Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Search
2.2. Eligibility Criteria
2.3. Data Collection
2.4. Methodological Quality of Included Studies
3. Results
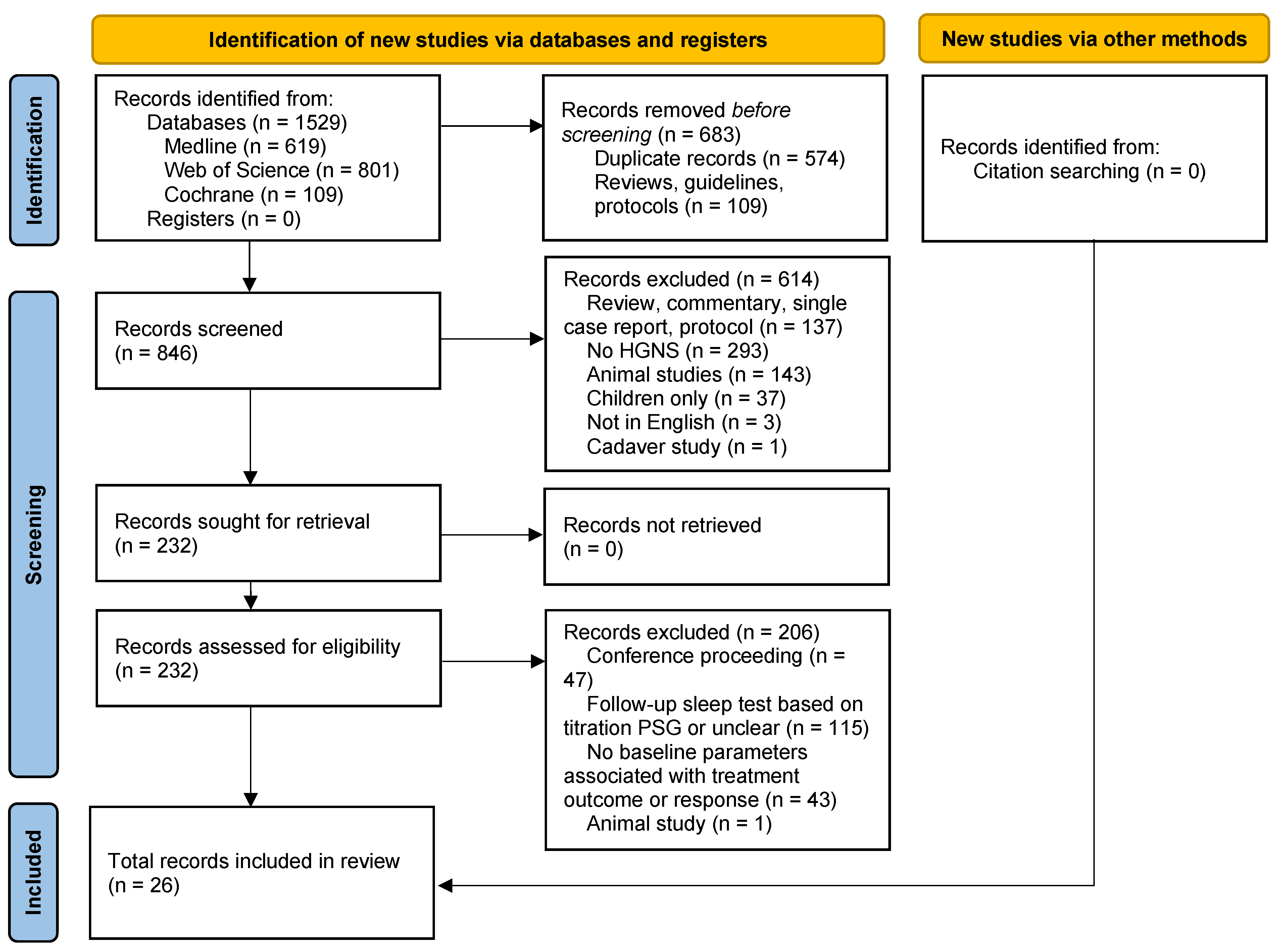
3.1. Search Results and Study Characteristics
3.2. Methodological Quality of Included Studies
3.3. Baseline Characteristics Associated with Treatment Response
3.3.1. Demographic Measurements
3.3.2. Clinical Measurements
3.3.3. Pathophysiological Measurements
3.3.4. Prior Upper Airway Surgery
4. Discussion
4.1. Baseline Characteristics
4.1.1. Demographic Measurements
4.1.2. Clinical Measurements
4.1.3. Pathophysiological Measurements
4.1.4. Other Measurements
4.2. Follow-Up
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A. Pathophysiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef]
- Dodds, S.; Williams, L.J.; Roguski, A.; Vennelle, M.; Douglas, N.J.; Kotoulas, S.-C.; Riha, R.L. Mortality and morbidity in obstructive sleep apnoea–hypopnoea syndrome: Results from a 30-year prospective cohort study. ERJ Open Res. 2020, 6, 00057–02020. [Google Scholar] [CrossRef]
- Ayas, N.T.; Taylor, C.M.; Laher, I. Cardiovascular consequences of obstructive sleep apnea. Curr. Opin. Cardiol. 2016, 31, 599–605. [Google Scholar] [CrossRef]
- Mitra, A.K.; Bhuiyan, A.R.; Jones, E.A. Association and Risk Factors for Obstructive Sleep Apnea and Cardiovascular Diseases: A Systematic Review. Diseases 2021, 9, 88. [Google Scholar] [CrossRef]
- Krüger, M.; Obst, A.; Ittermann, T.; Bernhardt, O.; Ivanovska, T.; Zygmunt, M.; Ewert, R.; Fietze, I.; Penzel, T.; Biffar, R.; et al. Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany. J. Clin. Med. 2023, 12, 2101. [Google Scholar] [CrossRef]
- Kapur, V.K. Obstructive Sleep Apnea: Diagnosis, Epidemiology, and Economics. Respir. Care 2010, 55, 1155–1167. [Google Scholar]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G.N. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Sullivan, C.; Berthon-Jones, M.; Issa, F.; Eves, L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981, 317, 862–865. [Google Scholar] [CrossRef]
- Vena, D.; Op de Beeck, S.; Mann, D.; Azarbarzin, A.; Marques, M.; Vanderveken, O.; Edwards, B.A.; Radmand, R.; Gell, L.; Messineo, L.; et al. Pharyngeal site of collapse and collapsibility estimated from airflow predict oral appliance treatment efficacy. Sleep Med. 2022, 100, S264–S265. [Google Scholar] [CrossRef]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral Appliance Treatment for Obstructive Sleep Apnea: An Update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Beyers, J.; Op de Beeck, S.; Dieltjens, M.; Willemen, M.; Verbraecken, J.A.; De Backer, W.A.; Van de Heyning, P.H. Development of a Clinical Pathway and Technical Aspects of Upper Airway Stimulation Therapy for Obstructive Sleep Apnea. Front. Neurosci. 2017, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Verbraecken, J.; Dieltjens, M.; Op de Beeck, S.; Vroegop, A.; Braem, M.; Vanderveken, O.; Randerath, W. Non-CPAP therapy for obstructive sleep apnoea. Breathe 2022, 18, 220164. [Google Scholar] [CrossRef]
- Randerath, W.J.; Verbraecken, J.; Andreas, S.; Bettega, G.; Boudewyns, A.; Hamans, E.; Jalbert, F.; Paoli, J.R.; Sanner, B.; Smith, I.; et al. Non-CPAP therapies in obstructive sleep apnoea. Eur. Respir. J. 2011, 37, 1000–1028. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; de Lange, J.; Hedner, J.; Ho, J.P.T.F.; Marklund, M.; Schiza, S.; Steier, J.; Verbraecken, J. Current and novel treatment options for obstructive sleep apnoea. ERJ Open Res. 2022, 8, 00126–02022. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Hoekema, A.; Weaver, E.M. Chapter 149—Upper Airway Surgery to Treat Obstructive Sleep-Disordered Breathing. In Principles and Practice of Sleep Medicine, 6th ed.; Kryger, M., Roth, T., Dement, W.