Elena, Vasile Daniel Tomoiagă, Sorin Marian Mârza, Ionel Papuc, Ioan Florin Beteg, Petru Cosmin Peștean, Mihai Musteață, Caroline Maria Lăcătuș, Raluca Marica, Paula Maria Pașca, and et al. 2024. "Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep" Life 14, no. 9: 1121. https://doi.org/10.3390/life14091121
APA StyleOlar, L. E., Tomoiagă, V. D., Mârza, S. M., Papuc, I., Beteg, I. F., Peștean, P. C., Musteață, M., Lăcătuș, C. M., Marica, R., Pașca, P. M., Purdoiu, R. C., & Lăcătuș, R. (2024). Computed Tomography and Fluorescence Spectroscopy Blood Plasma Analysis Study for Kynurenic Acid as a Diagnostic Approach to Chronic Coenurosis in Sheep. Life, 14(9), 1121. https://doi.org/10.3390/life14091121






