Low-Grade Glioma Clinical Trials in the United States: A Systematic Review
Abstract
1. Introduction
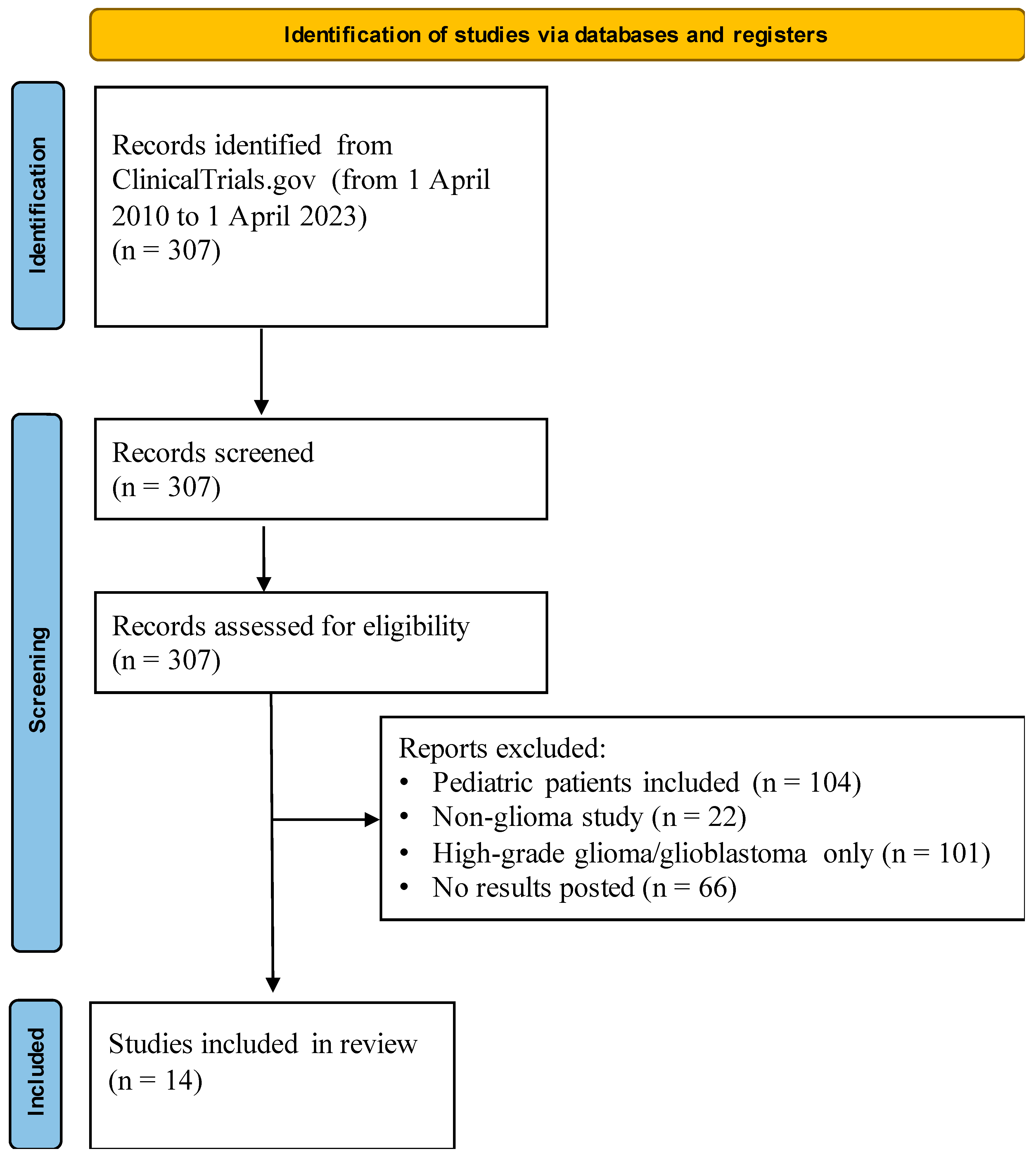
2. Materials and Methods
2.1. Data Collection
2.2. Trial Stratification and Analysis
2.3. Statistical Analysis
3. Results
3.1. Overview of Low-Grade Glioma Trials
Inclusion Criteria
3.2. Interventions and Primary Outcomes
Preliminary Results
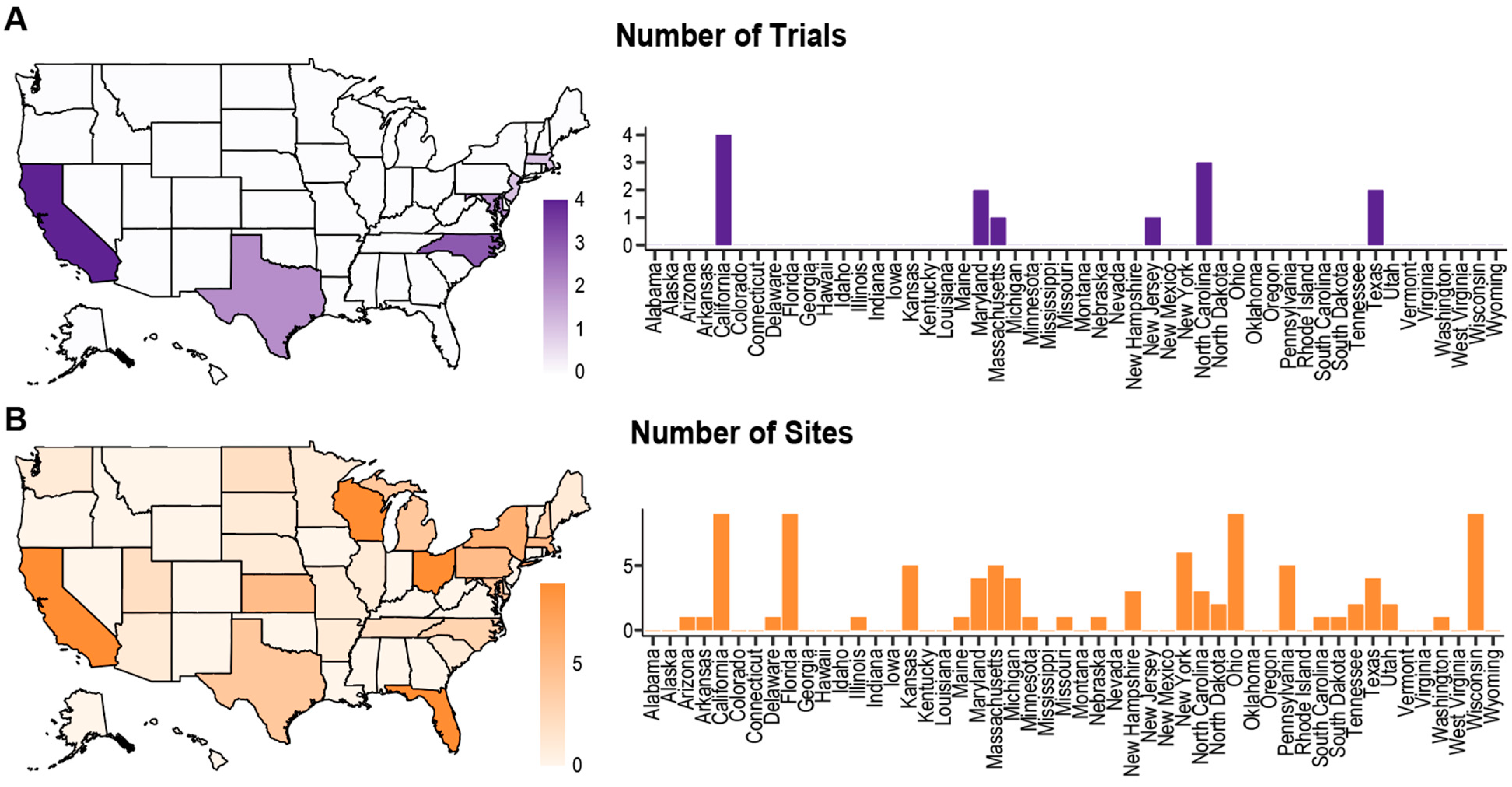
3.3. Demographic Recruitment and Reporting
3.3.1. Trial Characteristics
3.3.2. Race and Ethnicity
3.3.3. Sex and Age
3.3.4. Geographic Diversity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Youssef, G.; Miller, J.J. Lower Grade Gliomas. Curr. Neurol. Neurosci. Rep. 2020, 20, 21. [Google Scholar] [CrossRef]
- Cao, J.; Yan, W.; Zhan, Z.; Hong, X.; Yan, H. Epidemiology and risk stratification of low-grade gliomas in the United States, 2004-2019: A competing-risk regression model for survival analysis. Front. Oncol. 2023, 13, 1079597. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M.; Martino, J.; Lastra-Aras, E.; Dueñas-Polo, M.T. Diffuse low-grade glioma: A review on the new molecular classification, natural history and current management strategies. Clin. Transl. Oncol. 2017, 19, 931–944. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Pacanowski, M.; Bull, J.; Zhang, L. Racial/ethnic differences in drug disposition and response: Review of recently approved drugs. Clin. Pharmacol. Ther. 2015, 97, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.T.; Watkins, L.; Piña, I.L.; Elmer, M.; Akinboboye, O.; Gorham, M.; Jamerson, B.; McCullough, C.; Pierre, C.; Polis, A.B.; et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr. Probl. Cardiol. 2019, 44, 148–172. [Google Scholar] [CrossRef]
- Loree, J.M.; Anand, S.; Dasari, A.; Unger, J.M.; Gothwal, A.; Ellis, L.M.; Varadhachary, G.; Kopetz, S.; Overman, M.J.; Raghav, K. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. 2019, 5, e191870. [Google Scholar] [CrossRef]
- Flores, L.E.; Frontera, W.R.; Andrasik, M.P.; Del Rio, C.; Mondríguez-González, A.; Price, S.A.; Krantz, E.M.; Pergam, S.A.; Silver, J.K. Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials. JAMA Netw. Open 2021, 4, e2037640. [Google Scholar] [CrossRef]
- Flores, L.E.; Muir, R.; Weeks, I.; Burton Murray, H.; Silver, J.K. Analysis of Age, Race, Ethnicity, and Sex of Participants in Clinical Trials Focused on Eating Disorders. JAMA Netw. Open 2022, 5, e220051. [Google Scholar] [CrossRef]
- Duma, N.; Aguilera, J.V.; Paludo, J.; Haddox, C.L.; Velez, M.G.; Wang, Y.; Leventakos, K.; Hubbard, J.M.; Mansfield, A.S.; Go, R.S.; et al. Representation of Minorities and Women in Oncology Clinical Trials: Review of the Past 14 Years. J. Oncol. Pract. 2018, 14, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Association. 2015–2016 Drug Trials Snapshots Summary Report; U.S. Food and Drug Association: Silver Spring, MD, USA, 2016.
- National Cancer Institute. Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence—SEER Research Data, 8 Registries, Nov 2023 Sub (1975–2021)—Linked to County Attributes—Time Dependent (1990–2022) Income/Rurality, 1969–2022 Counties. Available online: www.seer.cancer.gov (accessed on 1 June 2024).
- Substance Abuse and Mental Health Services Administration. 2021 National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2023.
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. US Office of Budget and Management Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. Available online: https://orwh.od.nih.gov/toolkit/other-relevant-federal-policies/OMB-standards (accessed on 10 June 2024).
- National Institutes of Health. Temozolomide; 2023. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8206461/ (accessed on 10 August 2024).
- Moy, B.; Kirkpatrick, P.; Kar, S.; Goss, P. Lapatinib. Nat. Rev. Drug Discov. 2007, 6, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Winstead, E. Dabrafenib–Trametinib Combination Approved for Solid Tumors with BRAF Mutations; National Cancer Institute: London, UK, 2022. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-dabrafenib-trametinib-braf-solid-tumors (accessed on 10 August 2024).