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1463–1477.e1465. [Google Scholar]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Maurer, J.T.; Van de Heyning, P.; Lin, H.-S.; Baskin, J.; Anders, C.; Hohenhorst, W.; Woodson, B.T. Operative technique of upper airway stimulation: An implantable treatment of obstructive sleep apnea. Oper. Tech. Otolaryngol.-Head Neck Surg. 2012, 23, 227–233. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.-S.; Vroegop, A.V.; Anders, C.; Vries, N.d.; Heyning, P.H.V.d. Evaluation of Drug-Induced Sleep Endoscopy as a Patient Selection Tool for Implanted Upper Airway Stimulation for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2013, 9, 433–438. [Google Scholar] [CrossRef]
- Steffen, A.; Sommer, J.U.; Hofauer, B.; Maurer, J.T.; Hasselbacher, K.; Heiser, C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope 2018, 128, 509–515. [Google Scholar] [CrossRef]
- Thaler, E.; Schwab, R.; Maurer, J.; Soose, R.; Larsen, C.; Stevens, S.; Stevens, D.; Boon, M.; Huntley, C.; Doghramji, K.; et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope 2020, 130, 1333–1338. [Google Scholar] [CrossRef]
- Heiser, C.; Steffen, A.; Boon, M.; Hofauer, B.; Doghramji, K.; Maurer, J.T.; Sommer, J.U.; Soose, R.; Patrick, J.; Strollo, J.; et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur. Respir. J. 2019, 53, 1801405. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Strollo, P.J., Jr.; Soose, R.J. Upper Airway Stimulation for Obstructive Sleep Apnea: Past, Present, and Future. Sleep 2015, 38, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Kaplish, N. Hypoglossal Nerve Stimulator: A Novel Treatment Approach for OSA–Overview of Treatment, Including Diagnostic and Patient Criteria and Procedural Terminology Codes. Chest 2021, 160, 1406–1412. [Google Scholar] [CrossRef]
- Borowiecki, B.; Pollak, C.P.; Weitzman, E.D.; Rakoff, S.; Imperato, J. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope 1978, 88, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, K.; Van de Perck, E.; Kazemeini, E.; Willemen, M.; Van de Heyning, P.H.; Verbraecken, J.; Op de Beeck, S.; Vanderveken, O.M. Natural sleep endoscopy in obstructive sleep apnea: A systematic review. Sleep Med. Rev. 2021, 60, 101534. [Google Scholar] [CrossRef]
- Carrasco-Llatas, M.; Matarredona-Quiles, S.; De Vito, A.; Chong, K.B.; Vicini, C. Drug-Induced Sleep Endoscopy: Technique, Indications, Tips and Pitfalls. Healthcare 2019, 7, 93. [Google Scholar] [CrossRef]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Sanchez-Azofra, A.; Malhotra, A.; Owens, R.L.; Healy, W.J. Obstructive Sleep Apnea: Pathophysiology and Endotypes. ATS Sch. 2023, 4, 567–568. [Google Scholar] [CrossRef]
- Wellman, A.; Edwards, B.A.; Sands, S.A.; Owens, R.L.; Nemati, S.; Butler, J.; Passaglia, C.L.; Jackson, A.C.; Malhotra, A.; White, D.P. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J. Appl. Physiol. 2013, 114, 911–922. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; White, D.P.; Wellman, A. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Op de Beeck, S.; Vena, D.; Mann, D.; Azarbarzin, A.; Huyett, P.; Van de Perck, E.; Gell, L.K.; Alex, R.M.; Dieltjens, M.; Willemen, M.; et al. Polysomnographic airflow shapes and site of collapse during drug-induced sleep endoscopy. Eur. Respir. J. 2024, 63, 2400261. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. NICE Process and Methods Guides. In Methods for the Development of NICE Public Health Guidance; National Institute for Health and Care Excellence (NICE): London, UK, 2012. [Google Scholar]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The Efficacy of Surgical Modifications of the Upper Airway in Adults with Obstructive Sleep Apnea Syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef]
