Fractionated CO2 Laser in Combination with Topical Tacrolimus for Chronic Alopecia Areata: A Case Series Study
Abstract
1. Introduction
2. Materials and Methods
3. Results—Presentation of Cases
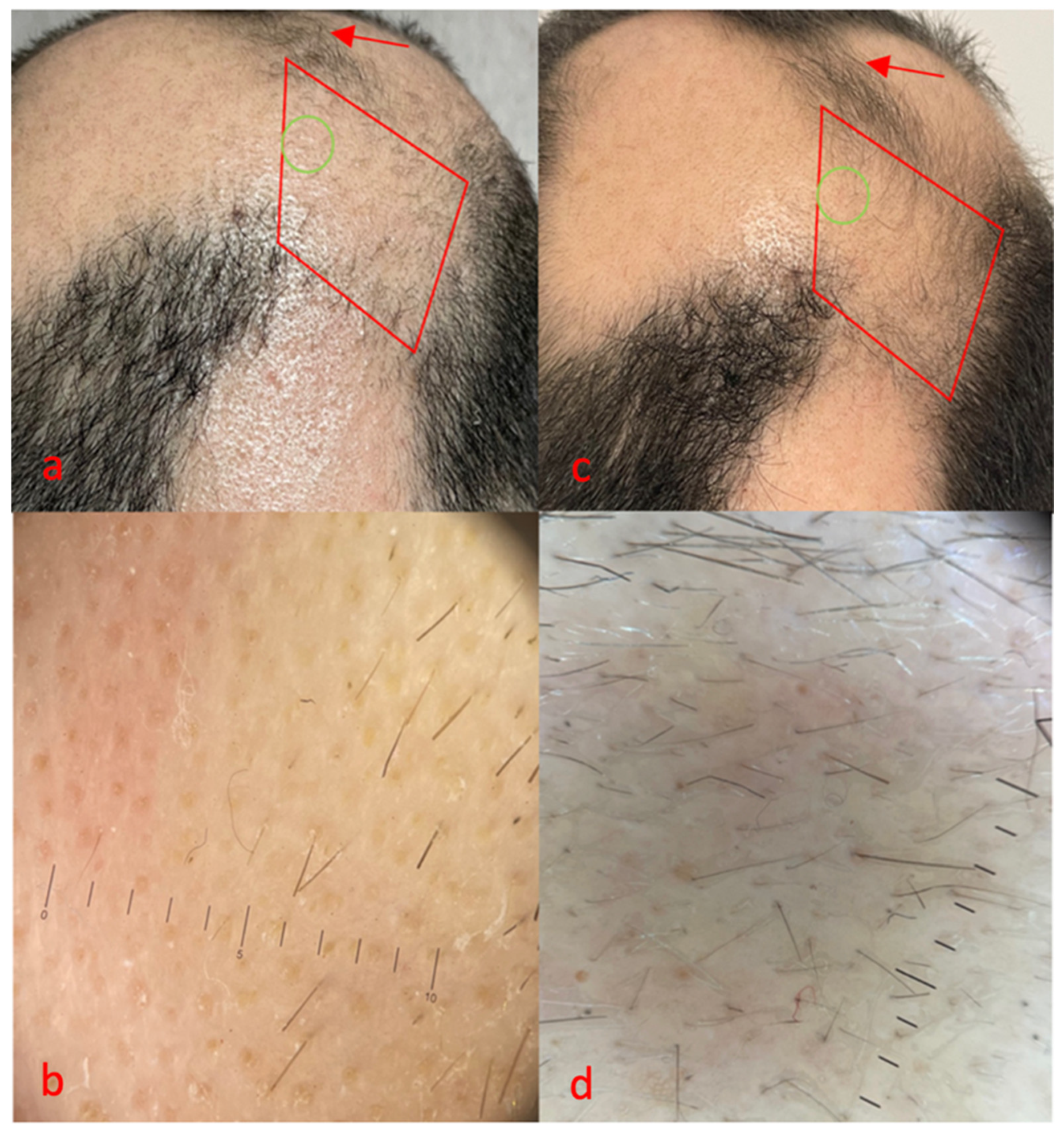
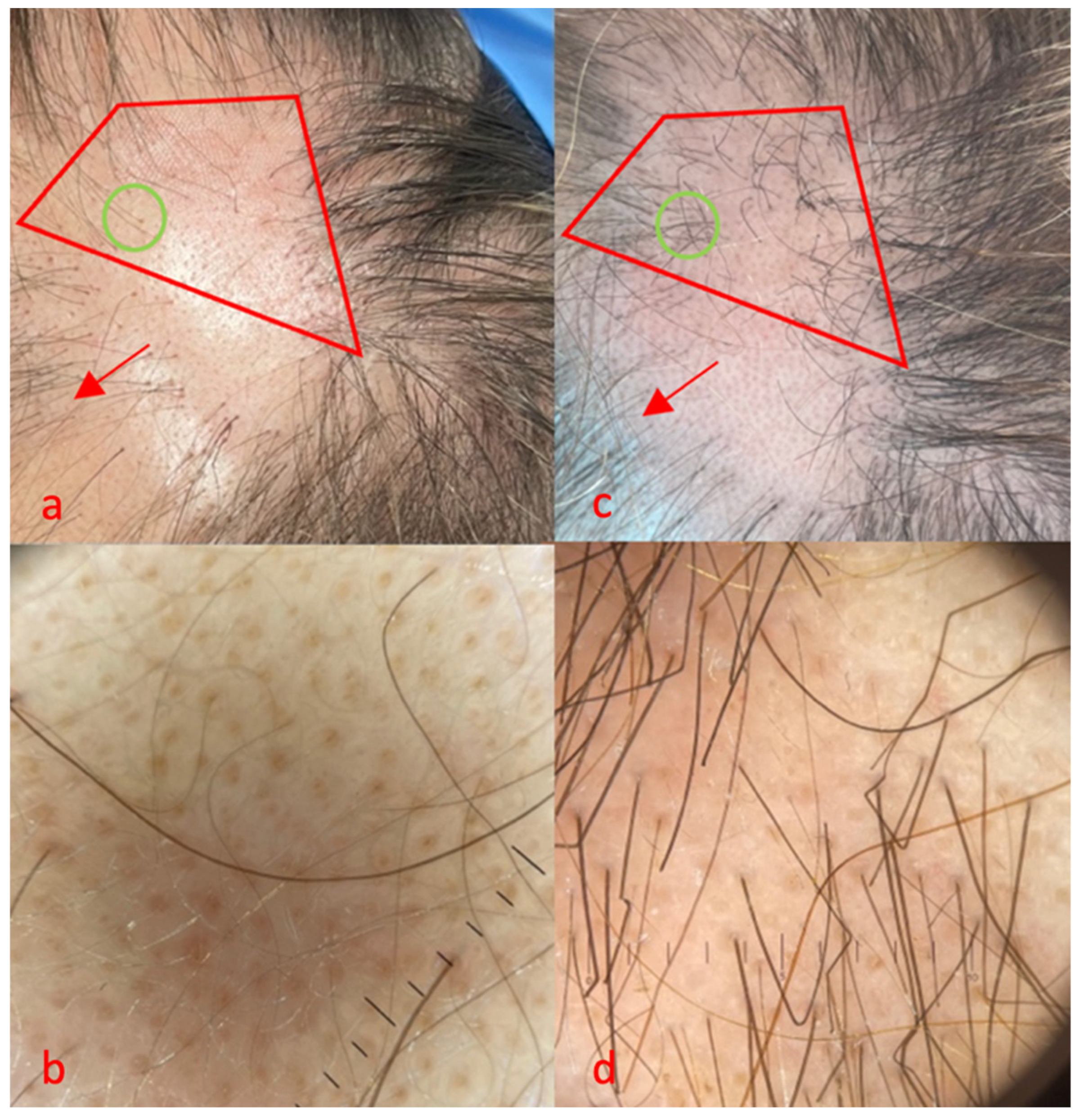
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, A.; Muscianese, M.; Piraccini, B.; Starace, M.; Carlesimo, M.; Mandel, V.D.; Alessandrini, A.; Calvieri, S.; Caro, G.; D’arino, A.; et al. Italian guidelines in diagnosis and treatment of alopecia areata. G. Ital. Dermatol. Venereol. 2019, 154, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Stefanato, C.M. Histopathology of alopecia: A clinicopathological approach to diagnosis. Histopathology 2010, 56, 24–38. [Google Scholar] [CrossRef]
- Nassar, A.; Elradi, M.; Radwan, M.; Albalat, W. Comparative evaluation of the efficacy of topical tacrolimus 0.03% and topical calcipotriol 0.005% mixed with betamethasone dipropionate versus topical clobetasol 0.05% in treatment of alopecia areata: A clinical and trichoscopic study. J. Cosmet. Dermatol. 2023, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Abd ElKawy, F.A.E.; Aly, S.H.M.; Ibrahim, S.M.A. Fractional CO2 laser versus microneedling as a transepidermal drug delivery system for the treatment of alopecia areata: A clinical dermoscopic evaluation. Dermatol. Ther. 2022, 35, e15553. [Google Scholar] [CrossRef] [PubMed]
- Meguid, A.M.A.; Ghazally, A.; Ahmed, A.M.; Bakr, R.M. Fractional carbon dioxide laser alone and as an assisted drug delivery for treatment of alopecia areata: A clinical, dermoscopic and immunohistochemical study. Arch. Dermatol. Res. 2023, 315, 1675–1688. [Google Scholar] [CrossRef]
- Ganjoo, S.; Thappa, D.M. Dermoscopic evaluation of therapeutic response to an intralesional corticosteroid in the treatment of alopecia areata. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 408–417. [Google Scholar]
- El-Husseiny, R.; Elframawy, S.; Abdallah, M. Comparative study between fractional carbon dioxide laser vs intralesional steroid injection in treatment of alopecia areata. Dermatol. Ther. 2020, 33, e13742. [Google Scholar] [CrossRef]