- Widhalm, G.; Olson, J.; Weller, J.; Bravo, J.; Han, S.J.; Phillips, J.; Hervey-Jumper, S.L.; Chang, S.M.; Roberts, D.W.; Berger, M.S. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J. Neurosurg. 2019, 133, 79–88. [Google Scholar] [CrossRef]
- Naughton, M.; Case, D.; Peiffer, A.; Chan, M.; Stieber, V.; Moore, D.; Falchuk, S.; Piephoff, J.; Edenfield, W.; Giguere, J.; et al. Quality of life of irradiated brain tumor survivors treated with donepezil or placebo: Results of the WFU CCOP research base protocol 91105. Neuro-Oncol. Pract. 2017, 5, 114–121. [Google Scholar] [CrossRef]
- Page, B.R.; Shaw, E.G.; Lu, L.; Bryant, D.; Grisell, D.; Lesser, G.J.; Monitto, D.C.; Naughton, M.J.; Rapp, S.R.; Savona, S.R.; et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro-Oncology 2015, 17, 1393–1401. [Google Scholar] [CrossRef]
- Fisher, B.J.; Hu, C.; Macdonald, D.R.; Lesser, G.J.; Coons, S.W.; Brachman, D.G.; Ryu, S.; Werner-Wasik, M.; Bahary, J.P.; Liu, J.; et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: Preliminary results of Radiation Therapy Oncology Group 0424. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 497–504. [Google Scholar] [CrossRef]
- Wahl, M.; Chang, S.M.; Phillips, J.J.; Molinaro, A.M.; Costello, J.F.; Mazor, T.; Alexandrescu, S.; Lupo, J.M.; Nelson, S.J.; Berger, M.; et al. Probing the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in gliomas: A phase 2 study of everolimus for recurrent adult low-grade gliomas. Cancer 2017, 123, 4631–4639. [Google Scholar] [CrossRef]
- Tabrizi, S.; Yeap, B.Y.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Dworkin, M.; Fullerton, B.C.; Daartz, J.; Royce, T.J.; Oh, K.S.; et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiother. Oncol. 2019, 137, 95–101. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Yuan, Y.; Wu, J.; Mendoza, T.; Vera, E.; Omuro, A.; Lieberman, F.; Robins, H.I.; Gerstner, E.R.; Wu, J.; et al. A phase II study of dose-dense temozolomide and lapatinib for recurrent low-grade and anaplastic supratentorial, infratentorial, and spinal cord ependymoma. Neuro-Oncology 2021, 23, 468–477. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Omuro, A.; Yuan, Y.; Mendoza, T.; Wall, K.; Grajkowska, E.; Vera, E.; Reyes, J.; Aldape, K.; Penas-Prado, M.; et al. JS01.5.A phase 2 trial of carboplatin and bevacizumab for recurrent adult ependymoma. a cern study. Neuro-Oncology 2023, 25, ii7. [Google Scholar] [CrossRef]
- Angione, A.; Patterson, J.; Akca, E.; Xu, J.; Xu, E.; Raab, V.; Elghawy, O.; Barsouk, A.A.; Sussman, J.H. A Cross-Sectional Analysis of Interventional Clinical Trials in High-Grade Glioma Therapy. Life 2024, 14, 926. [Google Scholar] [CrossRef]
- Sloan, A.E.; Okada, H.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. The role of emerging therapy in the management of patients with diffuse low grade glioma. J. Neuro-Oncol. 2015, 125, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Simon, R.; Foulkes, M.A.; Friedman, L.; Geller, N.L.; Gordon, D.J.; Mowery, R. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993—The perspective of NIH clinical trialists. Control Clin. Trials 1995, 16, 277–285; discussion 286–279, 293–309. [Google Scholar] [CrossRef]
- National Institutes of Health. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. 2017. Available online: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm#:~:text=It%20is%20the%20policy%20of,that%20inclusion%20is%20inappropriate%20with (accessed on 1 June 2024).
- U.S. Food and Drug Association. Drug Trials Snapshot: Summary Report; U.S. Food and Drug Association: Silver Spring, MD, USA, 2019.
- Taha, B.; Winston, G.; Tosi, U.; Hartley, B.; Hoffman, C.; Dahmane, N.; Mason, C.E.; Greenfield, J.P. Missing diversity in brain tumor trials. Neuro-Oncol. Adv. 2020, 2, vdaa059. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.M.; Penner, L.A.; Albrecht, T.L.; Heath, E.; Gwede, C.K.; Eggly, S. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control 2016, 23, 327–337. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Jensen, G.A. Diverging racial and ethnic disparities in access to physician care: Comparing 2000 and 2007. Med. Care 2012, 50, 327–334. [Google Scholar] [CrossRef]
