M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Renal Tissue Specimens
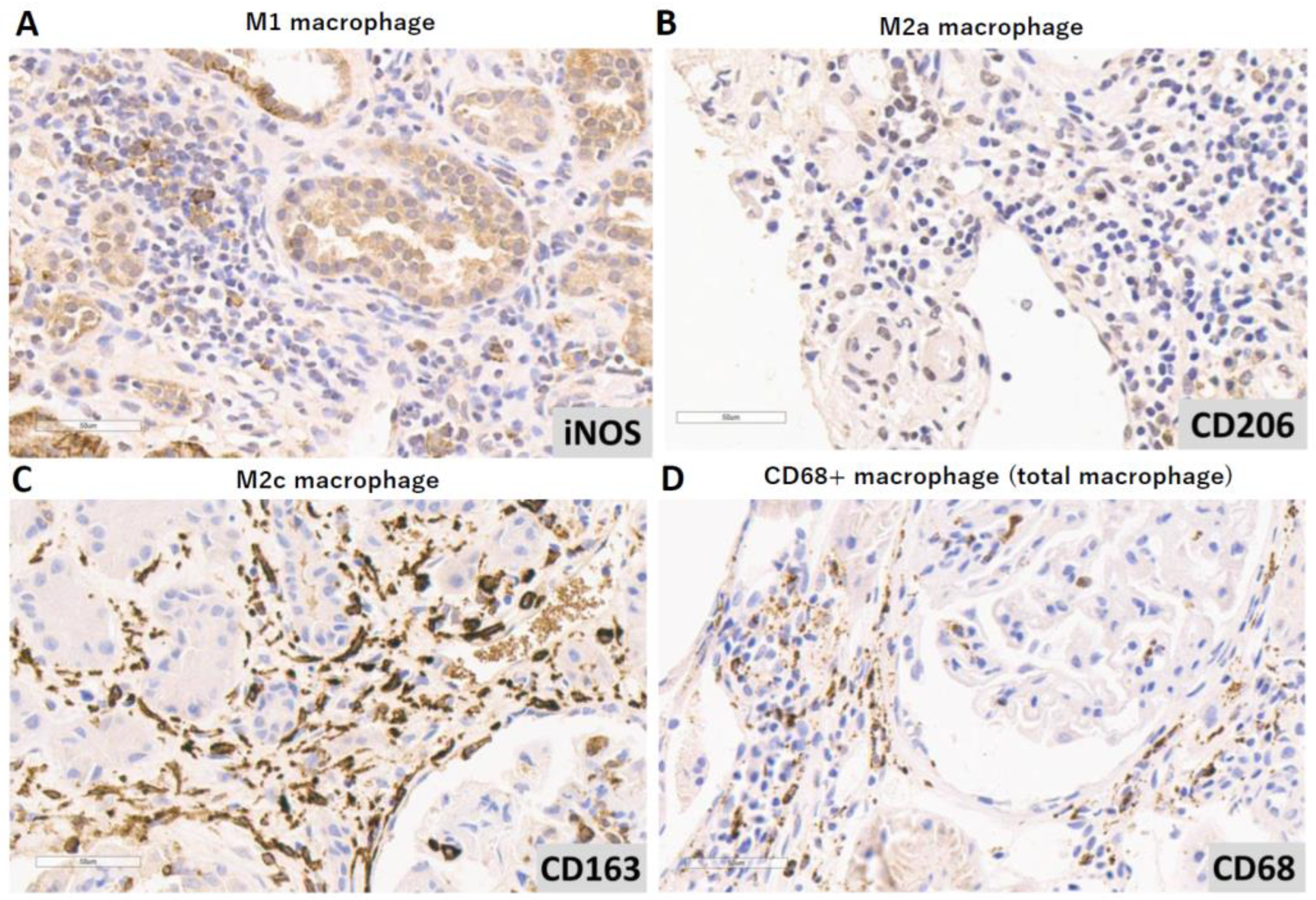
2.2. Immunohistochemical Analysis
2.3. Histopathological Evaluation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of SLE Patients
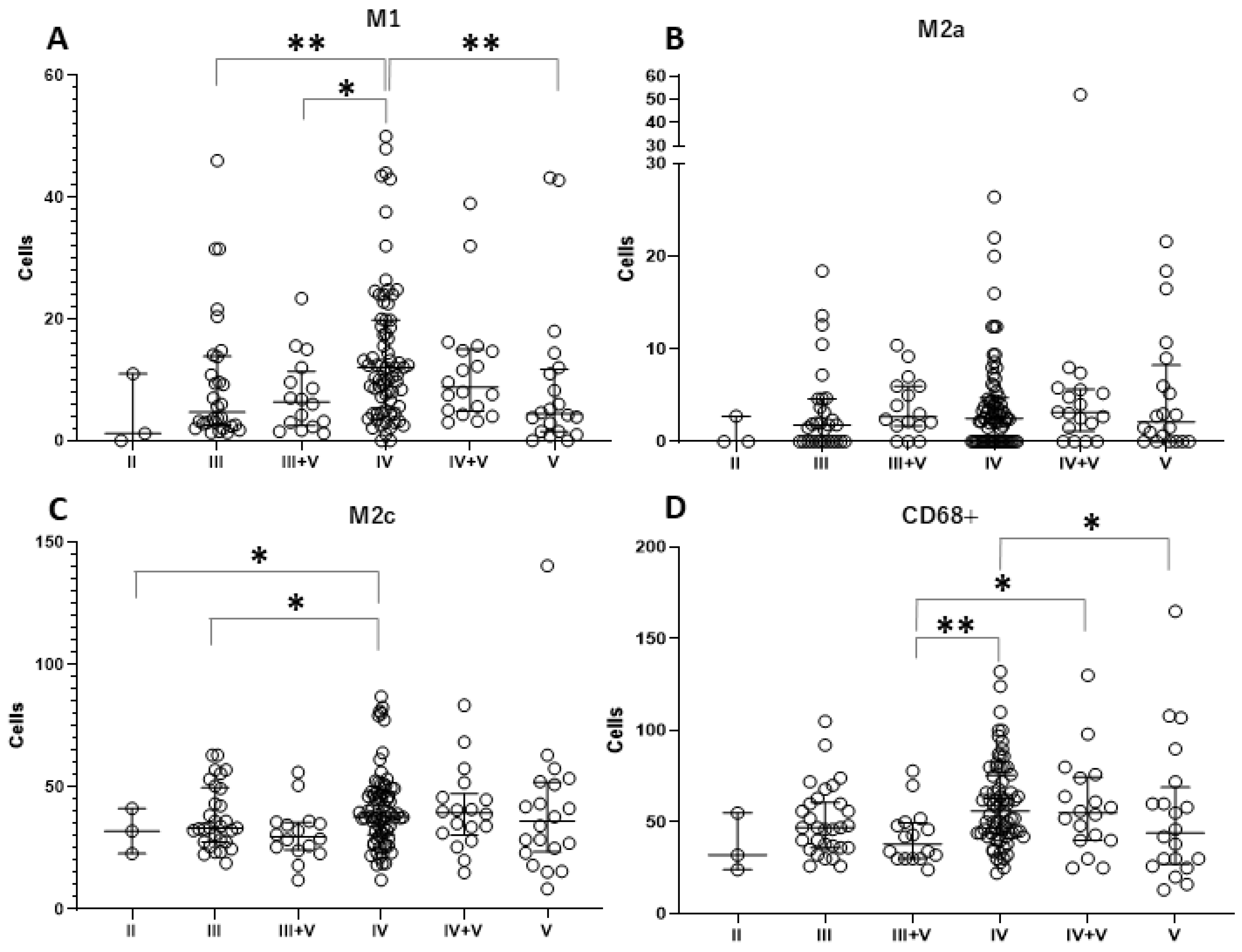
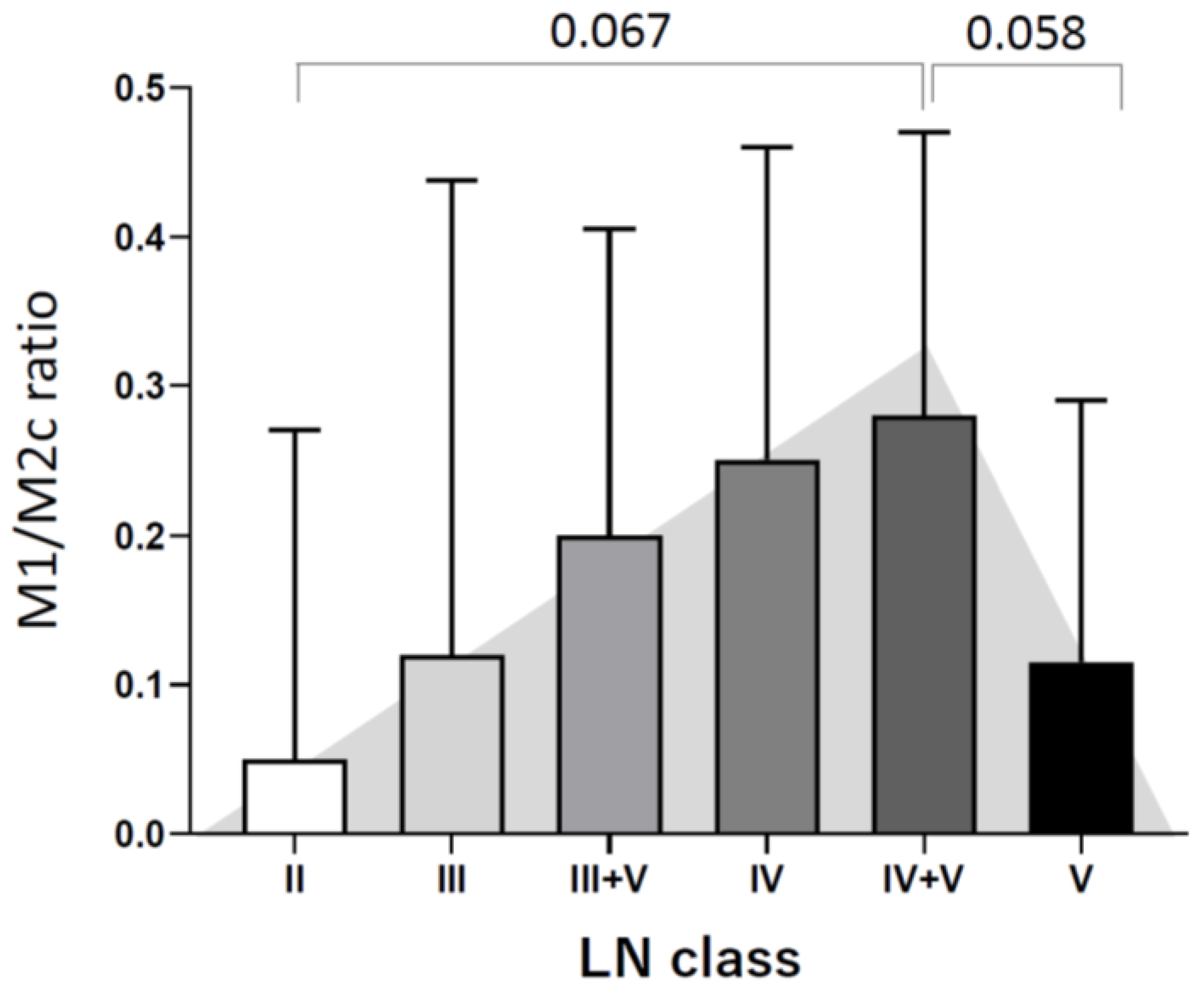
3.2. Different Tubulointerstitial Macrophage Subpopulations Among Various Lupus Nephritis Classifications
3.3. Tubulointerstitial Macrophages Are Associated with High Activity and Chronicity Indices
3.4. Tubulointerstitial Macrophages Are Associated with Decreased Renal Function
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, C.; Putterman, C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 2015, 11, 329–341. [Google Scholar] [CrossRef]
- Jakes, R.W.; Bae, S.C.; Louthrenoo, W.; Mok, C.C.; Navarra, S.V.; Kwon, N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: Prevalence, incidence, clinical features, and mortality. Arthritis Care Res. 2012, 64, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, N.; Henriksen, K.J.; Chang, A. Non-neoplastic Kidney Diseases in Adult Tumor Nephrectomy and Nephroureterectomy Specimens in a Southeast Asian Tertiary Medical Center. Vajira Med. J. J. Urban Med. 2024, 68, e265159. [Google Scholar] [CrossRef]
- Kasitanon, N.; Louthrenoo, W.; Sukitawut, W.; Vichainun, R. Causes of death and prognostic factors in Thai patients with systemic lupus erythematosus. Asian Pac. J. Allergy Immunol. 2002, 20, 85–91. [Google Scholar]
- Davidson, A.; Aranow, C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr. Opin. Intern. Med. 2006, 5, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllopoulou, A.; Franzke, C.W.; Seshan, S.V.; Perino, G.; Kalliolias, G.D.; Ramanujam, M.; Van Rooijen, N.; Davidson, A.; Ivashkiv, L.B. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc. Natl. Acad. Sci. USA 2010, 107, 3012–3017. [Google Scholar] [CrossRef]
