The Effect of Sleep Disruption on Cardiometabolic Health
Abstract
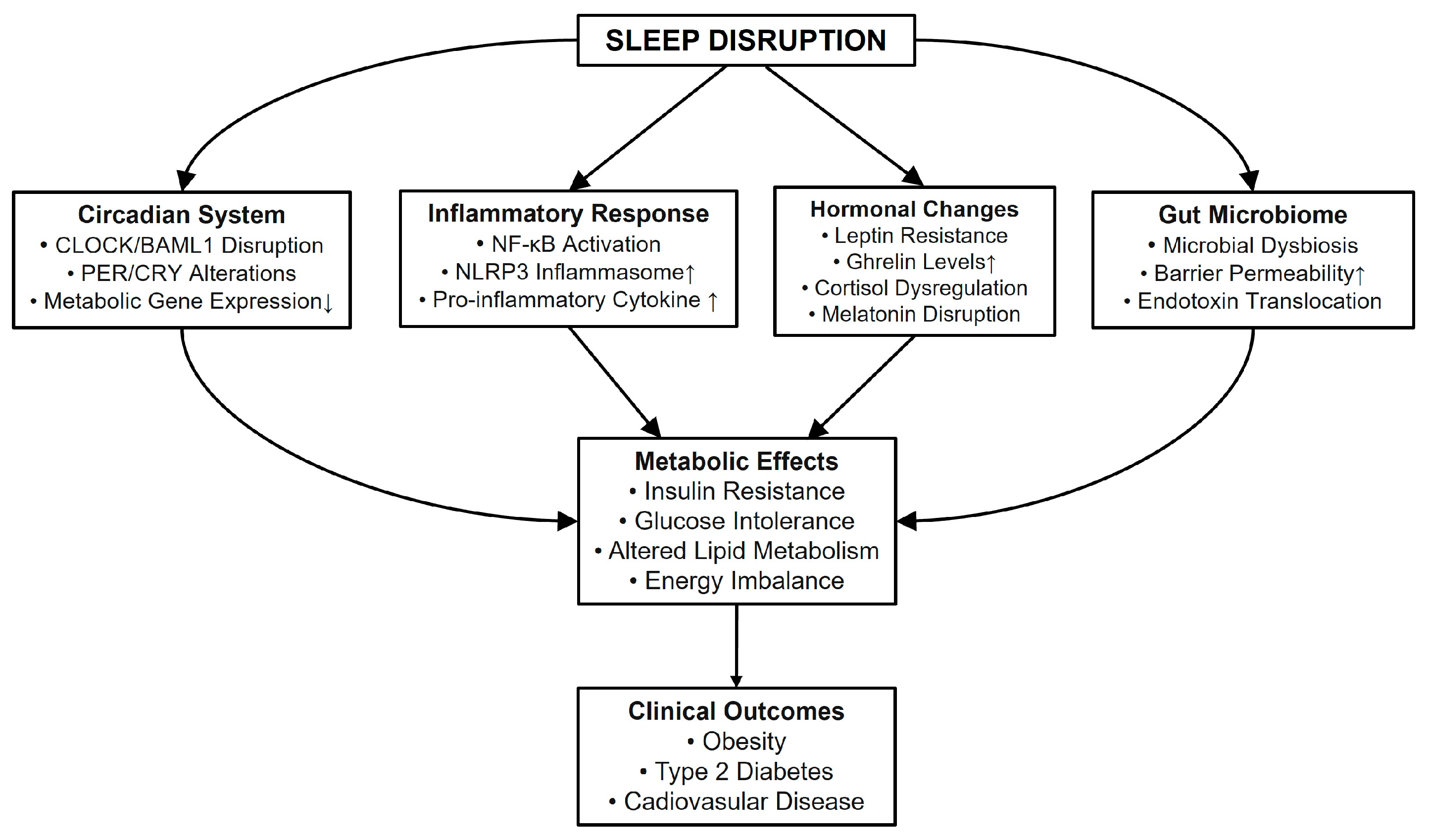
:1. Introduction
2. Short Sleep Duration and the Increased Risk of Obesity
3. Sleep Disruption and Increased Risk of Type 2 Diabetes
| Study | Population | Study Design | Follow-Up Period | Sleep Parameter Measured | Primary Outcomes | Risk Ratio (95% CI) | Reference |
|---|---|---|---|---|---|---|---|
| Nurses’ Health Study | 70,000 female nurses; US | Prospective cohort | 10 years | Self-reported sleep duration | T2DM incidence | 1.35 for ≤5 h sleep | [20] |
| National Health Interview Survey | 130,943 adults; Mixed ethnicity | Population survey | Various | Sleep duration and quality | Diabetes risk | Significant linear association | [21] |
| Korean Cohort | Middle-aged adults; Asian population | Prospective cohort | 8 years | OSA severity (AHI) | T2DM development | 1.5 for moderate–severe OSA | [34] |
| Wang et al. | 5953 participants; multiple ethnicities | Meta- analysis | Various | OSA presence and severity | Diabetes risk | 1.63 (1.09–2.45) | [35] |
| Qie et al. | 338,912 subjects; global population | Meta- analysis | Various | OSA severity levels | T2DM risk | 1.40 (1.32–1.48) | [36] |
4. Sleep Disruptions and Cardiovascular Morbidity and Mortality
4.1. Insomnia and Cardiovascular Disease
4.2. Short Sleep Duration and Cardiovascular Disease
4.3. Poor Sleep Quality and Cardiovascular Disease
| Sleep Parameter | Study Design | Sample Characteristics | Duration | Outcomes | Risk Ratio/HR (95% CI) | Reference |
|---|---|---|---|---|---|---|
| Insomnia | Meta-analysis of 13 studies | 122,501 adults; multiple countries | Various | CV mortality | 1.45 (1.29–1.62) | [38] |
| Insomnia symptoms | Prospective study | 52,610 adults | 11.4 years | Acute MI | 1.45 (1.18–1.80) | [39] |
| Short sleep (<6 h) | Meta-analysis | 474,684 adults | Various | CHD risk | 1.48 (1.22–1.80) | [43] |
| Poor sleep quality | UK Biobank | 205,654 adults | 13 years | CVD mortality | 1.13 (1.04–1.24) | [50] |
| Delta wave disruption | SHHS/MrOS combined analysis | 6251 participants | Extended follow-up | CVD mortality | SHHS: 1.94 (1.18–3.18); MrOS: 1.66 (1.12–2.47) | [51] |
5. Molecular Mechanism Underlying Sleep Disruption and Metabolic Dysfunction
5.1. Circadian Clock System and Metabolic Regulation
5.2. Impact of Sleep Disruption on the Circadian Clock System
5.3. Sleep Disruption and Glucose and Lipid Metabolism
| Component | Function | Expression Pattern | Effect of Disruption | Tissue Specificity | Metabolic Impact | Therapeutic Implications | Reference |
|---|---|---|---|---|---|---|---|
| CLOCK /BMAL1 | Core clock transcription factors; regulate metabolic genes | Peak expression during active phase | Altered amplitude and timing; disrupted rhythmicity | Hypothalamus, liver, adipose tissue, pancreas | Glucose dysregulation; lipid metabolism alterations | Chronotherapy potential; timing of interventions | [53,55] |
| PER/CRY | Negative feedback regulators; metabolic sensors | Peak during rest phase | Rhythm disruption; altered phase timing | Ubiquitous expression; tissue-specific patterns | Metabolic imbalance; energy homeostasis disruption | Timing of medication; meal timing importance | [55,64] |
