Enhancing Cartilage Repair: Surgical Approaches, Orthobiologics, and the Promise of Exosomes
Abstract
:1. Introduction
2. Cartilage Overview
2.1. Human Cartilage: An Overview
2.2. Articular Cartilage: Lessons from Early Chondrogenesis
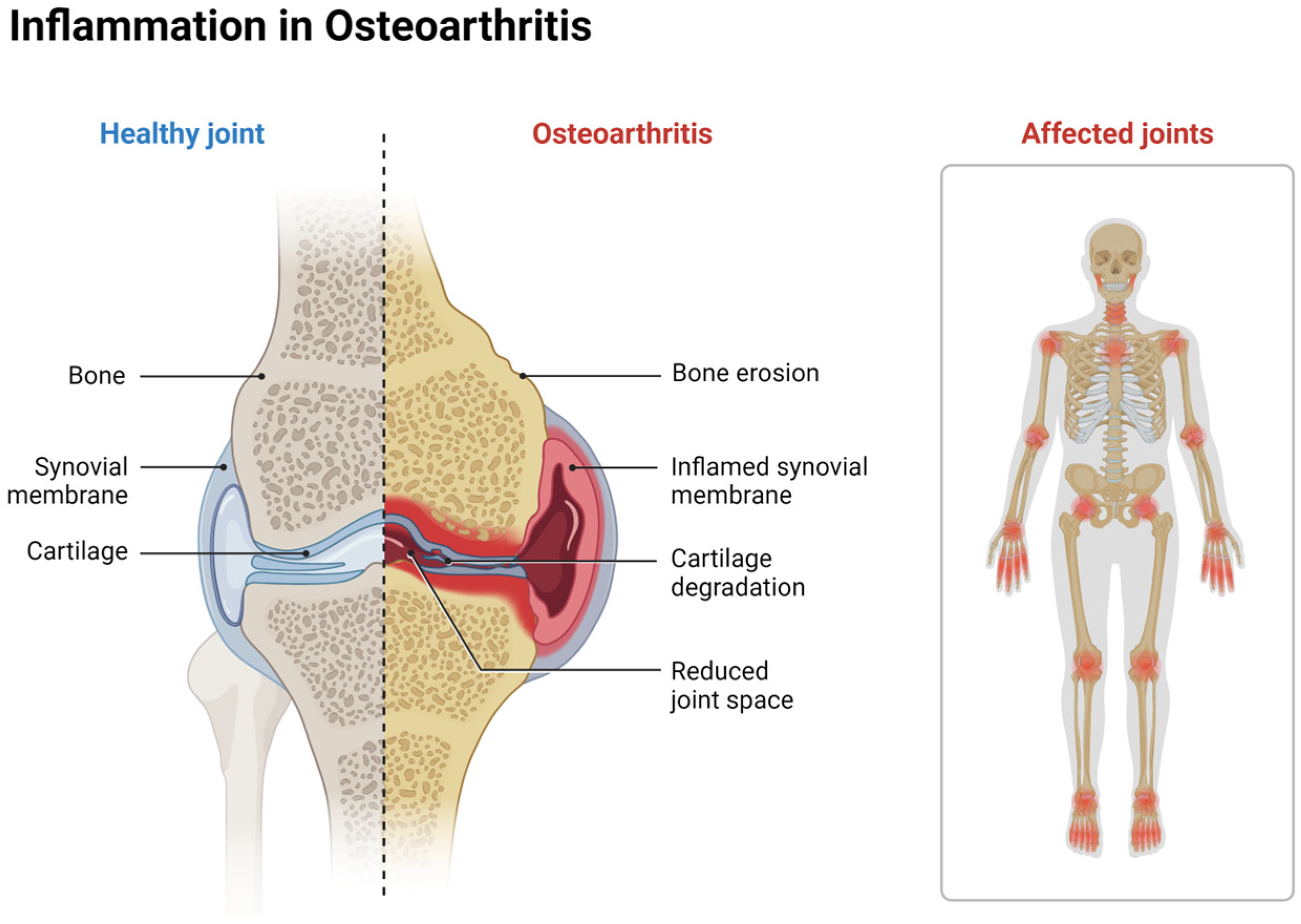
2.3. Cartilage Defects and Osteoarthritis
2.4. Tissue Response to Cartilage Damage
3. Cartilage Repair Techniques
3.1. Surgical Approaches for Cartilage Repair
3.1.1. Microfracture or Bone Marrow Stimulation
3.1.2. Cartilage Resurfacing
3.2. Orthobiologics for Cartilage Repair
3.2.1. Current Orthobiologic Treatments
3.2.2. Limitations of Orthobiologics
3.3. Microfracture Enhancement Therapy
3.4. Exosomes, an Emerging Therapeutic for Cartilage Repair
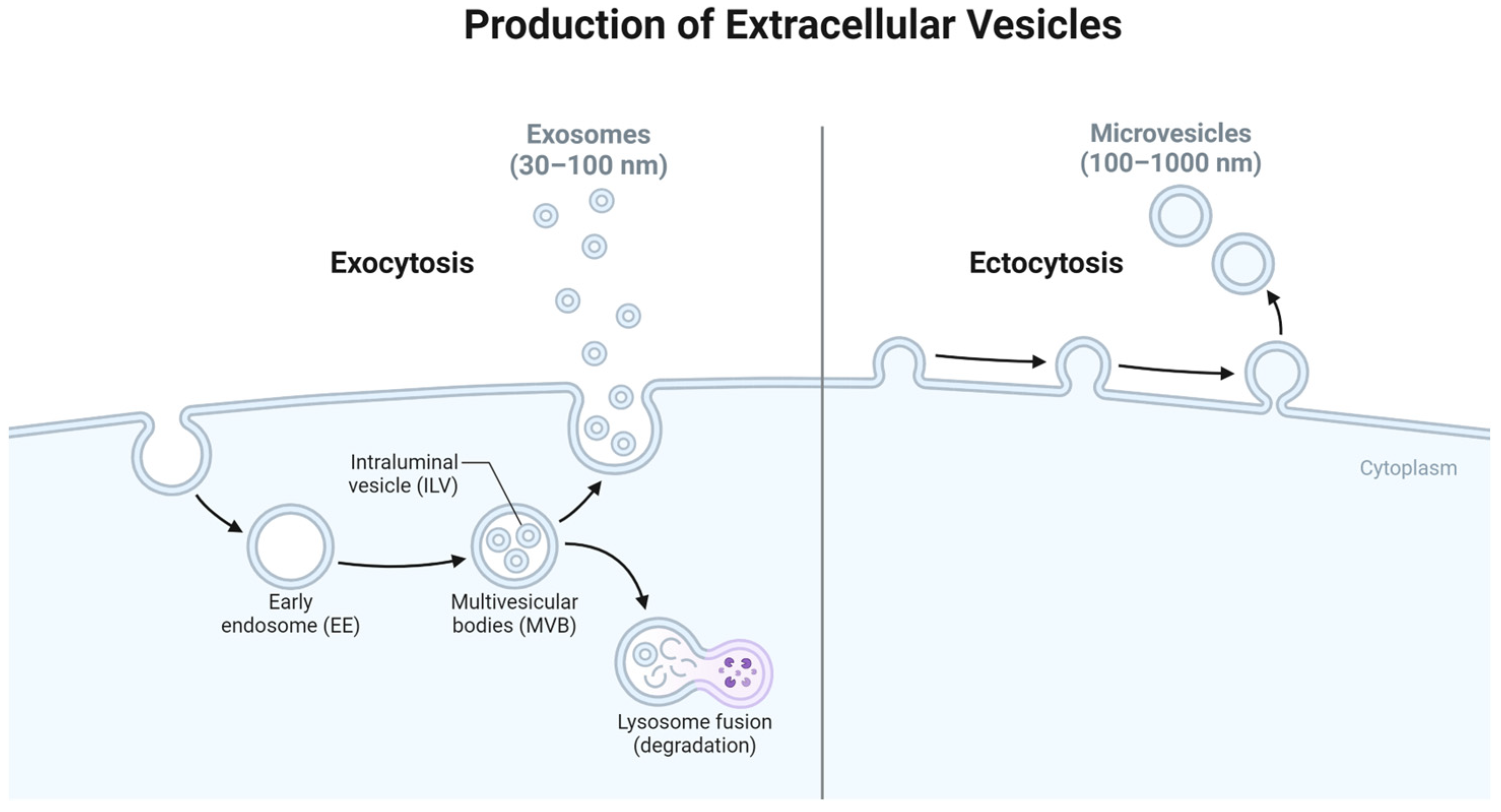
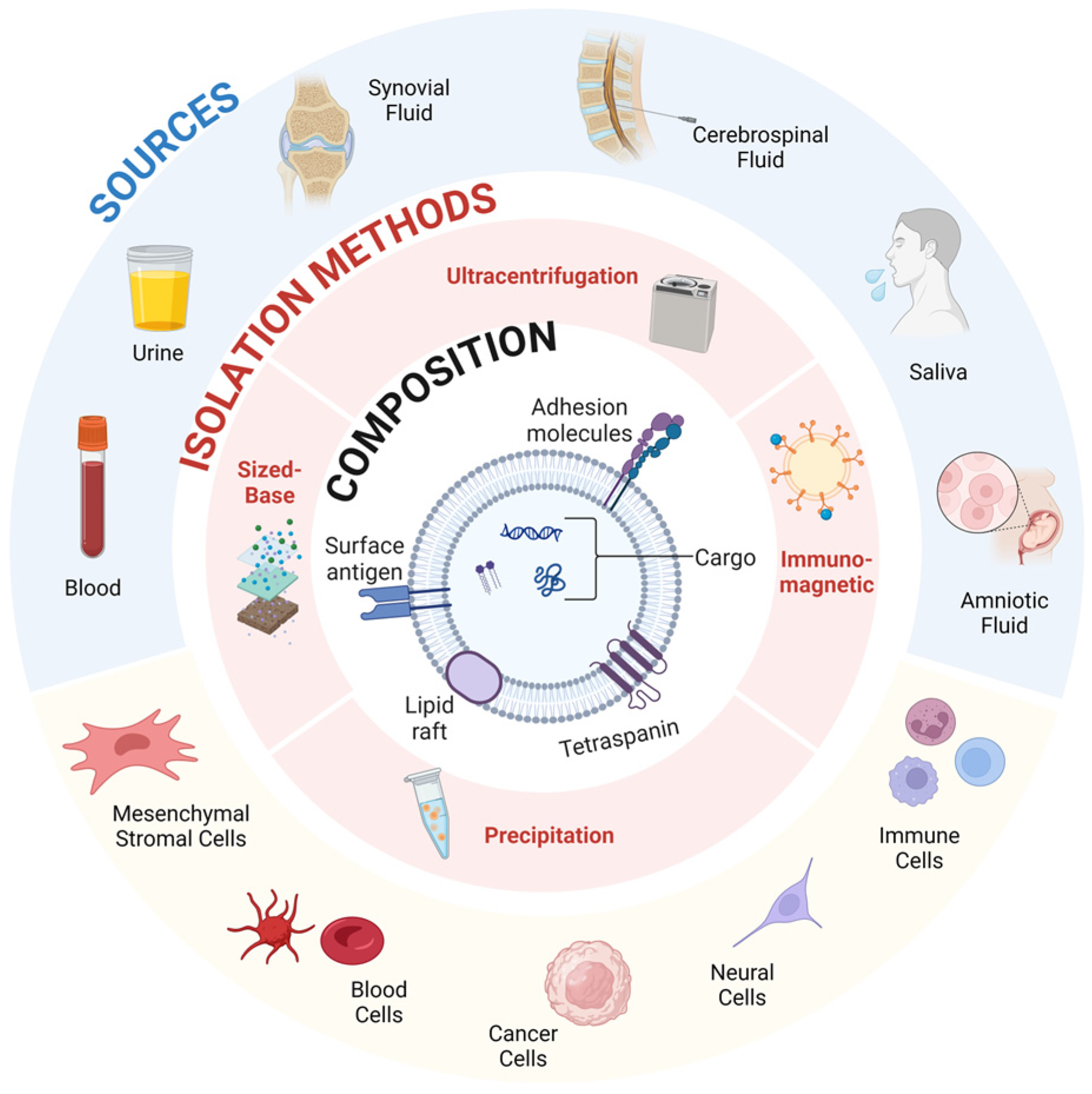
3.4.1. Introduction to Extracellular Vesicles
3.4.2. Origin of Exosomes
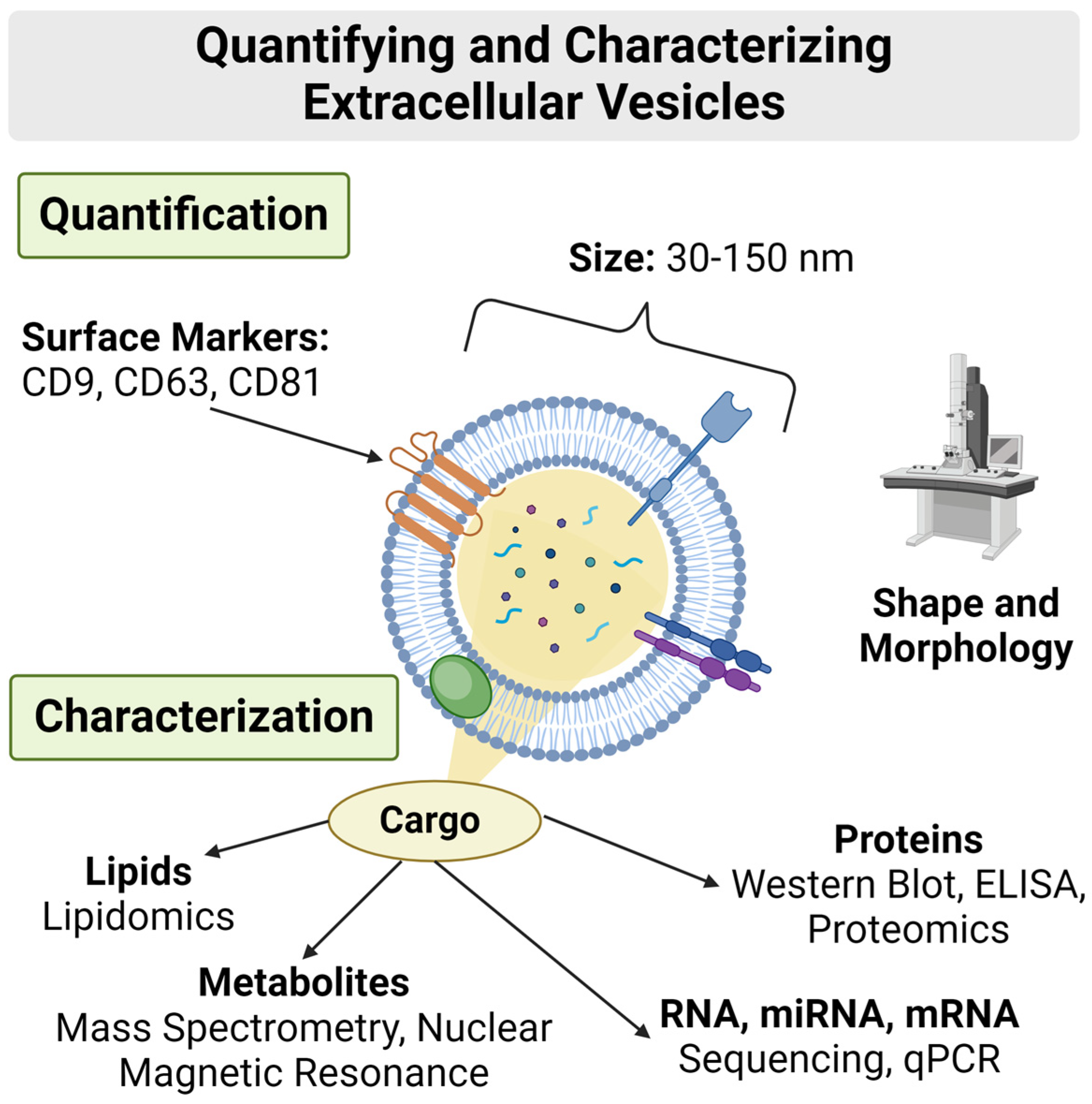
3.4.3. Composition
3.4.4. Targeting and Delivery
3.4.5. Impact
3.4.6. PRP-Derived Exosomes
MSC vs. MSC-Derived Exosome Treatment
BM-MSC Derived Exosomes Enhance Cartage Repair
mRNA and miRNA Cargo in Exosomes
Exosomes as a Novel Orthobiologic Direction
4. Conclusions
5. Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of articular cartilage defects: Therapeutic strategies and perspectives. J. Tissue Eng. 2023, 14, 1–27. [Google Scholar] [CrossRef]
- Wachsmuth, L.; Söder, S.; Fan, Z.; Finger, F.; Aigner, T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol. Histopathol. 2006, 21, 9. [Google Scholar]
- Stockwell, R. The cell density of human articular and costal cartilage. J. Anat. 1967, 101, 753. [Google Scholar]
- Mankin, H.J. The reaction of articular cartilage to injury and osteoarthritis. N. Engl. J. Med. 1974, 291, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Koyama, E.; Iwamoto, M. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell. Mol. Life Sci. 2005, 62, 2241–2256. [Google Scholar] [CrossRef]
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. Transl. Integr. 1996, 81, 535–545. [Google Scholar] [CrossRef]
- Newman, A.P. Articular cartilage repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [CrossRef]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef]
- Shum, L.; Nuckolls, G. The life cycle of chondrocytes in the developing skeleton. Arthritis Res. Ther. 2001, 4, 94. [Google Scholar] [CrossRef]
- Godman, G.C.; Porter, K.R. Chondrogenesis, studied with the electron microscope. J. Cell Biol. 1960, 8, 719–760. [Google Scholar] [CrossRef]
- Kosher, R.A.; Kulyk, W.M.; Gay, S.W. Collagen gene expression during limb cartilage differentiation. J. Cell Biol. 1986, 102, 1151–1156. [Google Scholar] [CrossRef]
- van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Differential effects of local application of BMP-2 or TGF-β1 on both articular cartilage composition and osteophyte formation. Osteoarthr. Cartil. 1998, 6, 306–317. [Google Scholar] [CrossRef]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 2015, 10, e0120857. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Nakama, G.; Scibetta, A.C.; Ravuri, S.K.; Goldman, J.L.; Lowe, W.R.; Rodkey, W.G.; et al. Biologically regulated marrow stimulation by blocking TGF-β1 with losartan oral administration results in hyaline-like cartilage repair: A rabbit osteochondral defect model. Am. J. Sports Med. 2020, 48, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Lópiz-Morales, Y.; Abarrategi, A.; Ramos, V.; Moreno-Vicente, C.; López-Durán, L.; López-Lacomba, J.L.; Marco, F. In vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regeneration. Eur. Cells Mater. 2010, 20, e78. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Louwerse, R.; Heyligers, I.; Wuisman, P.; Semeins, C.; Goei, S.; Burger, E. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J. Biomed. Mater. Res. 1998, 40, 614–620. [Google Scholar] [CrossRef]
- McQuillan, D.J.; Handley, C.J.; Campbell, M.A.; Bolis, S.; Milway, V.; Herington, A. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem. J. 1986, 240, 423–430. [Google Scholar] [CrossRef]