- Sahu, R.; Bethunaickan, R.; Singh, S.; Davidson, A. Structure and Function of Renal Macrophages and Dendritic Cells from Lupus-Prone Mice. Arthritis Rheumatol. 2014, 66, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Schwartz, Y.S.; Svistelnik, A.V. Functional phenotypes of macrophages and the M1–M2 polarization concept. Part I. Proinflammatory phenotype. Biochemistry 2012, 77, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wietecha, T.A.; Hudkins, K.L.; Kida, Y.; Spencer, M.W.; Pichaiwong, W.; Kojima, I.; Duffield, J.S.; Alpers, C.E. Macrophages are essential contributors to kidney injury in murine cryoglobulinemic membranoproliferative glomerulonephritis. Kidney Int. 2011, 80, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Arnold, C.E.; Whyte, C.S.; Gordon, P.; Barker, R.N.; Rees, A.J.; Wilson, H.M. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology 2013, 141, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.-S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Filardy, A.A.; Pires, D.R.; Nunes, M.P.; Takiya, C.M.; Freire-De-Lima, C.G.; Ribeiro-Gomes, F.L.; DosReis, G.A. Proinflammatory Clearance of Apoptotic Neutrophils Induces an IL-12lowIL-10high Regulatory Phenotype in Macrophages. J. Immunol. 2010, 185, 2044–2050. [Google Scholar] [CrossRef]
- Weening, J.J.; D’agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.N.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Nonsiri, P.; Srivilai, P.; Onkaew, S.; Benjakul, N. The effectiveness of using dye models for small tissue biopsies in the surgical pathology laboratory. Folia Histochem. Cytobiol. 