| GLUT2/Glucokinase | Glucose transport; nutrient sensing | Diurnal rhythm in expression | Reduced expression; impaired function | Liver, pancreas β-cells, intestine | Impaired glucose uptake; altered insulin secretion | Glucose management strategies | [23,56] |
| SREBP-1c | Lipid homeostasis regulation; transcriptional control | Circadian oscillation | Dysregulation; phase shifts | Liver, adipose tissue | Altered lipid metabolism; fat storage changes | Lipid-lowering interventions | [57] |
| AMPK | Energy sensor; metabolic regulator | Activity follows feeding/fasting cycles | Reduced activation; timing disruption | Multiple tissues | Energy metabolism disruption; substrate utilization | Exercise timing; nutrient sensing | [75,76,77] |
5.4. Sleep Disruption and Inflammation in Metabolic Dysfunction
6. Sleep Disruption and the Gut Microbiome in Metabolic Dysfunction
| Study Model | Population /Sample | Sleep Condition | Microbiome Changes | Metabolic Effects | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Human | Healthy young adults (n = 9) | 2 nights partial sleep deprivation | ↑Firmicutes/Bacteroidetes ratio; altered bacterial diversity | Impaired glucose metabolism; insulin sensitivity↓ | Altered microbial metabolite production; changed gut permeability | [113] |
| Mouse | C57BL/6J mice | Chronic sleep fragmentation | ↑Pro-inflammatory bacteria; ↓beneficial species; changed metabolic pathways | Insulin resistance; glucose intolerance; adipose tissue inflammation | Increased intestinal permeability; metabolic endotoxemia | [114] |
| Human/mouse | Humans and mice | Jet lag/circadian disruption | Dysbiosis; altered microbial rhythmicity | Glucose intolerance; weight gain; metabolic dysfunction | Circadian misalignment; disturbed feeding patterns | [117] |
| Mouse | Adult mice | Complete sleep deprivation | Altered diversity; changed bacterial composition | Anxiety-like behavior; metabolic changes | Gut–brain axis disruption; barrier dysfunction | [126] |
| Human | 118 middle-aged volunteers | Reduced REM sleep | Christensenellaceae family positively associated with REM duration; Enterobacteriaceae family negatively associated with REM duration; iron metabolism | Glucose profile alterations | Sleep-microbiome interaction | [127] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep. Med. 2017, 32, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Kocevska, D.; Lysen, T.S.; Dotinga, A.; Koopman-Verhoeff, M.E.; Luijk, M.; Antypa, N.; Biermasz, N.R.; Blokstra, A.; Brug, J.; Burk, W.J.; et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: A systematic review and meta-analysis. Nat. Hum. Behav. 2021, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Hansen, J.; Timmers, S.; Moonen-Kornips, E.; Duez, H.; Staels, B.; Hesselink, M.K.; Schrauwen, P. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci. Rep. 2016, 6, 35047. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef]
- Van Cauter, E.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef]
- Aurora, R.N.; Punjabi, N.M. Obstructive sleep apnoea and type 2 diabetes mellitus: A bidirectional association. Lancet Respir. Med. 2013, 1, 329–338. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Malhotra, A.; White, D.P.; Gottlieb, D.J.; Hu, F.B. Association between reduced sleep and weight gain in women. Am. J. Epidemiol. 2006, 164, 947–954. [Google Scholar] [CrossRef]