- Sah, R.L.; Chen, A.C.; Grodzinsky, A.J.; Trippel, S. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch. Biochem. Biophys. 1994, 308, 137–147. [Google Scholar] [CrossRef]
- Hung, G.; Galea-Lauri, J.; Mueller, G.; Georgescu, H.; Larkin, L.; Suchanek, M.; Tindal, M.; Robbins, P.; Evans, C. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994, 1, 64–69. [Google Scholar] [PubMed]
- Gigout, A.; Guehring, H.; Froemel, D.; Meurer, A.; Ladel, C.; Reker, D.; Bay-Jensen, A.; Karsdal, M.; Lindemann, S. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr. Cartil. 2017, 25, 1858–1867. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [PubMed]
- Schmidt, M.; Chen, E.; Lynch, S. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q. Adipokines: New therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- Dilley, J.E.; Bello, M.A.; Roman, N.; McKinley, T.; Sankar, U. Post-traumatic osteoarthritis: A review of pathogenic mechanisms and novel targets for mitigation. Bone Rep. 2023, 18, 101658. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Squires, E.; Bui, A.L.; Campbell, M.; Chapin, A.; Hamavid, H.; Horst, C.; Li, Z.; Matyasz, T.; Reynolds, A. Factors associated with increases in US health care spending, 1996–2013. JAMA 2017, 318, 1668–1678. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W. US health care spending by payer and health condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Anderson, D.D.; Chubinskaya, S.; Guilak, F.; Martin, J.A.; Oegema, T.R.; Olson, S.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J. Orthop. Res. 2011, 29, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar]
- Poulet, B.; Staines, K.A. New developments in osteoarthritis and cartilage biology. Curr. Opin. Pharmacol. 2016, 28, 8–13. [Google Scholar] [CrossRef]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Mene, P.; Savarino, V.; Fornasari, D. Management of Osteoarthritis: Expert Opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and mechanoregulation of endothelial cell functions. Compr. Physiol. 2019, 9, 873. [Google Scholar]
- Koenen, R.R. The prowess of platelets in immunity and inflammation. Thromb. Haemost. 2016, 116, 605–612. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants–a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Sillat, T.; Barreto, G.; Clarijs, P.; Soininen, A.; Ainola, M.; Pajarinen, J.; Korhonen, M.; Konttinen, Y.T.; Sakalyte, R.; Hukkanen, M. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013, 84, 585–592. [Google Scholar] [CrossRef]
- Kim, H.A.; Cho, M.L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2006, 54, 2152–2163. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Griffin, T.M.; Humphrey, M.B. Innate immune responses and osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Kandahari, A.M.; Yang, X.; Dighe, A.S.; Pan, D.; Cui, Q. Recognition of immune response for the early diagnosis and treatment of osteoarthritis. J. Immunol. Res. 2015, 2015, 192415. [Google Scholar] [CrossRef]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Ralphs, J. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004, 233, 1–46. [Google Scholar]
- Yasui, N.; Nimni, M.E. Cartilage collagens. In Collagen; CRC Press: Boca Raton, FL, USA, 2018; pp. 225–242. [Google Scholar]
- Sgaglione, N.A. The future of cartilage restoration. J. Knee Surg. 2004, 17, 235–243. [Google Scholar] [CrossRef]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular cartilage: Structural and developmental intricacies and questions. Curr. Osteoporos. Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef]
- Franke, O.; Durst, K.; Maier, V.; Göken, M.; Birkholz, T.; Schneider, H.; Hennig, F.; Gelse, K. Mechanical properties of hyaline and repair cartilage studied by nanoindentation. Acta Biomater. 2007, 3, 873–881. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the mechanical environment on the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef] [PubMed]
- Coutts, R.D.; Healey, R.M.; Ostrander, R.; Sah, R.L.; Goomer, R.; Amiel, D. Matrices for cartilage repair. Clin. Orthop. Relat. Res. 2001, 391, S271–S279. [Google Scholar] [CrossRef]
