The Interaction between Vergence and Accommodation Cues in the Assessment of Fusional Vergence Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants Selection
2.2. Fusional Vergence Range, Heterophoria, Near Point of Convergence, and Amplitude of Accommodation
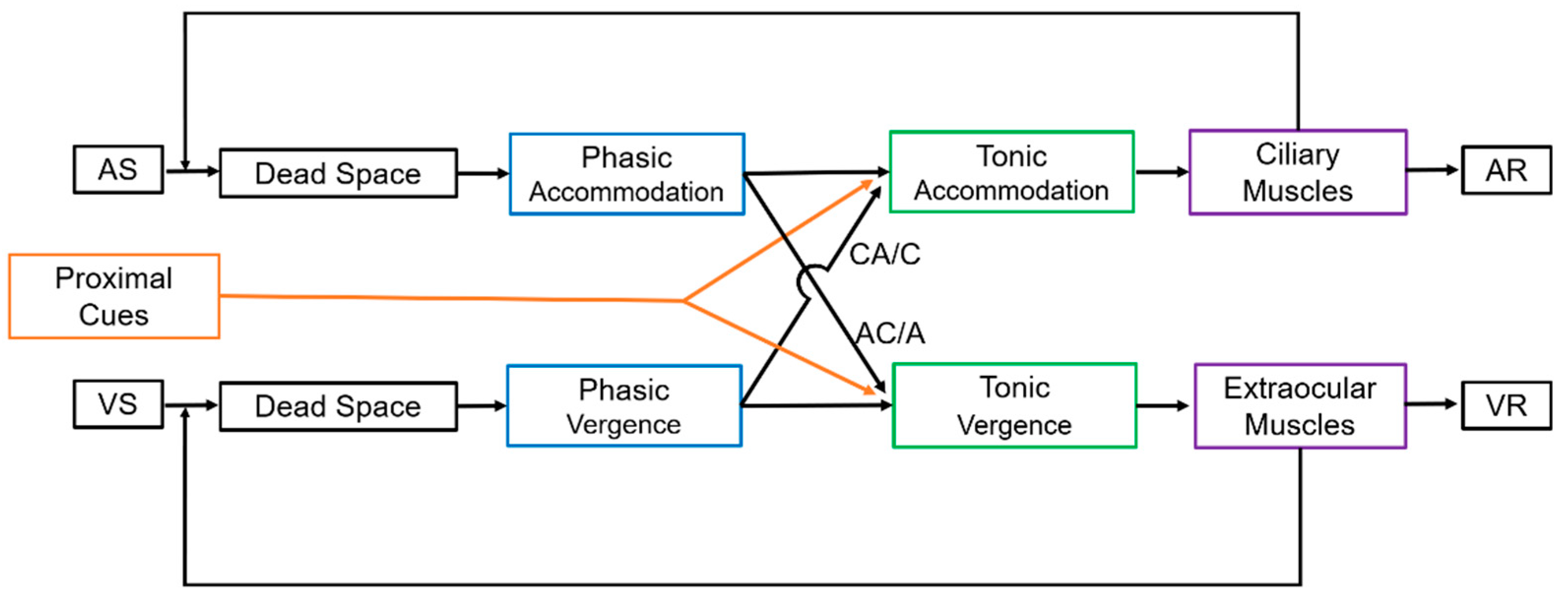
2.3. CA/C Stimulus Ratio
2.4. AC/A Stimulus Ratio
2.5. Proximal Cues (PCT Ratio)
2.6. Data Analysis
3. Results
3.1. Participants and Descriptive Values
3.2. Incidence of Blur Reporting during Fusional Vergence Test
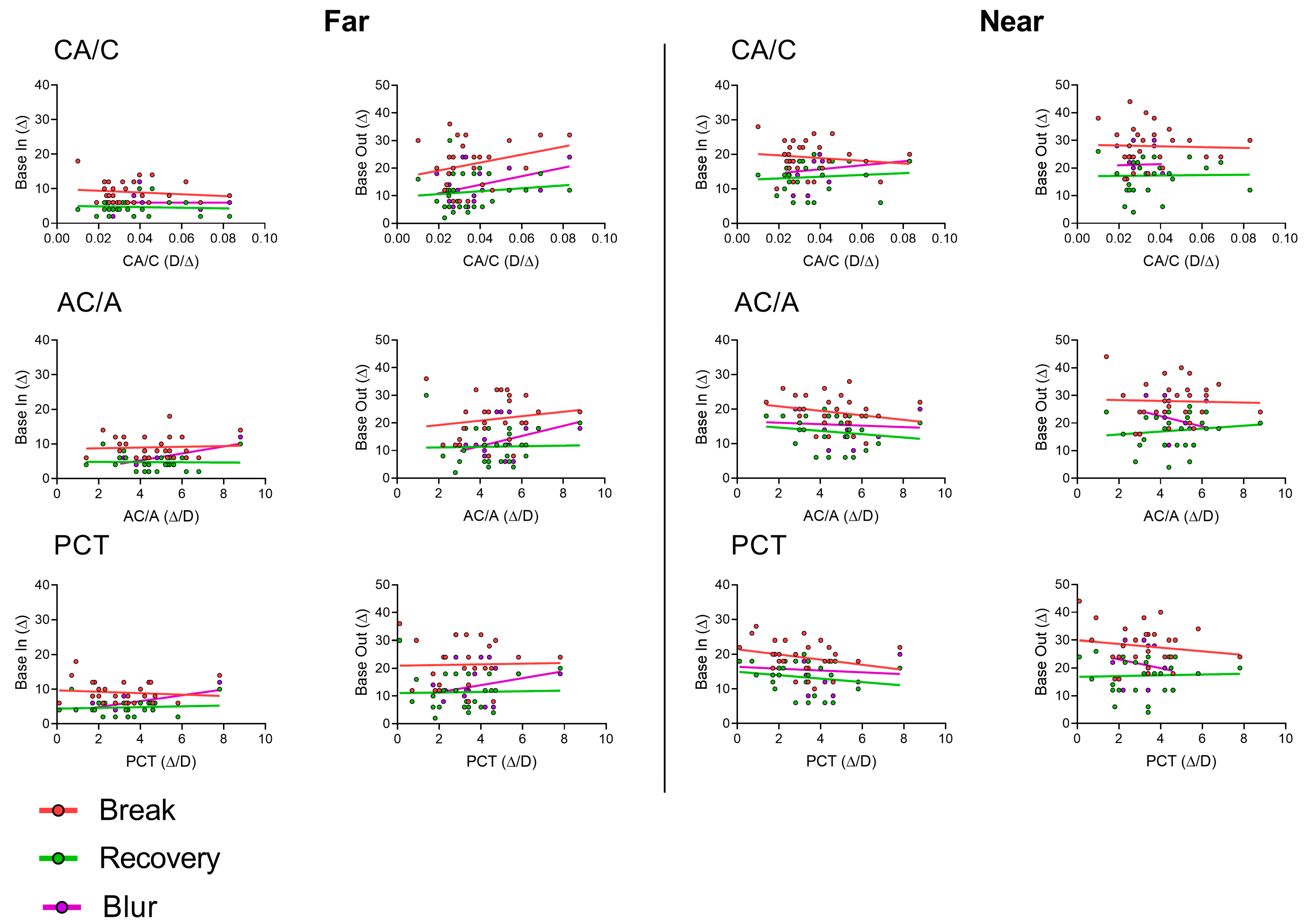
3.3. Correlation between CA/C and AC/A
3.4. Correlations between CA/C, AC/A, and PCT with Break and Recovery Values
3.5. AC/A, CA/C, and PCT Values and the Occurrence of Blur
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosvenor, T.; Grosvenor, T.P. Primary Care Optometry; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Rosenfield, M.; Logan, N. Optometry: Science, Techniques and Clinical Management; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Antona, B.; Barrio, A.; Barra, F.; Gonzalez, E.; Sanchez, I. Repeatability and agreement in the measurement of horizontal fusional vergences. Ophthalmic Physiol. Opt. 2008, 28, 475–491. [Google Scholar] [CrossRef]
- Fray, K.J. Fusional amplitudes: Developing testing standards. Strabismus 2017, 25, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lança, C.C.; Rowe, F.J. Measurement of fusional vergence: A systematic review. Strabismus 2019, 27, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Palomo Álvarez, C.; Puell, M.C.; Sánchez-Ramos, C.; Villena, C. Normal values of distance heterophoria and fusional vergence ranges and effects of age. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Gay, C.; Mestre, C.; Argiles, M.; Vinuela-Navarro, V.; Pujol, J. Feasibility of measuring fusional vergence amplitudes objectively. PLoS ONE 2023, 18, e0284552. [Google Scholar] [CrossRef]
- Rowe, F.J. Fusional vergence measures and their significance in clinical assessment. Strabismus 2010, 18, 48–57. [Google Scholar] [CrossRef]
- Sreenivasan, V.; Babinsky, E.E.; Wu, Y.; Candy, T.R. Objective measurement of fusional vergence ranges and heterophoria in infants and preschool children. Investig. Opthalmology Vis. Sci. 2016, 57, 2678–2688. [Google Scholar] [CrossRef]
- Moon, B.-Y.; Kim, S.-Y.; Yu, D.-S. Receiver operating characteristic curve analysis of clinical signs for screening of convergence insufficiency in young adults. PLoS ONE 2020, 15, e0228313. [Google Scholar] [CrossRef]