- Morshed, R.A.; Reihl, S.J.; Molinaro, A.M.; Kakaizada, S.; Young, J.S.; Schulte, J.D.; Butowski, N.; Taylor, J.; Bush, N.A.; Aghi, M.K.; et al. The influence of race and socioeconomic status on therapeutic clinical trial screening and enrollment. J. Neuro-Oncol. 2020, 148, 131–139. [Google Scholar] [CrossRef]
- Jirka, G.W.; Bisselou, K.S.M.; Smith, L.M.; Shonka, N. Evaluating the decisions of glioma patients regarding clinical trial participation: A retrospective single provider review. Med. Oncol. 2019, 36, 34. [Google Scholar] [CrossRef]
- Greenwald, P.; Cullen, J.W.; McKenna, J.W. Cancer prevention and control: From research through applications. J. Natl. Cancer Inst. 1987, 79, 389–400. [Google Scholar]
| Clinical Trial Number | Study Title | Interventional Agent Investigated | Primary Outcome | Sponsor | Number of Participants | Number of Recruitment Sites | Results Submitted Year | Phase | Funder Type |
|---|---|---|---|---|---|---|---|---|---|
| NCT00492089 | Bevacizumab in Reducing CNS Side Effects in Patients Who Have Undergone Radiation Therapy to the Brain for Primary Brain Tumor, Meningioma, or Head and Neck Cancer | Bevacizumab | Radiographic response rate (>25% Reduction in T2 Flair) at 6 weeks post-treatment | National Cancer Institute (NCI) | 11 | 1 | 2012 | Phase 2 | NIH |
| NCT00369785 | Donepezil in Treating Patients Who Have Undergone Radiation Therapy for Brain Tumors | Donepezil hydrochloride | Memory as measured by the Hopkins Verbal Learning Test (HVLT) | Wake Forest University Health Sciences | 198 | 16 | 2015 | Phase 3 | Other |
| NCT01032200 | Armodafinil in Treating Fatigue Caused By Radiation Therapy in Patients With Primary Brain Tumors | Armodafinil | Retention of participants 4 weeks post-radiotherapy | Wake Forest University Health Sciences | 54 | 1 | 2016 | Phase 2 | Other |
| NCT00114140 | Temozolomide and Radiation Therapy in Treating Patients With Gliomas | Temozolomide and Radiation Therapy | Overall Survival Rate at 3 Years Progression-free survival time Quality of life as measured by the Functional Assessment of Cancer Therapy Scale With Brain Module (FACT-BR) Phonemic verbal fluency as measured by the Controlled Oral Word Association test | Radiation Therapy Oncology Group | 136 | 47 | 2017 | Phase 2 | Network |
| NCT00823459 | Everolimus in Treating Patients with Recurrent Low-Grade Glioma | Everolimus | Progression-free survival at 6 Months | Susan Chang | 58 | 1 | 2017 | Phase 2 | Other |
| NCT00045110 | Erlotinib in Treating Patients with Recurrent Malignant Glioma or Recurrent or Progressive Meningioma | Erlotinib hydrochloride | Dose Limiting Toxicity (DLT) Maximum Tolerated Dose (MTD) 6 Months Progression-free survival | National Cancer Institute (NCI) | 136 | 7 | 2017 | Phase 1/2 | NIH |
| NCT00681473 | Late Effects of Proton Radiation Therapy in Patients with Low-Grade Glioma | Proton Radiation Therapy | Number of participants with late effects >3 months post radiotherapy | Massachusetts General Hospital | 20 | 1 | 2017 | NA | Other |
| NCT00313729 | Temozolomide in Treating Patients With Low-Grade Glioma | Temozolomide | Response rate at 1 year | University of California, San Francisco | 120 | 1 | 2018 | Phase 2 | Other |
| NCT01116661 | Safety Study of Aminolevulinic Acid (ALA) to Enhance Visualization and Resection of Tumors of the Brain | 5-Aminolevuline Acid (ALA) | Percentage of biopsies with tumorous content | University of California, San Francisco | 199 | 1 | 2018 | Phase 2 | Other |
| NCT00826241 | Dose-Dense Temozolomide + Lapatinib for Recurrent Ependymoma | Temozolomide and Lapatinib | Time to Progression up to 4 years | National Institutes of Health Clinical Center (CC) | 58 | 6 | 2019 | Phase 2 | NIH |