- Wang, L.; Lazebnik, M.; Detamore, M. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthr. Cartil. 2009, 17, 346–353. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Buchanan, J.L. Types of fibrocartilage. Clin. Podiatr. Med. Surg. 2022, 39, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Castorina, S.; Imbesi, R.; Leonardi, R.; Castrogiovanni, P. Current concepts in the treatment of cartilage damage. A review. Ital. J. Anat. Embryol. 2013, 118, 189–203. [Google Scholar] [PubMed]
- Smith, G.; Knutsen, G.; Richardson, J. A clinical review of cartilage repair techniques. J. Bone Jt. Surg. Br. Vol. 2005, 87, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture: Its history and experience of the developing surgeon. Cartilage 2010, 1, 78–86. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture Chondroplasty: Indications, Techniques, and Outcomes. Sports Med. Arthrosc. Rev. 2003, 11, 236–244. [Google Scholar] [CrossRef]
- Bugbee, W.D.; Convery, F.R. Osteochondral allograft transplantation. Clin. Sports Med. 1999, 18, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage restoration: Microfracture and osteochondral autograft transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Berrigan, W.A.; Mautner, K. Regulatory Considerations of Orthobiologic Procedures. Phys. Med. Rehabil. Clin. 2023, 34, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Chahla, J.; Wordie, S.J.; Shapiro, S.A.; Piuzzi, N.S.; Frank, R.M.; Halbrecht, J.; Okada, K.; Nakamura, N.; Mandelbaum, B. Regulatory and Ethical Aspects of Orthobiologic Therapies. Orthop. J. Sports Med. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Allickson, J. Emerging translation of regenerative therapies. Clin. Pharmacol. Ther. 2017, 101, 28–30. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Francis, W.R.; Whitaker, I.S. Transforming healthcare through regenerative medicine. BMC Med. 2016, 14, 115. [Google Scholar] [CrossRef]
- Boivin, J.; Tolsma, R.; Awad, P.; Kenter, K.; Li, Y. The biological use of platelet-rich plasma in skeletal muscle injury and repair. Am. J. Sports Med. 2023, 51, 1347–1355. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Jiang, W.; Zhu, Y.; Hu, Y.; Zhao, Y.; Song, X.; Zhao, J.; Zhang, W.; Peng, J. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Eng. Part B Rev. 2020, 26, 571–585. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert Opin. Biol. Ther. 2015, 15, 987–999. [Google Scholar] [CrossRef]
- Gulati, G.L.; Ashton, J.K.; Hyun, B.H. Structure and function of the bone marrow and hematopoiesis. Hematol./Oncol. Clin. 1988, 2, 495–511. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone marrow concentrate injections for the treatment of osteoarthritis: Evidence from preclinical findings to the clinical application. Int. Orthop. 2021, 45, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Potter, H.G.; Rickey, E.J.; Schnabel, L.V.; Foo, L.F.; Chong, L.R.; Stokol, T.; Cheetham, J.; Nixon, A.J. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. JBJS 2010, 92, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Nawabi, D.H.; Farshad-Amacker, N.A.; Jones, K.J.; Maak, T.G.; Potter, H.G.; Williams III, R.J. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: A comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am. J. Sports Med. 2016, 44, 91–98. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Tschon, M.; Borsari, V.; Nicoli Aldini, N.; Fini, M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013, 22, 181–192. [Google Scholar] [CrossRef]
- Kreulen, C.; Giza, E.; Shieh, A.; Singh, S.; Nathe, C.; Lian, E.; Haudenschild, D. Effects of Micronized Cartilage Matrix on Cartilage Repair in Osteochondral Lesions of the Talus. Foot Ankle Orthop. 2017, 2, 1–2. [Google Scholar] [CrossRef]
- Bernhardt, A.; Lode, A.; Boxberger, S.; Pompe, W.; Gelinsky, M. Mineralised collagen—An artificial, extracellular bone matrix—Improves osteogenic differentiation of bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2008, 19, 269–275. [Google Scholar] [CrossRef]
