The Influence of Specific Pathogen-Free and Conventional Environments on the Hematological Parameters of Pigs Bred for Xenotransplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Complete Blood Cell (CBC) Analysis
2.4. Statistical Analysis
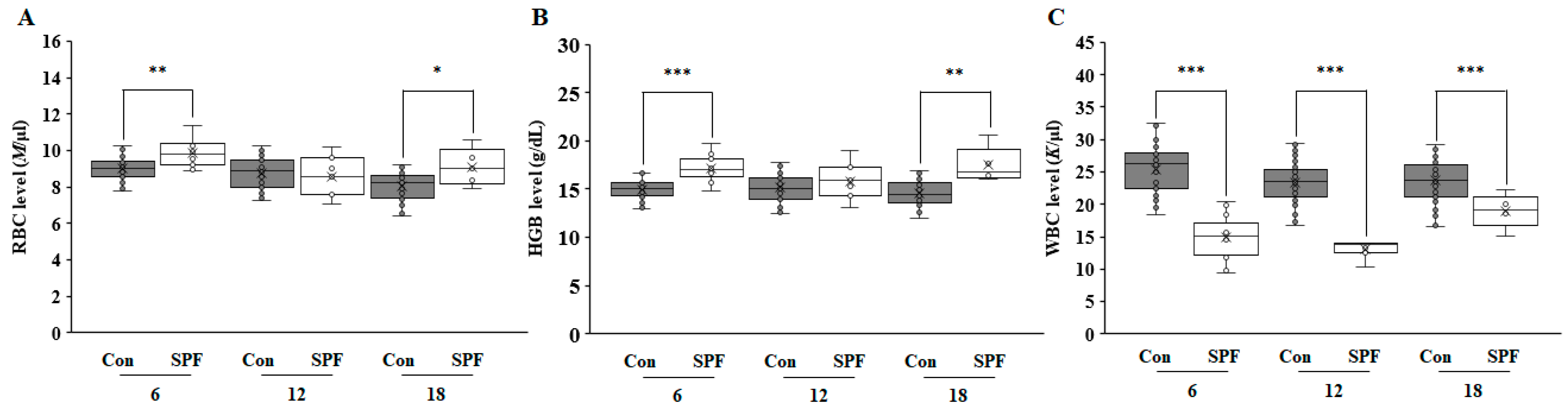
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burks, M.F.; Tumbleson, M.E.; Hicklin, K.W.; Hutcheson, D.P.; Middleton, C.C. Age and sex related changes of hematologic parameters in Sinclair(S-1) miniature swine. Growth 1977, 41, 51–62. [Google Scholar] [PubMed]
- Fisher, D.D.; Wilson, L.L.; Scholz, R.W. Environmental and genetic effects on hematologic characteristics of beef cows. Am. J. Vet. Res. 1980, 41, 1533–1536. [Google Scholar] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Stern, J.M.; Khalil, K.; Kim, J.I.; Narula, N.; Mangiola, M.; Weldon, E.P.; Kagermazova, L.; James, L.; Lawson, N.; et al. Pig-to-human heart xenotransplantation in two recently deceased human recipients. Nat. Med. 2023, 29, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Cozzi, E.; Spizzo, T.; Cowan, P.J.; Rayat, G.R.; Cooper, D.K.; Denner, J. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Executive summary. Xenotransplantation 2016, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Oh, K.B.; Kwon, D.-J.; Ock, S.-A.; Lee, J.-W.; Im, G.-S.; Lee, S.-S.; Lee, K.; Park, J.-K. Improvement of cloning efficiency in minipigs using post-thawed donor cells treated with roscovitine. Mol. Biotechnol. 2013, 55, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.H.; Leight, G.; Cone, J.; Schwarz, S.; Stuart, L.; Rosenberg, S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation 1976, 22, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Oh, K.B.; Roh, J.-H.; Jung, Y.-H.; Jung, S.-H.; Kang, M.-G.; Kim, M.-S.; Do, Y.J.; Oh, S.-I.; Kim, E.; et al. Comparison of hematological values of conventional pigs and transgenic pigs supressed in immune rejection response. Korean J. Vet. Serv. 2019, 42, 193–200. [Google Scholar] [CrossRef]
- Rispat, G.; Slaoui, M.; Weber, D.; Salemink, P.; Berthoux, C.; Shrivastava, R. Haematological and plasma biochemical values for healthy Yucatan micropigs. Lab. Anim. 1993, 27, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Lu, Y.; Xing, T.; Xu, D.; Zhang, K.; Zhang, S.; Wang, Y.; Yan, G.; Lan, G.; Liang, J. Blood metabolic and physiological profiles of Bama miniature pigs at different growth stages. Porc. Health Manag. 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Yamada, T.; Miura, N.; Takahashi, Y.; Yoshikawa, T.; Izumi, H.; Kawarasaki, T.; Miyoshi, N.; Tanimoto, A. Reference values of hematological and biochemical parameters for the world smallest microminipigs. J. Vet. Med. Sci. 2012, 74, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The baseline immunological and hygienic status of pigs impact disease severity of African swine fever. PLoS Pathog. 2022, 18, e1010522. [Google Scholar] [CrossRef] [PubMed]