- Hung, S.S.; Fisher, A.G.; Cermak, S.A. The performance of learning-disabled and normal young men on the test of visual-perceptual skills. Am. J. Occup. Ther. 1987, 41, 790–797. [Google Scholar] [CrossRef]
- Hung, G.K. Linear model of accommodation and vergence can account for discrepancies between AC/A measures using the fixation disparity and phoria methods. Ophthalmic Physiol. Opt. 1991, 11, 275–278. [Google Scholar] [CrossRef]
- Hung, G.K. Adaptation model of accommodation and vergence. Ophthalmic Physiol. Opt. 1992, 12, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Hung, G.K.; Ciuffreda, K.J.; Rosenfield, M. Proximal contribution to a linear static model of accommodation and vergence. Ophthalmic Physiol. Opt. 1996, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, J.; Thorn, F.; Held, R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom. Vis. Sci. 2005, 82, 273–278. [Google Scholar] [CrossRef]
- Arnoldi, K.A.; Reynolds, J.D. Diagnosis of pseudo-divergence excess exotropia secondary to high accommodative convergence to accommodation ratio. Am. Orthopt. J. 2006, 56, 133–137. [Google Scholar] [CrossRef]
- Brautaset, R.L.; Jennings, A.J.M. Effects of orthoptic treatment on the CA/C and AC/A ratios in convergence insufficiency. Investig. Opthalmology Vis. Sci. 2006, 47, 2876–2880. [Google Scholar] [CrossRef]
- Fukushima, T.; Torii, M.; Ukai, K.; Wolffsohn, J.S.; Gilmartin, B. The relationship between CA/C ratio and individual differences in dynamic accommodative responses while viewing stereoscopic images. J. Vis. 2009, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Hirani, K.J.; Firth, A.Y. Convergence accommodation to convergence (CA/C) ratio: Stability with different levels of convergence demand. Br. Ir. Orthopt. J. 2009, 6, 60–64. [Google Scholar] [CrossRef]
- Neveu, P.; Roumes, C.; Philippe, M.; Fuchs, P.; Priot, A.-E. Stereoscopic viewing can induce changes in the CA/C ratio. Investig. Opthalmology Vis. Sci. 2016, 57, 4321–4326. [Google Scholar] [CrossRef]
- Rosenfield, M.; Gilmartin, B. Assessment of the CA/C ratio in a myopic population. Am. J. Optom. Physiol. Opt. 1988, 65, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, M.; Ciuffreda, K.J.; Chen, H.-W. Effect of age on the interaction between the AC/A and CA/C ratios. Ophthalmic Physiol. Opt. 1995, 15, 451–455. [Google Scholar] [CrossRef]