- Monibi, F.A.; Bozynski, C.C.; Kuroki, K.; Stoker, A.M.; Pfeiffer, F.M.; Sherman, S.L.; Cook, J.L. Development of a micronized meniscus extracellular matrix scaffold for potential augmentation of meniscal repair and regeneration. Tissue Eng. Part C Methods 2016, 22, 1059–1070. [Google Scholar] [CrossRef]
- Shin, J.J.; Mellano, C.; Cvetanovich, G.L.; Frank, R.M.; Cole, B.J. Treatment of glenoid chondral defect using micronized allogeneic cartilage matrix implantation. Arthrosc. Tech. 2014, 3, e519–e522. [Google Scholar] [CrossRef]
- Lai, W.; Li, Y.; Mak, S.; Ho, F.; Chow, S.; Chooi, W.; Chow, C.; Leung, A.; Chan, B. Reconstitution of bone-like matrix in osteogenically differentiated mesenchymal stem cell–collagen constructs: A three-dimensional in vitro model to study hematopoietic stem cell niche. J. Tissue Eng. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, H.; Quade, M.; Lode, A.; Gelinsky, M.; Rammelt, S.; Vater, C. Chemotactic and angiogenic potential of mineralized collagen scaffolds functionalized with naturally occurring bioactive factor mixtures to stimulate bone regeneration. Int. J. Mol. Sci. 2021, 22, 5836. [Google Scholar] [CrossRef] [PubMed]
- Huebner, K.; Frank, R.M.; Getgood, A. Ortho-biologics for osteoarthritis. Clin. Sports Med. 2019, 38, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Karampikas, V.; Zikopoulos, A.; Sioutis, S.; Mastrokalos, D.; Koulalis, D.; Scarlat, M.M.; Hernigou, P. Orthobiologics: A review. Int. Orthop. 2023, 47, 1645–1662. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, F.; Liu, Y.; Wang, J.; Lu, W.; Jiang, W.; Zhu, W. Losartan protects against osteoarthritis by repressing the TGF-β1 signaling pathway via upregulation of PPARγ. J. Orthop. Transl. 2021, 29, 30–41. [Google Scholar] [CrossRef]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. Ther. 2014, 16, 427. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.-K.; Park, S.-K. Effects of fisetin, a plant-derived flavonoid, on response to oxidative stress, aging, and age-related diseases in Caenorhabditis elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar]
- Logan, C.A.; Gao, X.; Utsunomiya, H.; Scibetta, A.C.; Talwar, M.; Ravuri, S.K.; Ruzbarsky, J.J.; Arner, J.W.; Zhu, D.; Lowe, W.R. The beneficial effect of an intra-articular injection of losartan on microfracture-mediated cartilage repair is dose dependent. Am. J. Sports Med. 2021, 49, 2509–2521. [Google Scholar] [CrossRef]
- Yamaura, K.; Nelson, A.; Nishimura, H.; Rutledge, J.; Ravuri, S.; Bahney, C.; Philippon, M.; Huard, J. The effects of losartan or angiotensin II receptor antagonists on cartilage: A systematic review. Osteoarthr. Cartil. 2023, 31, 435–446. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Lowman, H. Therapeutic anti-VEGF antibodies. In Therapeutic Antibodies; Springer: Berlin/Heidelberg, Germany, 2008; pp. 131–150. [Google Scholar]
- Nagai, T.; Sato, M.; Kutsuna, T.; Kokubo, M.; Ebihara, G.; Ohta, N.; Mochida, J. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis Res. Ther. 2010, 12, R178. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Gao, X.; Cheng, H.; Deng, Z.; Nakama, G.; Mascarenhas, R.; Goldman, J.L.; Ravuri, S.K.; Arner, J.W.; Ruzbarsky, J.J. Intra-articular injection of bevacizumab enhances bone marrow stimulation–mediated cartilage repair in a rabbit osteochondral defect model. Am. J. Sports Med. 2021, 49, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Rodrigo, J.; Reddi, A.; Curtiss, S.; Grotkopp, E.; Chiu, M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthr. Cartil. 2006, 14, 1126–1135. [Google Scholar] [CrossRef]
- Yang, H.S.; La, W.-G.; Bhang, S.H.; Kim, H.-J.; Im, G.-I.; Lee, H.; Park, J.-H.; Kim, B.-S. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng. Part A 2011, 17, 1809–1818. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Huard, C.A.; Gao, X.; Dey Hazra, M.E.; Dey Hazra, R.-O.; Lebsock, K.; Easley, J.T.; Millett, P.J.; Huard, J. Effects of Fisetin treatment on cellular senescence of various tissues and organs of old sheep. Antioxidants 2023, 12, 1646. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017, 9, 955. [Google Scholar] [CrossRef]