- Bacaro, V.; Ballesio, A.; Cerolini, S.; Vacca, M.; Poggiogalle, E.; Donini, L.M.; Lucidi, F.; Lombardo, C. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 301–309. [Google Scholar] [CrossRef]
- Deng, X.; He, M.; He, D.; Zhu, Y.; Zhang, Z.; Niu, W. Sleep duration and obesity in children and adolescents: Evidence from an updated and dose-response meta-analysis. Sleep Med. 2021, 78, 169–181. [Google Scholar] [CrossRef]
- Knutson, K.L. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 731–743. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Depner, C.M.; Stothard, E.R.; Wright, K.P., Jr. Metabolic consequences of sleep and circadian disorders. Curr. Diabetes Rep. 2014, 14, 507. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef]
- Jackson, C.L.; Redline, S.; Kawachi, I.; Hu, F.B. Association between sleep duration and diabetes in black and white adults. Diabetes Care 2013, 36, 3557–3565. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Seetho, I.W.; Wilding, J.P. Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chron. Respir. Dis. 2014, 11, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef]
- Kachi, Y.; Nakao, M.; Takeuchi, T.; Yano, E. Association between insomnia symptoms and hemoglobin A1c level in Japanese men. PLoS ONE 2011, 6, e21420. [Google Scholar] [CrossRef]
- Kass, L.; Sanderson, J.C.; Desai, T.; Hurst, R. The relationship between the elevation of haemoglobin A1c level, sleep quality and sleep duration in clinically diagnosed pre-diabetic patients in a nationally representative sample. Diabetes Vasc. Dis. Res. 2022, 19, 14791641211067421. [Google Scholar] [CrossRef]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.L.; Perfect, M.M.; Janovsky, C.C.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: Systematic review and meta-analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Reichmuth, K.J.; Austin, D.; Skatrud, J.B.; Young, T. Association of sleep apnea and type II diabetes: A population-based study. Am. J. Respir. Crit. Care Med. 2005, 172, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Snoring as a risk factor for type II diabetes mellitus: A prospective study. Am. J. Epidemiol. 2002, 155, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Tomlinson, G.; Leung, R.S. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am. J. Respir. Crit. Care Med. 2014, 190, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, A.T.; Kim, S.; Thomas, R.J.; Lee, M.H.; Ku Lee, S.; Shin, C. Obstructive sleep apnoea and long-term risk of incident diabetes in the middle-aged and older general population. ERJ Open Res. 2023, 9, 00401–2022. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Y.; Zhang, Q.; Pan, F. Obstructive sleep apnoea and the risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Respirology 2013, 18, 140–146. [Google Scholar] [CrossRef]
- Qie, R.; Zhang, D.; Liu, L.; Ren, Y.; Zhao, Y.; Liu, D.; Liu, F.; Chen, X.; Cheng, C.; Guo, C.; et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of cohort studies. J. Diabetes 2020, 12, 455–464. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Vatten, L.J.; Platou, C.; Janszky, I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation 2011, 124, 2073–2081. [Google Scholar] [CrossRef]