- Op de Beeck, S.; Wellman, A.; Dieltjens, M.; Strohl, K.P.; Willemen, M.; Van de Heyning, P.H.; Verbraecken, J.A.; Vanderveken, O.M.; Sands, S.A.; Investigators, T.S.T. Endotypic Mechanisms of Successful Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2021, 203, 746–755. [Google Scholar] [CrossRef]
- Lee, C.H.; Seay, E.G.; Walters, B.K.; Scalzitti, N.J.; Dedhia, R.C. Therapeutic Positive Airway Pressure Level Predicts Response to Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 1165–1172. [Google Scholar] [CrossRef]
- Bosschieter, P.F.N.; Vries, N.d.; Mehra, R.; Manchanda, S.; Padhya, T.A.; Vanderveken, O.M.; Ravesloot, M.J.L. Similar effect of hypoglossal nerve stimulation for obstructive sleep apnea in 5 disease severity categories. J. Clin. Sleep Med. 2022, 18, 1657–1665. [Google Scholar] [CrossRef]
- Coca, K.K.; Heiser, C.; Huntley, C.; Boon, M.; de Vries, N.; Mamidala, M.; Gillespie, M.B. Hypoglossal Nerve Stimulation Usage by Therapy Nonresponders. Otolaryngol.–Head Neck Surg. 2022, 166, 786–790. [Google Scholar] [CrossRef]
- Gao, T.Z.; Ma, J.; Hall, D.; Pan, X.; Rings, V.K.; Zia, A.; Harris, M.K.; Chio, E.G. Hypoglossal Nerve Stimulation Therapy Outcomes in Apnea-Versus Hypopnea-Predominant Patients. Otolaryngol.–Head Neck Surg. 2023, 169, 1674–1682. [Google Scholar] [CrossRef]
- Kant, E.; Hardeman, J.A.; Copper, M.P. The effect of velar collapse patterns on unilateral upper airway stimulation therapy. Sleep Breath. 2024, 28, 877–885. [Google Scholar] [CrossRef]
- Kant, E.; Hardeman, J.A.; Stokroos, R.J.; Copper, M.P. Treatment of a floppy epiglottis with upper airway stimulation therapy. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.T.; Carden, K.A.; Wang, L.; Lindsell, C.J.; Ishman, S.L. Evaluation of Hypoglossal Nerve Stimulation Treatment in Obstructive Sleep Apnea. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Goding Jr, G.S.; Malhotra, A.; O’Donoghue, F.J.; Zammit, G.; Wheatley, J.R.; Catcheside, P.G.; Smith, P.L.; Schwartz, A.R.; Walsh, J.H.; et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J. Sleep Res. 2014, 23, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Heiser, C.; Steffen, A.; Boon, M.; Hofauer, B.; Doghramji, K.; Maurer, J.T.; Sommer, J.U.; Soose, R.J.; Schwab, R.; et al. Previous Surgery and Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Otolaryngol.–Head Neck Surg. 2019, 161, 897–903. [Google Scholar] [CrossRef]
- Lee, C.H.; Seay, E.G.; Reese, J.W.; Wu, X.; Schwab, R.J.; Keenan, B.; Dedhia, R.C. Clinical Radiographic Predictors of Response to Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Otolaryngol.–Head Neck Surg. 2021, 164, 1122–1127. [Google Scholar] [CrossRef]
- Mulholland, G.B.; Dedhia, R.C. Success of Hypoglossal Nerve Stimulation Using Mandibular Advancement During Sleep Endoscopy. Laryngoscope 2020, 130, 2917–2921. [Google Scholar] [CrossRef]
- Ong, A.A.; Murphey, A.W.; Nguyen, S.A.; Soose, R.J.; Woodson, B.T.; Vanderveken, O.M.; de Vries, N.; Gillespie, M.B. Efficacy of Upper Airway Stimulation on Collapse Patterns Observed during Drug-Induced Sedation Endoscopy. Otolaryngol.–Head Neck Surg. 2016, 154, 970–977. [Google Scholar] [CrossRef]
- Renslo, B.; Hobelmann, K.; Sagalow, E.S.; Ananth, A.; Boon, M.; Huntley, C. Palatal Coupling Maneuvers Do Not Predict Hypoglossal Nerve Stimulator Treatment Efficacy. Laryngoscope 2023, 133, 431–436. [Google Scholar] [CrossRef]