2023, 61, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Chalmers, S.A.; Chitu, V.; Herlitz, L.C.; Sahu, R.; Stanley, E.R.; Putterman, C. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 2015, 57, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, J.; Redecke, V.; Guy, C.; Zhou, J.; Wu, R.; Ippagunta, S.K.; Tillman, H.; Walker, P.D.; Vogel, P.; Häcker, H. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J. Clin. Investig. 2019, 129, 2251–2265. [Google Scholar] [CrossRef] [PubMed]
- Kosałka-Węgiel, J.; Jakieła, B.; Dziedzic, R.; Milewski, M.; Siwiec-Koźlik, A.; Zaręba, L.; Bazan-Socha, S.; Sanak, M.; Musiał, J.; Korkosz, M. Circulating B Lymphocyte Subsets in Patients with Systemic Lupus Erythematosus. Medicina 2024, 60, 1994. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.-H.; Xu, D.-L.; Gao, B. Significance of CD163-Positive Macrophages in Proliferative Glomerulonephritis. Am. J. Med Sci. 2015, 350, 387–392. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, S.M.; Linge, C.P.; Gullstrand, B.; Lood, C.; Johansson, Å.; Ohlsson, S.; Lundqvist, A.; Bengtsson, A.A.; Carlsson, F.; Hellmark, T. Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin. Immunol. 2014, 152, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Olmes, G.; Büttner-Herold, M.; Ferrazzi, F.; Distel, L.; Amann, K.; Daniel, C. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res. Ther. 2016, 18, 90. [Google Scholar] [CrossRef] [PubMed]

| Variable | |
|---|---|
| Age (years), mean ± SD | 33.14 ± 13.35 |
| Sex, n (%) | |
| Male | 141 (88.1) |
| Female | 19 (11.9) |
| ISN/RPS classification, n (%) | |
| Class II | 3 (1.9) |
| Class III | 30 (18.8) |
| Class III + V | 16 (10.0) |
| Class IV | 73 (45.6) |
| Class IV + V | 18 (11.3) |
| Class V | 20 (12.5) |
| Laboratory data, median (IQR) | |
| Serum creatinine before treatment | 1.18 (0.75–1.86) |