- Jarrin, D.C.; Alvaro, P.K.; Bouchard, M.A.; Jarrin, S.D.; Drake, C.L.; Morin, C.M. Insomnia and hypertension: A systematic review. Sleep Med. Rev. 2018, 41, 3–38. [Google Scholar] [CrossRef]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Alfonso-Miller, P.; Fernandez-Mendoza, J.; Shetty, S.; Shenoy, S.; Combs, D. Sleep: Important considerations for the prevention of cardiovascular disease. Curr. Opin. Cardiol. 2016, 31, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Cherubini, J.M.; Cheng, J.L.; Williams, J.S.; MacDonald, M.J. Sleep deprivation and endothelial function: Reconciling seminal evidence with recent perspectives. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H29–H35. [Google Scholar] [CrossRef]
- Hall, M.H.; Mulukutla, S.; Kline, C.E.; Samuelsson, L.B.; Taylor, B.J.; Thayer, J.F.; Krafty, R.T.; Frank, E.; Kupfer, D.J. Objective Sleep Duration Is Prospectively Associated With Endothelial Health. Sleep 2017, 40, zsw003. [Google Scholar] [CrossRef]
- Jaspan, V.N.; Greenberg, G.S.; Parihar, S.; Park, C.M.; Somers, V.K.; Shapiro, M.D.; Lavie, C.J.; Virani, S.S.; Slipczuk, L. The Role of Sleep in Cardiovascular Disease. Curr. Atheroscler. Rep. 2024, 26, 249–262. [Google Scholar] [CrossRef]
- Bertisch, S.M.; Pollock, B.D.; Mittleman, M.A.; Buysse, D.J.; Bazzano, L.A.; Gottlieb, D.J.; Redline, S. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018, 41, zsy047. [Google Scholar] [CrossRef]
- Hu, W.; Han, Q.; Chu, J.; Sun, N.; Li, T.; Feng, Z.; He, Q.; Ma, Z.; Wang, Y.; Shen, Y. Mechanism of the association between sleep quality and mortality in middle-aged and older adults: A prospective study analysis of the UK Biobank. Arch. Gerontol. Geriatr. 2023, 113, 105051. [Google Scholar] [CrossRef]
- Ai, S.; Ye, S.; Li, G.; Leng, Y.; Stone, K.L.; Zhang, M.; Wing, Y.K.; Zhang, J.; Liang, Y.Y. Association of Disrupted Delta Wave Activity During Sleep With Long-Term Cardiovascular Disease and Mortality. J. Am. Coll. Cardiol. 2024, 83, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Lo Sasso, G.; Moschetta, A.; Schibler, U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Gooley, J.J.; Chua, E.C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J. Genet. Genom. 2014, 41, 231–250. [Google Scholar] [CrossRef]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Maury, E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef] [PubMed]
- Moller-Levet, C.S.; Archer, S.N.; Bucca, G.; Laing, E.E.; Slak, A.; Kabiljo, R.; Lo, J.C.; Santhi, N.; von Schantz, M.; Smith, C.P.; et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc. Natl. Acad. Sci. USA 2013, 110, E1132–E1141. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Bartolini, D.; Arcangeletti, M.; Ciffolilli, S.; Murdolo, G.; Piroddi, M.; Papa, S.; Reiter, R.J.; Galli, F. Melatonin regulates mesenchymal stem cell differentiation: A review. J. Pineal Res. 2014, 56, 382–397. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Sato, T.; Sato, S. Circadian Regulation of Metabolism: Commitment to Health and Diseases. Endocrinology 2023, 164, bqad086. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Osler, M.E.; Voisin, S.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Zierath, J.R.; Schioth, H.B.; Benedict, C. Acute Sleep Loss Induces Tissue-Specific Epigenetic and Transcriptional Alterations to Circadian Clock Genes in Men. J. Clin. Endocrinol. Metab. 