- Schwab, R.J.; Wang, S.H.; Verbraecken, J.; Vanderveken, O.M.; Van de Heyning, P.; Vos, W.G.; DeBacker, J.W.; Keenan, B.T.; Ni, Q.; DeBacker, W. Anatomic predictors of response and mechanism of action of upper airway stimulation therapy in patients with obstructive sleep apnea. Sleep 2018, 41, zsy021. [Google Scholar] [CrossRef]
- Seay, E.G.; Keenan, B.T.; Schwartz, A.R.; Dedhia, R.C. Evaluation of Therapeutic Positive Airway Pressure as a Hypoglossal Nerve Stimulation Predictor in Patients with Obstructive Sleep Apnea. JAMA Otolaryngol.–Head Neck Surg. 2020, 146, 691–698. [Google Scholar] [CrossRef]
- Suurna, M.V.; Steffen, A.; Boon, M.; Chio, E.; Copper, M.; Patil, R.D.; Green, K.; Hanson, R.; Heiser, C.; Huntley, C.; et al. Impact of Body Mass Index and Discomfort on Upper Airway Stimulation: ADHERE Registry 2020 Update. Laryngoscope 2021, 131, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Van de Heyning, P.H.; Badr, M.S.; Baskin, J.Z.; Cramer Bornemann, M.A.; De Backer, W.A.; Dotan, Y.; Hohenhorst, W.; Knaack, L.; Lin, H.-S.; Maurer, J.T.; et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 2012, 122, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Modik, O.; Sturm, J.J.; Metkus, J.; Oaks-Leaf, R.; Kaplan, A.; Ni, Q.; Suurna, M. Neurophysiological profiles of responders and nonresponders to hypoglossal nerve stimulation: A single-institution study. J. Clin. Sleep Med. 2022, 18, 1327–1333. [Google Scholar] [CrossRef]
- Wirth, M.; Bautz, M.; von Meyer, F.; Hofauer, B.; Strassen, U.; Heiser, C. Obstruction level associated with outcome in hypoglossal nerve stimulation. Sleep Breath. 2022, 26, 419–427. [Google Scholar] [CrossRef]
- Withrow, K.; Evans, S.; Harwick, J.; Kezirian, E.; Strollo, P.; Investigators, A.R. Upper Airway Stimulation Response in Older Adults with Moderate to Severe Obstructive Sleep Apnea. Otolaryngol.–Head Neck Surg. 2019, 161, 714–719. [Google Scholar] [CrossRef]
- Yu, J.L.; Younes, M. Relation between arousability and outcome of upper airway stimulation in the Stimulation for Apnea Reduction (STAR) Trial. J. Clin. Sleep Med. 2021, 17, 797–801. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2018, 40, 1149–1157. [Google Scholar] [CrossRef]
- Punjabi, N.M. The Epidemiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Huyett, P.; Kent, D.T.; D’Agostino, M.A.; Green, K.K.; Soose, R.J.; Kaffenberger, T.M.; Woodson, B.T.; Huntley, C.; Boon, M.S.; Heiser, C.; et al. Drug-Induced Sleep Endoscopy and Hypoglossal Nerve Stimulation Outcomes: A Multicenter Cohort Study. Laryngoscope 2021, 131, 1676–1682. [Google Scholar] [CrossRef]
- Wellman, A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Passaglia, C.L.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J. Appl. Physiol. 2011, 110, 1627–1637. [Google Scholar] [CrossRef]
- Op de Beeck, S.; Vena, D.; Mann, D.; Azarbarzin, A.; Gell, L.; Van de Perck, E.; Alex, R.M.; Dieltjens, M.; Willemen, M.; Verbraecken, J.; et al. Identifying the site and pattern of pharyngeal collapse using polysomnographic airflow shapes. Sleep Med. 2022, 100, S252–S253. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Cistulli, P.A.; Sutherland, K.; Madronio, M.; Eckert, D.J.; Hess, L.; Edwards, B.A.; Wellman, A.; Sands, S.A. Polysomnographic Endotyping to Select Patients with Obstructive Sleep Apnea for Oral Appliances. Ann. Am.Thorac. Soc. 2019, 16, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, R.C.; Woodson, B.T. Standardized Reporting for Hypoglossal Nerve Stimulation Outcomes. J. Clin. Sleep Med. 2018, 14, 1835–1836. [Google Scholar] [CrossRef] [PubMed]