- Salah, M.; Samy, N.; Fawzy, M.M.; Farrag, A.R.; Shehata, H.; Hany, A. The Effect of the Fractional Carbon Dioxide Laser on Improving Minoxidil Delivery for the Treatment of Androgenetic Alopecia. J. Lasers Med. Sci. 2020, 11, 29–36. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Campanati, A.; Goteri, G.; Zizzi, A.; Marconi, B.; Ganzetti, G.; Minardi, D.; Di Primio, R.; Offidani, A.; et al. VEGF, survivin and NOS overexpression in psoriatic skin: Critical role of nitric oxide synthases. J. Dermatol. Sci. 2009, 54, 205–208. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Bernardini, M.L.; Simoncini, C.; Biagini, G.; Offidani, A. Expression of vascular endothelial growth factor, apoptosis inhibitors (survivin and p16) and CCL27 in alopecia areata before and after diphencyprone treatment: An immunohistochemical study. Br. J. Dermatol. 2004, 150, 940–948. [Google Scholar] [CrossRef]
- Radi, G.; Simonetti, O.; Rizzetto, G.; Diotallevi, F.; Molinelli, E.; Offidani, A. Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare 2021, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, H.; Srinivas, R.T.; Kuppuswamy, S.K.; Keloji, H.; Ravikumar, P.T. A comparative study of fractional CO2 laser with topical triamcinolone acetonide versus intralesional triamcinolone acetonide in the treatment of alopecia areata. J. Cutan. Aesthet. Surg. 2024, 17, 34–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majid, I.; Jeelani, S.; Imran, S. Fractional carbon dioxide laser in combination with topical corticosteroid application in resistant alopecia areata: A case series. J. Cutan. Aesthet. Surg. 2018, 11, 217–221. [Google Scholar] [CrossRef]
- Halim, D.A.; Nayer, M.; El-Samanoudy, S.I.; Raheem, H.M.A.; Ragab, N. Evaluation of fractional carbon dioxide laser alone versus its combination with betamethasone valerate in treatment of alopecia areata, a clinical and dermoscopic study. Arch. Dermatol. Res. 2023, 315, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Soror, O.A.; Abdel-Bary, A.; Khalil, B.M. Fractional carbon dioxide laser-assisted topical steroid delivery versus intralesional steroid in the treatment of alopecia areata. J. Egypt. Women’s Dermatol. Soc. 2021, 18, 43–51. [Google Scholar] [CrossRef]