2015, 100, E1255–E1261. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, E.; Cakiroglu, F.P. The importance of AMPK in obesity and chronic diseases and the relationship of AMPK with nutrition: A literature review. Crit. Rev. Food Sci. Nutr. 2023, 63, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Ehrmann, D.A.; Van Cauter, E.; Tasali, E.; Brady, M.J. Impaired insulin signaling in human adipocytes after experimental sleep restriction: A randomized, crossover study. Ann. Intern. Med. 2012, 157, 549–557. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, D.B.; Yoon, D.W.; Kim, J. Melatonin Improves Glucose Homeostasis and Insulin Sensitivity by Mitigating Inflammation and Activating AMPK Signaling in a Mouse Model of Sleep Fragmentation. Cells 2024, 13, 470. [Google Scholar] [CrossRef]
- Zhu, Q.; An, Y.A.; Scherer, P.E. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022, 32, 351–364. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Copinschi, G.; Leproult, R.; Spiegel, K. The important role of sleep in metabolism. Front. Horm. Res. 2014, 42, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Ghosh, S.; Bhola, A.; Verma, P.; Amist, A.D.; Sharma, H.; Sachdeva, P.; Sinha, J.K. Sleep and Immune System Crosstalk: Implications for Inflammatory Homeostasis and Disease Pathogenesis. Ann. Neurosci. 2024, 09727531241275347. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Carroll, J.E.; Carrillo, C.; Olmstead, R.; Witarama, T.; Breen, E.C.; Yokomizo, M.; Seeman, T.; Irwin, M.R. Sleep deprivation and divergent toll-like receptor-4 activation of cellular inflammation in aging. Sleep 2015, 38, 205–211. [Google Scholar] [CrossRef]
- Wisor, J.P.; Clegern, W.C.; Schmidt, M.A. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep 2011, 34, 1335–1345. [Google Scholar] [CrossRef]
- Xu, Y.P.; Tao, Y.N.; Wu, Y.P.; Zhang, J.; Jiao, W.; Wang, Y.H.; Chen, T. Sleep deprivation aggravates brain injury after experimental subarachnoid hemorrhage via TLR4-MyD88 pathway. Aging 2021, 13, 3101–3111. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Diaz-Garcia, E.; Garcia-Tovar, S.; Alfaro, E.; Jaureguizar, A.; Casitas, R.; Sanchez-Sanchez, B.; Zamarron, E.; Fernandez-Lahera, J.; Lopez-Collazo, E.; Cubillos-Zapata, C.; et al. Inflammasome Activation: A Keystone of Proinflammatory Response in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Yangzhong, X.; Hua, S.; Wen, Y.; Bi, X.; Li, M.; Zheng, Y.; Sun, S. NLRP3 Inflammasome Triggers Inflammation of Obstructive Sleep Apnea. Curr. Mol. Med. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Yousefi, Z.; Ghafori, S.S.; Hassanzadeh, G. Sleep deprivation and NLRP3 inflammasome: Is there a causal relationship? Front. Neurosci. 2022, 16, 1018628. [Google Scholar] [CrossRef] [PubMed]
- Pourcet, B.; Duez, H. Circadian Control of Inflammasome Pathways: Implications for Circadian Medicine. Front. Immunol. 2020, 11, 1630. [Google Scholar] [CrossRef]
- Mosavat, M.; Mirsanjari, M.; Arabiat, D.; Smyth, A.; Whitehead, L. The Role of Sleep Curtailment on Leptin Levels in Obesity and Diabetes Mellitus. Obes. Facts 2021, 14, 214–221. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Hosoi, T.; Ozawa, K. Possible Pharmacological Approach Targeting Endoplasmic Reticulum Stress to Ameliorate Leptin Resistance in Obesity. Front. Endocrinol. 2016, 7, 59. [Google Scholar] [CrossRef]