- Tsuetaki, T.K.; Schor, C.M. Clinical method for measuring adaptation of tonic accommodation and vergence accommodation. Am. J. Optom. Physiol. Opt. 1987, 64, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Horwood, A.M.; Riddell, P.M. The clinical near gradient stimulus AC/A ratio correlates better with the response CA/C ratio than with the response AC/A ratio. Strabismus 2013, 21, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.E.; Seidel, D.; Day, M.; Gray, L.S. Quantifying interactions between accommodation and vergence in a binocularly normal population. Vis. Res. 2014, 105, 121–129. [Google Scholar] [CrossRef]
- Benjamin, W.J. Borish’s Clinical Refraction; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Horwood, A.M.; Riddell, P.M. The use of cues to convergence and accommodation in naïve, uninstructed participants. Vis. Res. 2008, 48, 1613–1624. [Google Scholar] [CrossRef]
- Horwood, A.M.; Riddell, P.M. Decreased accommodation during decompensation of distance exotropia. Br. J. Ophthalmol. 2012, 96, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Horwood, A.M.; Riddell, P.M. Evidence that convergence rather than accommodation controls intermittent distance exotropia. Acta Ophthalmol. 2012, 90, e109–e117. [Google Scholar] [CrossRef] [PubMed]
- Horwood, A.M.; Riddell, P.M. Accommodation and vergence response gains to different near cues characterize specific esotropias. Strabismus 2013, 21, 155–164. [Google Scholar] [CrossRef]
- Horwood, A.M.; Riddell, P.M. Disparity-driven vs blur-driven models of accommodation and convergence in binocular vision and intermittent strabismus. J. AAPOS 2014, 18, 576–583. [Google Scholar] [CrossRef]
- Mestre, C.; Neupane, S.; Manh, V.; Tarczy-Hornoch, K.; Candy, T.R. Vergence and accommodation responses in the control of intermittent exotropia. Ophthalmic Physiol. Opt. 2023, 43, 598–614. [Google Scholar] [CrossRef]
- Joubert, C.; Bedell, H.E. Proximal vergence and perceived distance. Optom. Vis. Sci. 1990, 67, 29–35. [Google Scholar] [CrossRef]
- Wick, B. Clinical factors in proximal vergence. Am. J. Optom. Physiol. Opt. 1985, 62, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wick, B.; Bedell, H.E. Magnitude and velocity of proximal vergence. Investig. Opthalmology Vis. Sci. 1989, 30, 755–760. [Google Scholar]
- Fogt, N.; Toole, A.J.; Rogers, D.L. A review of proximal inputs to the near response. Clin. Exp. Optom. 2016, 99, 30–38. [Google Scholar] [CrossRef]