| Serum creatinine after 1-year treatment | 0.7 (0.55–0.88) |
| 24 h urine protein before treatment | 1071 (621–1917) |
| Urine protein dipstick test (before treatment), n (%) | |
| 1+ | 10 (6.3) |
| 2+ | 29 (18.1) |
| 3+ | 46 (28.7) |
| 4+ | 75 (46.9) |
| Macrophage Sub-Populations | LN Classification; Median (IQR) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| II | III | III + V | IV | IV + V | V | ||
| M1 (iNOS+) | 1 (0.6–6.1) | 4.7 (2.6–13.8) | 6.4 (2.7–10.8) | 12 (5.6–19.8) | 9 (5–14.8) | 4 (1.5–11.5) | 0.003 * |
| M2a (CD206+) | 0 (0–1.38) | 1.8 (0–4.6) | 2.7 (1.7–6) | 2.5 (0–4.6) | 3 (1.5–5.2) | 2 (0–7.5) | 0.53 |
| M2c (CD163+) | 32 (27.4–36.6) | 33.3 (27.6–49.4) | 29.7 (24.8–35.4) | 37.8 (31.2–48) | 40 (31.2–45.8) | 34 (24–47.1) | 0.13 |
| Macrophage (CD68+) | 32 (28–43.5) | 47 (36–60) | 38 (30–49) | 56 (44–75) | 55 (40–74) | 42 (28–60) | 0.009 * |
| Subgroup Comparison | M1 (iNOS+) | M2a (CD206+) | M2c (CD163+) | Total Macrophages (CD68+) |
|---|---|---|---|---|
| II vs. III | 0.150 | 0.397 | 0.153 | 0.178 |
| II vs. III + V | 0.198 | 0.142 | 0.113 | 0.653 |
| II vs. IV | 0.064 | 0.189 | 0.040 * | 0.074 |
| II vs. IV + V | 0.088 | 0.148 | 0.069 | 0.131 |
| II vs. V | 0.337 | 0.208 | 0.182 | 0.565 |
| III vs. III + V | 0.936 | 0.173 | 0.364 | 0.111 |
| III vs. IV | 0.009 * | 0.206 | 0.016 * | 0.053 |
| III vs. IV + V | 0.064 | 0.214 | 0.135 | 0.263 |
| III vs. V | 0.607 | 0.435 | 0.936 | 0.388 |
| III + V vs. IV | 0.012 * | 0.697 | 0.328 | 0.002 * |
| III + V vs. IV + V | 0.105 | 0.800 | 0.639 | 0.040 * |
| III + V vs. V | 0.556 | 0.665 | 0.432 | 0.868 |
| IV vs. IV + V | 0.417 | 0.778 | 0.677 | 0.679 |
| IV vs. V | 0.007 * | 1.000 | 0.051 | 0.037 * |
| Variable | Macrophage Subpopulation, n (%) | ||||
|---|---|---|---|---|---|
| M1 (iNOS+) | M2a (CD206+) | M2c (CD163+) | Total Macrophages (CD68+) | ||