- Koliaki, C.; Kokkinos, A.; Tentolouris, N.; Katsilambros, N. The effect of ingested macronutrients on postprandial ghrelin response: A critical review of existing literature data. Int. J. Pept. 2010, 2010, 710852. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Goldberg, R.B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009, 94, 3171–3182. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, F.; Li, J.; Cai, H.; Strom, S.; Bisello, A.; Kelley, D.E.; Friedman-Einat, M.; Skibinski, G.A.; McCrory, M.A.; et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat. Med. 2006, 12, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, K.; Mineo, C.; Yuhanna, I.S.; Chambliss, K.L.; Quon, M.J.; Bonvini, E.; Shaul, P.W. C-reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine-based inhibition motif of FcgammaRIIB and SHIP-1. Circ. Res. 2009, 104, 1275–1282. [Google Scholar] [CrossRef]
- Li, Y.; Tan, J.; Miao, Y.; Zhang, Q. MicroRNA in extracellular vesicles regulates inflammation through macrophages under hypoxia. Cell Death Discov. 2021, 7, 285. [Google Scholar] [CrossRef]
- Nelson, M.C.; O’Connell, R.M. MicroRNAs: At the Interface of Metabolic Pathways and Inflammatory Responses by Macrophages. Front. Immunol. 2020, 11, 1797. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, P.Y.; Su, M.C.; Chin, C.H.; Liou, C.W.; Wang, T.Y.; Lin, Y.Y.; Lee, C.P.; Lin, M.C.; Hsiao, C.C. miR-21-5p Under-Expression in Patients with Obstructive Sleep Apnea Modulates Intermittent Hypoxia with Re-Oxygenation-Induced-Cell Apoptosis and Cytotoxicity by Targeting Pro-Inflammatory TNF-alpha-TLR4 Signaling. Int. J. Mol. Sci. 2020, 21, 999. [Google Scholar] [CrossRef]
- Karuga, F.F.; Jaromirska, J.; Malicki, M.; Sochal, M.; Szmyd, B.; Bialasiewicz, P.; Strzelecki, D.; Gabryelska, A. The role of microRNAs in pathophysiology and diagnostics of metabolic complications in obstructive sleep apnea patients. Front. Mol. Neurosci. 2023, 16, 1208886. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Moya, P.R.; Wendland, J.R.; Salemme, J.; Fried, R.L.; Murphy, D.L. miR-15a and miR-16 regulate serotonin transporter expression in human placental and rat brain raphe cells. Int. J. Neuropsychopharmacol. 2013, 16, 621–629. [Google Scholar] [CrossRef]
- Dos Santos, A.; Galie, S. The Microbiota-Gut-Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients 2024, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Pala, B.; Pennazzi, L.; Nardoianni, G.; Fogacci, F.; Cicero, A.F.G.; Di Renzo, L.; Barbato, E.; Tocci, G. Gut Microbiota Dysbiosis and Sleep Disorders: Culprit in Cardiovascular Diseases. J. Clin. Med. 2024, 13, 3254. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schurmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Lopez, D.E.; Lashinger, L.M.; Weinstock, G.M.; Bray, M.S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 2021, 33, 873–887. [Google Scholar] [CrossRef]