- Gao, X.; Hambright, S.; Whitney, K.; Huard, M.; Murata, Y.; Nolte, P.; Stake, I.; Huard, C.; Ravuri, S.; Philippon, M. Paper 40: Improved Cartilage Healing with Microfracture Augmented with Fisetin & Bone Marrow Aspirate Concentrate in Acute Osteochondral Defect. Orthop. J. Sports Med. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, K.-C.; Harn, H.-J.; Lin, S.-Z.; Ding, D.-C. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplant. 2018, 27, 349–363. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New players in cell–cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Saadeldin, I.M. Role of Exosomes in Biological Communication Systems; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.L.; Cho, W.C.S.; Yee, B.K.; Kwan, Y.W.; Tai, W.C.S. Exosomes in inflammation and inflammatory disease. Proteomics 2019, 19, 1800149. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Indiveri, C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta 2019, 488, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their role in pathogenesis, diagnosis and treatment of diseases. Cancers 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, H.-T.; Wang, S.; Huang, S.-L.; Zheng, Y.; Wang, C.-Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of exosomes through receptor-mediated endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Corrado, C.; Raimondo, S.; Chiesi, A.; Ciccia, F.; De Leo, G.; Alessandro, R. Exosomes as intercellular signaling organelles involved in health and disease: Basic science and clinical applications. Int. J. Mol. Sci. 2013, 14, 5338–5366. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, 2103222. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Wu, S.-C.; Kuo, P.-J.; Rau, C.-S.; Wu, Y.-C.; Wu, C.-J.; Lu, T.-H.; Lin, C.-W.; Tsai, C.-W.; Hsieh, C.-H. Subpopulations of exosomes purified via different exosomal markers carry different microRNA contents. Int. J. Med. Sci. 2021, 18, 1058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cai, Y.; Jiang, Y.; Lin, X. Exosomes in osteoarthritis and cartilage injury: Advanced development and potential therapeutic strategies. Int. J. Biol. Sci. 2020, 16, 1811. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kodam, S.P.; Ullah, M. Diagnostic and therapeutic potential of extracellular vesicles. Technol. Cancer Res. Treat. 2021, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stoorvogel, W. Resolving sorting mechanisms into exosomes. Cell Res. 2015, 25, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Xu, P.; Ma, J.; Zhang, R. Compositional variation and functional mechanism of exosomes in the articular microenvironment in knee osteoarthritis. Cell Transplant. 2020, 29, 1–10. [Google Scholar] [CrossRef]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barcelo, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential disease biomarkers and new therapeutic targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- de Gassart, A.; Géminard, C.; Hoekstra, D.; Vidal, M. Exosome secretion: The art of reutilizing nonrecycled proteins? Traffic 2004, 5, 896–903. [Google Scholar] [CrossRef]
- Jin, D.; Yang, F.; Zhang, Y.; Liu, L.; Zhou, Y.; Wang, F.; Zhang, G.-J. ExoAPP: Exosome-oriented, aptamer nanoprobe-enabled surface proteins profiling and detection. Anal. Chem. 2018, 90, 14402–14411. [Google Scholar] [CrossRef] [PubMed]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef]
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Compañ, A.; Guillén, M.I. Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.-J.; Liu, D.; Pan, L.-Y.; Wang, W.-Y.; Ding, Y.-L.; Zhang, Y.-Y.; Ye, R.-X.; Zhou, Y.; An, S.-B.; Xiao, W.-F. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 2022, 10, 949690. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: Insights from drug delivery to clinical perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Vader, P.; van Dommelen, S.M.; van Solinge, W.W.; Schiffelers, R.M. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar]