| NCT01635283 | Vaccine for Patients With Newly Diagnosed or Recurrent Low-Grade Glioma | Tumor lysate-pulsed autologous dendritic cell vaccine | Progression-free Survival (PFS) up to 44 months | Jonsson Comprehensive Cancer Center | 5 | 1 | 2019 | Phase 2 | Other |
| NCT01295944 | Carboplatin and Bevacizumab for Recurrent Ependymoma | Carboplatin and Bevacizumab | Progression-free Survival (PFS) after 1 year | National Cancer Institute (NCI) | 35 | 2 | 2021 | Phase 2 | NIH |
| NCT02193347 | IDH1 Peptide Vaccine for Recurrent Grade II Glioma | PEPIDH1M vaccine | Toxicity rate | Katy Peters, MD, PhD | 24 | 1 | 2021 | Phase 1 | Other |
| NCT02034110 | Efficacy and Safety of the Combination Therapy of Dabrafenib and Trametinib in Subjects With BRAF V600E- Mutated Rare Cancers | Dabrafenib and Trametinib | Overall response rate up to 92 months post-treatment | Novartis Pharmaceuticals | 13 | 41 | 2022 | Phase 2 | Industry |
| Characteristic | Number of Trials (% of All Trials), n = 14 |
|---|---|
| Intervention Type | |
| Drug | 11 (78.6%) |
| Radiation | 1 (7.1%) |
| Surgical | 0 (0%) |
| Biologic | 3 (21.4%) |
| Other | 0 (0%) |
| Utilizes Multiple Investigational Agents | 4 (28.6%) |
| Utilizes a Multimodal Interventional Strategy | 2 (14.3%) |
| Randomized | 3 (21.4%) |
| Testing Site Number | |
| Single Testing Site | 8 (57.1%) |
| Multiple Testing Sites | 6 (42.9%) |
| Testing Site Location | |
| Testing Sites in Multiple States | 6 (42.9%) |
| Multiple Testing Sites Across Single State | 4 (28.6%) |
| Includes International Testing Sites | 2 (14.3%) |
| Investigational Institution Type | |
| Private Investigational Institution | 5 (35.7%) |
| Public Investigational Institution | 5 (35.7%) |
| Mixed Investigational Institution | 4 (28.6%) |
| Demographics Reported | |
| Reports Race | 8 (57.1%) |
| Reports Race Consistent with OMB Standards | 6 (42.9%) |
| Reports Ethnicity | 6 (42.9%) |
| Reports Sex | 14 (100%) |
| Reports Age | 14 (100%) |
| Phase | |
| Phase 1 | 1 (7.1%) |
| Phase 1/2 | 1 (7.1%) |
| Phase 2 | 10 (71.4%) |
| Phase 3 | 1 (7.1%) |
| Funding Source | |
| NIH Funded | 4 (28.6%) |
| Industry Funded | 1 (7.1%) |
| Network Funded | 1 (7.1%) |
| Other Funder | 8 (57.1%) |
| Characteristic | Number of Trial Participants (% of Reported) n = 1067 | Number of LGG Patients in U.S. (%) n = 7306 | p-Value |
|---|---|---|---|
| Race | |||
| White | 558 (92.7%) | 6219 (85.9%) | <0.00001 * |
| Black or African American | 22 (3.7%) | 418 (5.8%) | 0.032 * |
| Asian or Pacific Islander | 20 (3.3%) | 536 (7.4%) | 0.00016 * |
| American Indian or Alaska Native | 1 (0.2%) | 69 (1.0%) | 0.051 |
| Other/Not Reported | 466 | 64 | |
| Ethnicity | |||
| Hispanic or Latino | 10 (2.6%) | 1228 (16.8%) | <0.00001 * |
| Not Hispanic or Latino | 382 (97.4%) | 6078 (83.2%) | |
| Not Reported | 685 | - | |
| Sex | |||
| Male | 575 (54.3%) | 4154 (56.9%) | 0.11184 |
| Female | 483 (45.7%) | 3152 (43.1%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, E.; Patterson, J.; Angione, A.; Li, A.; Wu, D.W.; Akca, E.; Elghawy, O.; Barsouk, A.; Sussman, J.H. Low-Grade Glioma Clinical Trials in the United States: A Systematic Review. Life 2024, 14, 1133. https://doi.org/10.3390/life14091133
Xu E, Patterson J, Angione A, Li A, Wu DW, Akca E, Elghawy O, Barsouk A, Sussman JH. Low-Grade Glioma Clinical Trials in the United States: A Systematic Review. Life. 2024; 14(9):1133. https://doi.org/10.3390/life14091133
Chicago/Turabian StyleXu, Emily, Jonathan Patterson, Angelo Angione, Alexander Li, David W. Wu, Ebrar Akca, Omar Elghawy, Alexander Barsouk, and Jonathan H. Sussman. 2024. "Low-Grade Glioma Clinical Trials in the United States: A Systematic Review" Life 14, no. 9: 1133. https://doi.org/10.3390/life14091133
APA StyleXu, E., Patterson, J., Angione, A., Li, A., Wu, D. W., Akca, E., Elghawy, O., Barsouk, A., & Sussman, J. H. (2024). Low-Grade Glioma Clinical Trials in the United States: A Systematic Review. Life, 14(9), 1133. https://doi.org/10.3390/life14091133