| Activity index | |||||
| Spearman’s rho a | 0.41 ** | 0.12 | 0.07 | 0.22 * | |
| p-value | <0.001 | 0.13 | 0.40 | 0.005 | |
| Chronicity index | |||||
| Spearman’s rho a | 0.44 ** | 0.48 ** | 0.18 * | 0.42 ** | |
| p-value | <0.001 | <0.001 | 0.024 | <0.001 | |
| Interstitial inflammation | |||||
| Spearman’s rho a | 0.43 ** | 0.41 ** | 0.23 * | 0.44 ** | |
| p-value | <0.001 | <0.001 | 0.004 | <0.001 | |
| Variable | Macrophage Subpopulation, n (%) | ||||
|---|---|---|---|---|---|
| M1 (iNOS+) | M2a (CD206+) | M2c (CD163+) | Total Macrophages (CD68+) | ||
| Serum creatinine before treatment | |||||
| Spearman’s rho a | 0.27 * | 0.17 * | 0.28 ** | 0.34 * | |
| p-value | <0.001 | 0.036 | <0.001 | <0.001 | |
| Serum creatinine after 1-year treatment | |||||
| Spearman’s rho a | 0.27 ** | 0.15 | 0.22 * | 0.30 ** | |
| p-value | <0.001 | 0.063 | 0.007 | <0.001 | |
| 24 h urine protein before treatment | |||||
| Spearman’s rho a | 0.14 | 0.09 | 0.13 | 0.17 * | |
| p-value | 0.082 | 0.287 | 0.115 | 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavanisakun, C.; Keawvichit, R.; Benjakul, N. M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis. Life 2025, 15, 55. https://doi.org/10.3390/life15010055
Chavanisakun C, Keawvichit R, Benjakul N. M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis. Life. 2025; 15(1):55. https://doi.org/10.3390/life15010055
Chicago/Turabian StyleChavanisakun, Chutima, Rassamon Keawvichit, and Nontawat Benjakul. 2025. "M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis" Life 15, no. 1: 55. https://doi.org/10.3390/life15010055
APA StyleChavanisakun, C., Keawvichit, R., & Benjakul, N. (2025). M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis. Life, 15(1), 55. https://doi.org/10.3390/life15010055