- Friendship, R.M.; Lumsden, J.H.; McMillan, I.; Wilson, M.R. Hematology and biochemistry reference values for Ontario swine. Can. J. Comp. Med. 1984, 48, 390–393. [Google Scholar] [PubMed]
- Faustini, M.; Munari, E.; Colombani, C.; Russo, V.; Maffeo, G.; Vigo, D. Haematology and plasma biochemistry of Stamboek pre-pubertal gilts in Italy: Reference values. J. Vet. Med. A 2000, 47, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Atsma, F.; Veldhuizen, I.; de Kort, W.; van Kraaij, M.; Jong, P.P.-D.; Deinum, J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 2012, 60, 936–941. [Google Scholar] [CrossRef] [PubMed]
| Facilities | Month | No. of Pig | RBC (M/uL) | HGB (g/dL) | WBC (k/uL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | |||
| Convent-ional | 6 | 58 | 8.99 ± 0.59 | 7.78 | 10.26 | 14.92 ± 0.91 | 12.90 | 16.70 | 25.44 ± 3.51 | 18.37 | 32.45 |
| 12 | 52 | 8.76 ± 0.82 | 7.29 | 10.28 | 15.15 ± 1.39 | 12.50 | 17.80 | 23.33 ± 3.08 | 16.82 | 29.45 | |
| 18 | 49 | 8.03 ± 0.73 | 6.43 | 9.24 | 14.53 ± 1.27 | 12.00 | 16.90 | 23.72 ± 3.34 | 16.56 | 29.14 | |
| Normal reference * | 5.00 | 8.00 | 10.70 | 16.70 | 11.00 | 22.00 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.K.; Lee, H.-C.; Lee, S.; Lee, H.; Kim, S.E.; Lee, M.; No, J.-G.; Oh, K.B.; Lee, P. The Influence of Specific Pathogen-Free and Conventional Environments on the Hematological Parameters of Pigs Bred for Xenotransplantation. Life 2024, 14, 1132. https://doi.org/10.3390/life14091132
Lee WK, Lee H-C, Lee S, Lee H, Kim SE, Lee M, No J-G, Oh KB, Lee P. The Influence of Specific Pathogen-Free and Conventional Environments on the Hematological Parameters of Pigs Bred for Xenotransplantation. Life. 2024; 14(9):1132. https://doi.org/10.3390/life14091132
Chicago/Turabian StyleLee, Won Kil, Hwi-Cheul Lee, Seunghoon Lee, Haesun Lee, Sang Eun Kim, Minguk Lee, Jin-Gu No, Keon Bong Oh, and Poongyeon Lee. 2024. "The Influence of Specific Pathogen-Free and Conventional Environments on the Hematological Parameters of Pigs Bred for Xenotransplantation" Life 14, no. 9: 1132. https://doi.org/10.3390/life14091132
APA StyleLee, W. K., Lee, H.-C., Lee, S., Lee, H., Kim, S. E., Lee, M., No, J.-G., Oh, K. B., & Lee, P. (2024). The Influence of Specific Pathogen-Free and Conventional Environments on the Hematological Parameters of Pigs Bred for Xenotransplantation. Life, 14(9), 1132. https://doi.org/10.3390/life14091132