- Yee, B.E.; Tong, Y.; Goldenberg, A.; Hata, T. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 1018–1021. [Google Scholar] [CrossRef]
- Dabek, R.J.; Roh, D.S.; Ozdemir, D.; Austen, W.G., Jr.; Bojovic, B. Fractional Laser-assisted Hair Regrowth and Microneedling for the Treatment of Alopecia Areata: A Review. Cureus 2019, 11, e4943. [Google Scholar] [CrossRef]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef]
- Cho, S.; Choi, M.J.; Zheng, Z.; Goo, B.; Kim, D.Y.; Cho, S.B. Clinical effects of non-ablative and ablative fractional lasers on various hair disorders: A case series of 17 patients. J. Cosmet. Laser Ther. 2013, 15, 74–79. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, M.N.; Kim, B.J.; Kim, C.W. Treatment of alopecia areata with fractional photothermolysis laser. Int. J. Dermatol. 2010, 49, 845–847. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzetto, G.; De Simoni, E.; Gioacchini, H.; Molinelli, E.; Offidani, A.; Simonetti, O. Fractionated CO2 Laser in Combination with Topical Tacrolimus for Chronic Alopecia Areata: A Case Series Study. Life 2024, 14, 1128. https://doi.org/10.3390/life14091128
Rizzetto G, De Simoni E, Gioacchini H, Molinelli E, Offidani A, Simonetti O. Fractionated CO2 Laser in Combination with Topical Tacrolimus for Chronic Alopecia Areata: A Case Series Study. Life. 2024; 14(9):1128. https://doi.org/10.3390/life14091128
Chicago/Turabian StyleRizzetto, Giulio, Edoardo De Simoni, Helena Gioacchini, Elisa Molinelli, Annamaria Offidani, and Oriana Simonetti. 2024. "Fractionated CO2 Laser in Combination with Topical Tacrolimus for Chronic Alopecia Areata: A Case Series Study" Life 14, no. 9: 1128. https://doi.org/10.3390/life14091128
APA StyleRizzetto, G., De Simoni, E., Gioacchini, H., Molinelli, E., Offidani, A., & Simonetti, O. (2024). Fractionated CO2 Laser in Combination with Topical Tacrolimus for Chronic Alopecia Areata: A Case Series Study. Life, 14(9), 1128. https://doi.org/10.3390/life14091128