- Rodeo, S.A. Exosomes: The New Kid on the Block in Orthobiologics; SAGE Publications: Los Angeles, CA, USA, 2023; Volume 51, pp. 3363–3366. [Google Scholar]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chen, J.; Qian, D.; Gao, P.; Qin, T.; Jiang, T.; Yi, J.; Xu, T.; Huang, Y. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J. Nanobiotechnol. 2022, 20, 56. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef] [PubMed]
- Bei, H.P.; Hung, P.M.; Yeung, H.L.; Wang, S.; Zhao, X. Bone-a-petite: Engineering exosomes towards bone, osteochondral, and cartilage repair. Small 2021, 17, 2101741. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-β and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Shebani-Rad, S. The basic science of platelet-rich plasma (PRP): What clinicians need to know. Sports Med. Arthrosc. Rev. 2013, 21, 180–185. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275. [Google Scholar]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Schipani, E. Bone marrow mesenchymal stem cells. J. Cell. Biochem. 2010, 109, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.R.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Jones, E.; McGonagle, D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology 2008, 47, 126–131. [Google Scholar] [CrossRef]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-q.; Kong, L.; Liu, C.; Xu, H.-G. Human bone marrow mesenchymal stem cell-derived exosomes attenuate IL-1β-induced annulus fibrosus cell damage. Am. J. Med. Sci. 2020, 360, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef] [PubMed]
- Yano, F.; Ohba, S.; Murahashi, Y.; Tanaka, S.; Saito, T.; Chung, U.-I. Runx1 contributes to articular cartilage maintenance by enhancement of cartilage matrix production and suppression of hypertrophic differentiation. Sci. Rep. 2019, 9, 7666. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; McVicar, A.; Yang, H.-L.; Li, Y.-P.; Chen, W. Runx1 is a key regulator of articular cartilage homeostasis by orchestrating YAP, TGFβ, and Wnt signaling in articular cartilage formation and osteoarthritis. Bone Res. 2022, 10, 63. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Lu, K.; Li, H.-Y.; Yang, K.; Wu, J.-L.; Cai, X.-W.; Zhou, Y.; Li, C.-Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 108. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [Google Scholar] [CrossRef]
- Velez, C. Unraveling the Mystery of Non-Coding Genomic Content: Evolution, Regulation, and Functional Significance. Master’s Thesis, SUNY Downstate Health Sciences University, Brooklyn, NY, USA, 2023. [Google Scholar]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Pérez Lanzón, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N. Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. 2020, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, X.; Guan, Y.; Chen, Q.; Zhao, T.; Sun, C.; Wei, L. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development. FASEB J. 2014, 28, 3930. [Google Scholar] [CrossRef]
- Wang, K.; Li, F.; Yuan, Y.; Shan, L.; Cui, Y.; Qu, J.; Lian, F. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol. Ther.-Nucleic Acids 2020, 22, 1078–1091. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, Z.; Zhang, Z.; Wu, P.; Zhao, X.; Fu, M.; Sheng, P.; Kang, Y.; Liao, W. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4877–4886. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Mao, G.; Hu, S.; Zhang, Z.; Wu, P.; Zhao, X.; Lin, R.; Liao, W.; Kang, Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med. 2018, 22, 5354–5366. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B.; Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, K.; Ge, G.; Zhang, D.; Bai, J.; Guo, X.; Zhou, J.; Xu, T.; Xu, M.; Long, X. Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021, 37, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, W.; Huang, G.; He, A.; Hou, C.; Long, Y.; Yang, Z.; Liao, W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthr. Cartil. 2016, 24, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, K.; Su, S.; Li, J.; Li, C. miR-486-5p is upregulated in osteoarthritis and inhibits chondrocyte proliferation and migration by suppressing SMAD2. Mol. Med. Rep. 2018, 18, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Park, C.H. Microfracture for cartilage repair in the knee: Current concepts and limitations of systematic reviews. Ann. Transl. Med. 2019, 7, S108. [Google Scholar] [CrossRef]