- Woodson, B.T.; Soose, R.J.; Gillespie, M.B.; Strohl, K.P.; Maurer MD, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Badr, M.S.; Lin, H.-s.; et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea. Otolaryngol.–Head Neck Surg. 2016, 154, 181–188. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.-s.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol.–Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Bliwise, D.L.; Quyyumi, A.A.; Thaler, E.R.; Boon, M.S.; Huntley, C.T.; Seay, E.G.; Tangutur, A.; Strollo, P.J.; Gurel, N.; et al. Hypoglossal Nerve Stimulation and Cardiovascular Outcomes for Patients with Obstructive Sleep Apnea: A Randomized Clinical Trial. JAMA Otolaryngol.–Head Neck Surg. 2024, 150, 39–48. [Google Scholar] [CrossRef]
- Crossley, J.R.; Wallerius, K.; Hoa, M.; Davidson, B.; Giurintano, J.P. Association Between Conflict of Interest and Published Position on Hypoglossal Nerve Stimulation for Sleep Apnea. Otolaryngol.–Head Neck Surg. 2021, 165, 375–380. [Google Scholar] [CrossRef]
| First Author | Year | Trial (If Applicable) | Design | Primary Outcome | Sample (R:NR) | Follow-Up Sleep Study (Months) | Baseline Characteristics | Responder Definition | Device | COI |
|---|---|---|---|---|---|---|---|---|---|---|
| Bosschieter [39] | 2022 | ADHERE | Retrospective analysis of prospective data | Change in AHI after 12 months of HGNS therapy for each group | 890 (593:297) | 12 | AHI (severity-groups) | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Coca [40] | 2022 | ADHERE | Retrospective analysis of prospective data | HGNS adherence in responders and non-responders | 717 (497:220) | 12 | Age, sex, BMI, AHI, ODI, ESS | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Gao [41] | 2023 | / | Retrospective case series | HGNS outcomes in apnea- and hypopnea-predominance | 56 | 12 | Apnea or hypopnea predominancy | ≥50% AHI reduction + AHI < 20/h | N/A | Yes |
| Heiser [23] | 2019 | ADHERE | Retrospective analysis of prospective data | Predictors of responsiveness and adherence of therapy use | 227 | 12 | Age, sex, BMI, AHI | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Kant (a) [42] | 2024 | / | Retrospective | Change in AHI after 12 months of HGNS therapy for each group | 56 (C-AP); 10 (AP/CCC) | 12 | Site of collapse | ≥50% AHI reduction + AHI < 20/h | Inspire | No |
| Kant (b) [43] | 2024 | / | Retrospective | Change in AHI during HGNS therapy for groups with and without floppy epiglottis | 75 (53:22) | 12 | Site of collapse | ≥50% AHI reduction + AHI < 20/h | Inspire | No |
| Kent [44] | 2019 | STAR, GPMS, ADHERE, US cohort study | Prospective and retrospective | OSA severity at baseline associated with postoperative HGNS outcomes | 584 (450:134) | 12 | Age, sex, BMI, AHI, neck circumference | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Kezirian [45] | 2014 | / | Prospective | Change in AHI after 12 months of HGNS therapy | 31 (17:14) | 12 | BMI | ≥50% AHI reduction + AHI < 20/h | Apnex | Yes |
| Kezirian [46] | 2019 | ADHERE | Retrospective analysis of prospective data | % responders at final visit for groups with prior upper airway surgery | 147 (116:31) | 12 | Prior upper airway surgery | ≥50% AHI reduction + AHI <20/h | Inspire | Yes |
| Lee [38] | 2019 | / | Pro- and retrospective | Change in AHI during HGNS therapy for low and high PAP group | 56 (31:25) | >6 | Therapeutic PAP level | ≥50% AHI reduction + AHI < 20/h (+ analyses on AHI < 10/h and < 5/h) | Inspire | No |
| Lee [47] | 2021 | / | Prospective and retrospective | Radiographic predictors of HGNS response during cephalometry | 51 (24:27) | 3–12 | Age, BMI, AHI | ≥50% AHI reduction + AHI < 20/h | Inspire | No |
| Mulholland [48] | 2020 | / | Prospective | Change in palate and oropharynx during mandibular advancement | 46 | N/A | Site of collapse | N/A | Inspire | No |