- Fogt, N. Comparisons of proximal vergence measures. Vis. Dev. Rehabil. 2020, 6, 252. [Google Scholar] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Murray, C.; Newsham, D. The normal accommodative convergence/accommodation (AC/A) ratio. J. Binocul. Vis. Ocul. Motil. 2018, 68, 140–147. [Google Scholar] [CrossRef]
- Gwiazda, J.; Grice, K.; Thorn, F. Response AC/A ratios are elevated in myopic children. Ophthalmic Physiol. Opt. 1999, 19, 173–179. [Google Scholar] [CrossRef]
- Bhoola, H.; Bruce, A.S.; Atchison, D.A. Validity of clinical measures of the AC/A ratio. Clin. Exp. Optom. 1995, 78, 3–10. [Google Scholar] [CrossRef]
- Rainey, B.B.; Goss, D.A.; Kidwell, M.; Feng, B. Reliability of the response AC/A ratio determined using nearpoint autorefraction and simultaneous heterophoria measurement. Clin. Exp. Optom. 1998, 81, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Mani, R.; Hussaindeen, J.R. Changes in stimulus and response AC/A ratio with vision therapy in Convergence Insufficiency. J. Optom. 2017, 10, 169–175. [Google Scholar] [CrossRef]
| Far (6.0 m) | Near (0.4 m) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BI | BO | BI | BO | |||||||||
| b | B | r | b | B | r | b | B | r | b | B | r | |
| Median | 6.00 | 8.00 | 4.00 | 12.00 | 20.00 | 12.00 | 18.00 | 20.00 | 14.00 | 21.00 | 28.00 | 18.00 |
| IQR (25%) | 4.00 | 6.00 | 3.50 | 8.00 | 14.00 | 6.00 | 12.00 | 16.00 | 10.00 | 12.00 | 24.00 | 12.00 |
| IQR (75%) | 7.00 | 12.00 | 6.00 | 18.00 | 30.00 | 18.00 | 18.00 | 22.00 | 18.00 | 28.50 | 32.00 | 22.00 |
| Far (6.0 m) | Near (0.4 m) | ||||||
|---|---|---|---|---|---|---|---|
| BI | BO | BI | BO | ||||
| Blur | No blur | Blur | No blur | Blur | No blur | Blur | No blur |
| 10 37.0% | 17 63.0% | 20 74.1% | 7 25.9% | 15 55.5% | 12 44.5% | 10 37.0% | 17 63.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argilés, M.; Cardona, G. The Interaction between Vergence and Accommodation Cues in the Assessment of Fusional Vergence Range. Life 2024, 14, 1185. https://doi.org/10.3390/life14091185
Argilés M, Cardona G. The Interaction between Vergence and Accommodation Cues in the Assessment of Fusional Vergence Range. Life. 2024; 14(9):1185. https://doi.org/10.3390/life14091185
Chicago/Turabian StyleArgilés, Marc, and Genis Cardona. 2024. "The Interaction between Vergence and Accommodation Cues in the Assessment of Fusional Vergence Range" Life 14, no. 9: 1185. https://doi.org/10.3390/life14091185
APA StyleArgilés, M., & Cardona, G. (2024). The Interaction between Vergence and Accommodation Cues in the Assessment of Fusional Vergence Range. Life, 14(9), 1185. https://doi.org/10.3390/life14091185