- Budhiparama, N.C.; Putramega, D.; Lumban-Gaol, I. Orthobiologics in knee osteoarthritis, dream or reality? Arch. Orthop. Trauma Surg. 2024. [Google Scholar] [CrossRef]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, S311–S324. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The use of scaffolds in cartilage regeneration. Crit. Rev.™ Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Woodfield, T.; Bezemer, J.; Pieper, J.; Van Blitterswijk, C.; Riesle, J. Scaffolds for tissue engineering of cartilage. Crit. Rev.™ Eukaryot. Gene Expr. 2002, 12, 28p. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, Y.; Wei, X.; He, J.; Yang, S.; Dickson, G.; Tang, J.; Xiang, J.; Song, C.; Li, G. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng. Part C Methods 2010, 16, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, P.; Ci, Z.; Hao, X.; Bai, B.; Zhang, W.; Jiang, H.; Zhou, G. Acellular cartilage matrix biomimetic scaffold with immediate enrichment of autologous bone marrow mononuclear cells to repair articular cartilage defects. Mater. Today Bio 2022, 15, 100310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Zhou, G.; Liu, Y.; Cao, Y. Biological evaluation of acellular cartilaginous and dermal matrixes as tissue engineering scaffolds for cartilage regeneration. Front. Cell Dev. Biol. 2021, 8, 624337. [Google Scholar] [CrossRef]
- Saurav, S.; Sharma, P.; Kumar, A.; Tabassum, Z.; Girdhar, M.; Mamidi, N.; Mohan, A. Harnessing Natural Polymers for Nano-Scaffolds in Bone Tissue Engineering: A Comprehensive Overview of Bone Disease Treatment. Curr. Issues Mol. Biol. 2024, 46, 585–611. [Google Scholar] [CrossRef]
- Debieux, P.; Mameri, E.S.; Medina, G.; Wong, K.L.; Keleka, C.C. Acellular scaffolds, cellular therapy and next generation approaches for knee cartilage repair. J. Cartil. Jt. Preserv. 2024, 4, 100180. [Google Scholar] [CrossRef]
- Demmer, W.; Schinacher, J.; Wiggenhauser, P.S.; Giunta, R.E. Use of Acellular Matrices as Scaffolds in Cartilage Regeneration: A Systematic Review. Adv. Wound Care 2024. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Zhang, J.; Yuan, Y.; Wang, J. Exosomes: A novel therapeutic agent for cartilage and bone tissue regeneration. Dose-Response 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Benrashed, M.A.; Alyousef, N.I.; AlQahtani, N.H.; AlMaimouni, Y.K.; Khan, M.; Khan, A.S. Conventional to advanced endodontics: Use of bioactive materials. In Biomaterials in Endodontics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 169–194. [Google Scholar]
- Farmani, A.R.; Nekoofar, M.H.; Ebrahimi-Barough, S.; Azami, M.; Najafipour, S.; Moradpanah, S.; Ai, J. Preparation and in vitro osteogenic evaluation of biomimetic hybrid nanocomposite scaffolds based on gelatin/plasma rich in growth factors (PRGF) and lithium-doped 45s5 bioactive glass nanoparticles. J. Polym. Environ. 2023, 31, 870–885. [Google Scholar] [CrossRef]
- Thamaraiselvi, T.; Rajeswari, S. Biological evaluation of bioceramic materials—A review. Carbon 2004, 24, 172. [Google Scholar]
- Zhang, X.; Tang, Y.; Liu, S.; Zhang, Y. Influence of lithium ion doping and mitoxantrone hydrochloride loading on the structure and in vitro biological properties of mesoporous bioactive glass microspheres in the treatment of multiple myeloma. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134168. [Google Scholar] [CrossRef]


| Growth Factor | Importance | Sources |
|---|---|---|
| Transforming Growth Factor-β (TGF-β) | TGF-β is essential for autonomous cartilage formation. In the absence of TGF-β, cartilage is not formed. However, it has been demonstrated that downregulation of TGF-β can lead to less fibrotic cartilage repair, suggesting that there is an optimum concentration level. | [20,21] |