| Ong [49] | 2016 | STAR | Retrospective analysis of prospective data | Collapse patterns during baseline DISE predictive of HGNS response | 124(84:40) | 12 | Age, sex, BMI, AHI, site of collapse, neck size | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Op de Beeck [37] | 2021 | STAR | Retrospective analysis of prospective data | Pathophysiological mechanisms underlying favorable versus incomplete responses to HGNS therapy | 91 (53:38) | 12 | Age, sex, BMI, AHI, ODI, arousal threshold, collapsibility, muscle responsiveness, loop gain, SASHB | ≥50% AHI reduction + AHI < 10/h (+ sensitivity analyses on other definitions) | Inspire | Yes |
| Renslo [50] | 2023 | / | Retrospective | Maneuvers during DISE predictive of HGNS outcome | 171 (61:110) | N/A | Age, gender, AHI, oxygen nadir, site of collapse | ≥50% AHI reduction + AHI < 15/h | N/A | No |
| Schwab [51] | 2018 | / | Prospective | Anatomic predictors (site of collapse) of HGNS response | 13 (7:6) | 12 | Age, BMI, AHI | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Seay [52] | 2020 | / | Prospective | Therapeutic nasal PAP levels at the soft palate during DISE, predictive of HGNS response | 27 (18:9) | >3 | Age, sex, BMI, AHI, site of collapse | ≥50% AHI reduction | Inspire | No |
| Steffen [21] | 2018 | GPMS | Prospective | Change in AHI, ESS and FOSQ after 12 months of HGNS therapy | 56 (41:15) | 12 | Age, BMI, AHI, ODI, ESS, neck circumference, prior upper airway surgery | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Suurna [53] | 2021 | ADHERE | Retrospective analysis of prospective data | Change in AHI and ESS after 12 months of HGNS therapy, specifically in BMI ≤ 32 and between 32 and 35 | 535 | 12 | BMI | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Thaler [22] | 2020 | ADHERE | Retrospective analysis of prospective data | Change in AHI after 12 months of HGNS therapy and predictors | 382 (265:117) | 12 | Age, sex, BMI, AHI | ≥50% AHI reduction + AHI <20/h (+ sensitivity analyses on ≥50% AHI reduction + AHI < 10/h, and AHI < 10) | Inspire | Yes |
| Vanderveken [20] | 2013 | / | Prospective | Collapse patterns during baseline DISE predictive of HGNS response | 21 (13:8) | 6 | Site of collapse | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Van de Heyning [54] | 2012 | / | Prospective | Change in AHI and ODI after 6 months of HGNS therapy | 20 (6:14) | 6 | AHI, BMI, ESS | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Wang [55] | 2022 | / | Retrospective | Data from intra-operative tongue muscle activation associated with post-operative HGNS response | 46 (28:18) | 6-12 | Age, sex, BMI | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| Wirth [56] | 2022 | / | Prospective | Manometric sites of collapse predictive of HGNS response | 26 (11:15) | 3-12 | AHI | ≥50% AHI reduction + AHI < 15/h | N/A | Yes |
| Withrow [57] | 2019 | ADHERE | Retrospective analysis of prospective data | Change in AHI during HGNS therapy for old and young group | 250 | 12 | Age | N/A | Inspire | Yes |
| Yu [58] | 2021 | STAR | Retrospective analysis of prospective data | Change in AHI after 12 months of HGNS therapy | 105 (72:33) | 12 | Age, sex, BMI, AHI, ESS, neck circumference | ≥50% AHI reduction + AHI < 20/h | Inspire | Yes |
| First Author | Quality Assessment of Included Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Bosschieter 2022 [39] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Coca 2022 [40] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Gao 2023 [41] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Heiser 2019 [23] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Kant (a) 2024 [42] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Kant (b) 2024 [43] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Kent 2019 [44] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Kezirian 2014 [45] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Kezirian 2019 [46] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Lee 2019 [38] | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Lee 2021 [47] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Mulholland 2020 [48] | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Ong 2016 [49] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Op de Beeck 2021 [37] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Renslo 2023 [50] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Schwab 2018 [51] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Seay 2020 [52] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Steffen 2018 [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Suurna 2021 [53] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Thaler 2020 [22] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Vanderveken 2013 [20] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Van de Heyning 2012 [54] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wang 2022 [55] | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Wirth 2022 [56] | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Withrow 2019 [57] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Yu 2021 [58] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Baseline Characteristic | Association | # of Studies | References |
|---|---|---|---|
| Demographic | |||
| Age | Older age was associated with AHI reduction or treatment response | 1 | [23] |
| Older patients (≥65 years) had a larger AHI reduction than younger patients (<65 years) | 1 | [57] | |
| No association between age and treatment outcome | 1 | [22] | |
| Older age was associated with AHI reduction or treatment response | 2 | [44,49] | |
| No association between age and treatment outcome | 9 | [21,37,40,47,50,51,52,55,58] | |
| Sex | Female sex was associated with AHI reduction or treatment response | 1 | [22] |
| No association between sex and treatment outcome | 1 | [23] | |
| No association between sex and treatment outcome | 6 | [37,40,44,49,55,58] | |
| Female sex was associated with AHI reduction or treatment response | 1 | [52] | |
| Male gender was associated with AHI reduction in univariable analysis, but not in multivariable analysis | 1 | [50] | |
| BMI | Lower baseline BMI was associated with increased AHI reduction or treatment response | 2 | [22,23] |
| No difference in AHI reduction between groups with BMI ≤ 32 kg/m2 and 32 < BMI ≤ 35 kg/m2 | 1 | [53] | |
| No association between baseline BMI and treatment outcome | 6 | [21,47,49,51,52,58] | |
| Lower baseline BMI was associated with increased AHI reduction or treatment response | 6 | [37,40,44,50,54,55] | |
| Non-significant AHI reduction in group with BMI > 35 kg/m2, but significant AHI reduction in group with BMI ≤ 35 kg/m2 | 1 | [45] | |
| Clinical | |||
| AHI | No association between baseline AHI and treatment outcome | 3 | [22,23,39] |
| No association between baseline AHI and treatment outcome | 9 | [21,37,40,47,49,51,52,56,58] | |
| Higher baseline AHI was associated with increased AHI reduction or treatment response | 2 | [44,50] | |
| Lower baseline AHI was associated with increased treatment response | 1 | [54] | |
| ODI | No association between baseline ODI and treatment outcome | 2 | [37,40] |
| Lower baseline ODI was associated with treatment response | 1 | [21] | |
| ESS | No association between ESS and treatment response | 5 | [21,40,52,54,58] |
| Neck circumference | No association between neck circumference and treatment response | 4 | [21,44,49,58] |