| Bone Morphogenic Protein (BMP) | BMPs are prominent growth factors for regenerating osteochondral tissue. Often expressed throughout the whole chondrogenic process, BMP2 has been shown to improve subchondral bone, while BMP4 has been shown to be superior in hyaline cartilage formation. BMP7 has demonstrated efficacy in promoting cartilage differentiation, proliferation, and retention of ECM. Many BMPs have been studied, all sharing similar roles in cartilage formation and maintenance. | [22,23] |
| Insulin-Like Growth Factor (IGF) | IGF-1 has been linked to increases in proteoglycan and collagen synthesis, as well as to the reduction of ECM degradation. | [24,25] |
| Interleukin-1 (IL-1) | IL-1 inflammatory cytokine has been shown to lead to cartilage degradation. IL-1 receptor antagonist reduces proteoglycan breakdown. | [26] |
| Fibroblast Growth Factor (FGF) | Recombinant human FGF-18 has been shown to stimulate chondrocyte proliferation and SOX-9 expression, as well as a marked decrease in type I collagen expression. | [27] |
| Vascular Endothelial Growth Factor (VEGF) | Increased levels of VEGF have been correlated to OA progression. Specifically, VEGF appears to be involved in endochondral ossification, osteocyte formation, synovitis, and pain. Anti-VEGF treatments show promise for protecting cartilage from degradation and reducing the progression of OA. | [28] |
| Platelet-Derived Growth Factor (PDGF) | PDGF is highly expressed in the early stages of wound healing and prevalent in platelets and Platelet Rich Plasma (PRP). PDGF plays a role in chondrocyte proliferation and inhibits the endochondral maturation process. | [29] |
| Tumor Necrosis Factor-alpha (TNF-α) | Another inflammatory cytokine, TNF-α, plays a role in OA progression. TNF-α inhibition has shown efficacy in reducing the progression of OA. | [30] |
| Interventional Drug | Common Use | Known Benefits | Targeted Use | Sources |
|---|---|---|---|---|
| Losartan | High blood pressure |
|
| [96] |
| Avastin | Generic cancer treatment |
|
| [97] |
| Fisetin | Supplement |
|
| [98] |
| miRNA | Orthopedic Function | Sources |
|---|---|---|
| miRNA-1 | Promote the growth of cartilage through HDAC4. | [187] |
| miRNA-31 | Factor important for growth and proliferation of chondrocytes, and found to be chondroprotective in OA models. | [188] |
| miRNA-92a | Promote proliferation of cartilage progenitor cells through PI3K. | [189] |
| miRNA-92a-3p | Regulates cartilage development and homeostasis through Wnt5a. | [190] |
| miRNA-95-5p | Regulates cartilage development and homeostasis through HDAC2. | [191] |
| miRNA-100-5p | Maintains cartilage homeostasis through mTOR. | [192] |
| miRNA-135b | Promotes chondrocyte proliferation and cartilage repair through SP1. | [193] |
| miRNA-140-5p | Enhances proliferation and migration of chondrocytes through RALA. | [194] |
| miRNA-145 | Promotes chondrogenesis in periosteal cells. | [195] |
| miRNA-155-5p | Shown to play a role in cell proliferation and apoptosis. Downregulated in OA. | [196] |
| miRNA-221 | Suppresses pro-inflammatory cytokines. | [195] |
| miRNA-320 | Promote the growth of cartilage through mmp13. | [197] |
| miRNA-486-5p | Upregulated in OA. Linked to cartilage degradation. | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, J.; Knezic, N.; Layne, J.; Gohring, G.; Christiansen, J.; Rothrauff, B.; Huard, J. Enhancing Cartilage Repair: Surgical Approaches, Orthobiologics, and the Promise of Exosomes. Life 2024, 14, 1149. https://doi.org/10.3390/life14091149
Singer J, Knezic N, Layne J, Gohring G, Christiansen J, Rothrauff B, Huard J. Enhancing Cartilage Repair: Surgical Approaches, Orthobiologics, and the Promise of Exosomes. Life. 2024; 14(9):1149. https://doi.org/10.3390/life14091149
Chicago/Turabian StyleSinger, Jacob, Noah Knezic, Jonathan Layne, Greta Gohring, Jeff Christiansen, Ben Rothrauff, and Johnny Huard. 2024. "Enhancing Cartilage Repair: Surgical Approaches, Orthobiologics, and the Promise of Exosomes" Life 14, no. 9: 1149. https://doi.org/10.3390/life14091149