| Apnea- or hypopnea-dependent | No association between apnea or hypopnea dependency (meaning either apnea or hypopnea predominancy) and treatment response | 1 | [41] |
| Oxygen nadir | Oxygen nadir was associated with AHI reduction in multivariable analysis, but not in univariable analysis | 1 | [50] |
| Pathophysiological | |||
| Site of collapse | No association between degree of oropharyngeal or tongue base collapse, and treatment response | 1 * | [49] |
| No association between presence of a floppy epiglottis and treatment response | 1 | [43] | |
| Absence of complete concentric collapse at the palate was associated with increased treatment response | 1 | [20] | |
| Complete collapse at the palate and lateral walls was associated with poorer response compared to patients who had partial collapse at the palate and lateral walls | 1 ** | [48] | |
| No association between either palatal or tongue base response during jaw thrust or chin lift and change in AHI | 1 | [50] | |
| Lower proportion of antero-posterior or latero-lateral collapse at the palate was associated with increased treatment response | 1 * | [49] | |
| Lower proportion of antero-posterior or latero-lateral collapse at the epiglottis was associated with increased treatment response | 1 * | [49] | |
| A decreased oropharyngeal response during mandibular advancement was associated with increased AHI reduction during HGNS | 1 ** | [48] | |
| A decreased palato-oropharyngeal response during mandibular advancement was associated with increased AHI reduction during HGNS | 1 ** | [48] | |
| No association between degree of palatal collapse and treatment response | 1 | [52] | |
| Higher baseline VOTE-score was associated with increased treatment response | 1* | [49] | |
| Endotypes (arousal threshold, muscle responsiveness, loop gain, collapsibility) | Higher arousal threshold was associated with treatment response. Higher muscle responsiveness was associated with treatment response, especially in patients with mild collapsibility. Lower loop gain was associated with treatment response, particularly in patients with milder upper airway collapsibility. Lower collapsibility was associated with treatment failure, specifically in patients with high loop gain and low arousal threshold. | 1 | [37] |
| Therapeutic PAP level | Low baseline PAP (<8 cm H2O) was associated with AHI reduction and treatment response | 1 | [38] |
| SASHB | No association between baseline SASHB and treatment response | 1 | [37] |
| Other | |||
| Prior upper airway surgery | No association between prior upper airway surgery and treatment outcome | 1 | [46] |
| No association between prior upper airway surgery and treatment outcome | 1 | [21] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukanov, E.; Van Loo, D.; Dieltjens, M.; Verbraecken, J.; Vanderveken, O.M.; Op de Beeck, S. Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review. Life 2024, 14, 1129. https://doi.org/10.3390/life14091129
Tukanov E, Van Loo D, Dieltjens M, Verbraecken J, Vanderveken OM, Op de Beeck S. Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review. Life. 2024; 14(9):1129. https://doi.org/10.3390/life14091129
Chicago/Turabian StyleTukanov, Eldar, Dorine Van Loo, Marijke Dieltjens, Johan Verbraecken, Olivier M. Vanderveken, and Sara Op de Beeck. 2024. "Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review" Life 14, no. 9: 1129. https://doi.org/10.3390/life14091129
APA StyleTukanov, E., Van Loo, D., Dieltjens, M., Verbraecken, J., Vanderveken, O. M., & Op de Beeck, S. (2024). Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review. Life, 14(9), 1129. https://doi.org/10.3390/life14091129