- Gil-Hernandez, E.; Ruiz-Gonzalez, C.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Rueda-Ruzafa, L.; Sanchez-Labraca, N.; Roman, P. Effect of gut microbiota modulation on sleep: A systematic review and meta-analysis of clinical trials. Nutr. Rev. 2023, 81, 1556–1570. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Swanson, G.R.; Burgess, H.J.; Keshavarzian, A. Sleep disturbances and inflammatory bowel disease: A potential trigger for disease flare? Expert Rev. Clin. Immunol. 2011, 7, 29–36. [Google Scholar] [CrossRef]
- Sobolewska-Wlodarczyk, A.; Wlodarczyk, M.; Szemraj, J.; Stec-Michalska, K.; Fichna, J.; Wisniewska-Jarosinska, M. Circadian rhythm abnormalities—Association with the course of inflammatory bowel disease. Pharmacol. Rep. 2016, 68, 847–851. [Google Scholar] [CrossRef]
- Parkar, N.; Dalziel, J.E.; Spencer, N.J.; Janssen, P.; McNabb, W.C.; Young, W. Slowed gastrointestinal transit is associated with an altered caecal microbiota in an aged rat model. Front. Cell Infect. Microbiol. 2023, 13, 1139152. [Google Scholar] [CrossRef]
- Khanijow, V.; Prakash, P.; Emsellem, H.A.; Borum, M.L.; Doman, D.B. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol. Hepatol. (N. Y.) 2015, 11, 817–825. [Google Scholar] [PubMed]
- Meyer, R.K.; Duca, F.A. RISING STARS: Endocrine regulation of metabolic homeostasis via the intestine and gut microbiome. J. Endocrinol. 2023, 258, e230019. [Google Scholar] [CrossRef] [PubMed]
- Iesanu, M.I.; Zahiu, C.D.M.; Dogaru, I.A.; Chitimus, D.M.; Pircalabioru, G.G.; Voiculescu, S.E.; Isac, S.; Galos, F.; Pavel, B.; O’Mahony, S.M.; et al. Melatonin-Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants 2022, 11, 2244. [Google Scholar] [CrossRef]
- Ortega, V.A.; Mercer, E.M.; Giesbrecht, G.F.; Arrieta, M.C. Evolutionary Significance of the Neuroendocrine Stress Axis on Vertebrate Immunity and the Influence of the Microbiome on Early-Life Stress Regulation and Health Outcomes. Front. Microbiol. 2021, 12, 634539. [Google Scholar] [CrossRef]
- Zhu, X.; Cai, J.; Wang, Y.; Liu, X.; Chen, X.; Wang, H.; Wu, Z.; Bao, W.; Fan, H.; Wu, S. A High-Fat Diet Increases the Characteristics of Gut Microbial Composition and the Intestinal Damage Associated with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 16733. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, X.; Li, D.; Xu, L.; Zhou, G.; Xu, M.; Peng, L.; Sun, G.; Pan, F.; Li, Y.; et al. Sleep deprivation-induced anxiety-like behaviors are associated with alterations in the gut microbiota and metabolites. Microbiol. Spectr. 2024, 12, e0143723. [Google Scholar] [CrossRef]
- Arnoriaga-Rodriguez, M.; Leal, Y.; Mayneris-Perxachs, J.; Perez-Brocal, V.; Moya, A.; Ricart, W.; Fernandez-Balsells, M.; Fernandez-Real, J.M. Gut Microbiota Composition and Functionality Are Associated With REM Sleep Duration and Continuous Glucose Levels. J. Clin. Endocrinol. Metab. 2023, 108, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The Role of Gut Microbiome in Sleep Quality and Health: Dietary Strategies for Microbiota Support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, S.J.; Ham, S.L.; Kim, J.; Bang, H.J.; Park, J.S.; Jang, H.H.; Kim, T.Y.; Park, J.W.; Seo, Y.R.; et al. Gut Bacterial Metabolites from Tryptophan and Phenylalanine Induce Melatonin Synthesis and Extend Sleep Duration in Mice. ACS Omega 2024, 9, 43875–43883. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Deyang, T.; Baig, M.A.I.; Dolkar, P.; Hediyal, T.A.; Rathipriya, A.G.; Bhaskaran, M.; PandiPerumal, S.R.; Monaghan, T.M.; Mahalakshmi, A.M.; Chidambaram, S.B. Sleep apnoea, gut dysbiosis and cognitive dysfunction. FEBS J. 2024, 291, 2519–2544. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, K.Y.; Wang, N.R.; Zhang, L.; Zhang, J.P. Effect of probiotics and paraprobiotics on patients with sleep disorders and sub-healthy sleep conditions: A meta-analysis of randomized controlled trials. Front. Neurol. 2024, 15, 1477533. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Melo, N.C.; Cuevas-Sierra, A.; Souto, V.F.; Martinez, J.A. Biological Rhythms, Chrono-Nutrition, and Gut Microbiota: Epigenomics Insights for Precision Nutrition and Metabolic Health. Biomolecules 2024, 14, 559. [Google Scholar] [CrossRef]
| Study Name | Population | Study Design | Follow-Up Duration | Key Findings | Effect Size/Risk Ratio | Reference |
|---|---|---|---|---|---|---|
| Nurses’ Health Study | 60,000 female nurses aged 30–55; US-based | Prospective cohort | 16 years | ≤5 h sleep: +1.14 kg weight gain; each hour reduction: +0.35 kg gain; higher risk of obesity | 0.35 kg per hour sleep reduction; linear relationship | [14] |
| Whitehall II | 5021 British civil servants; mixed gender | Prospective cohort | 5 years | Cross-sectional association but no prospective changes in BMI | No significant prospective effect | [3] |
| Bacaro et al. | 154,936 participants | Meta-analysis | Various | ≤6.5 h sleep increased obesity risk | OR = 1.41 (95% CI: 1.18–1.69) | [15] |
| Deng et al. | 57,848 children/adolescents | Meta-analysis | Various | Short sleep increased obesity risk | RR = 1.57 (95% CI: 1.36–1.81) | [16] |
| Pathway | Function | Metabolic Consequences | Therapeutic Targets | Biomarkers | Reference |
|---|---|---|---|---|---|
| NF-κB signaling | Inflammatory response regulation and immune homeostasis | Insulin resistance development; enhanced adipose inflammation; Increased hepatic lipogenesis | IKK inhibitors; natural compounds; anti-inflammatory agents | p-NF-κB; nuclear p65; IκB levels | [25,84] |
| NLRP3 inflammasome | Innate immune response and metabolic sensing | Impaired insulin signaling; pancreatic β-cell dysfunction; enhanced adipose inflammation | NLRP3 inhibitors; IL-1β antagonists; ROS scavengers | IL-1β levels; IL-18 levels; ASC specks | [90,91,92] |
| TLR4 signaling | Pattern recognition and innate immunity modulation | Systemic insulin resistance; altered lipid metabolism; disrupted glucose homeostasis | TLR4 antagonists; pathway inhibitors; metabolic targets | TLR4 expression; inflammatory markers | [87,88] |
| Pro-inflammatory cytokines | Immune signaling and metabolic regulation | Impaired insulin signaling; reduced glucose uptake; altered metabolism | Cytokine inhibitors; receptor antagonists; anti-inflammatory agents | TNF-α; IL-6; IL-1β | [25,26,85] |
| CRP pathway | Acute phase protein production and inflammatory response | Leptin resistance; reduced insulin sensitivity | Anti-inflammatory agents; metabolic modulators | hs-CRP; inflammatory panels | [103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Lee, D.-B.; Yoon, D.-W.; Yoo, S.-L.; Kim, J. The Effect of Sleep Disruption on Cardiometabolic Health. Life 2025, 15, 60. https://doi.org/10.3390/life15010060
Hong S, Lee D-B, Yoon D-W, Yoo S-L, Kim J. The Effect of Sleep Disruption on Cardiometabolic Health. Life. 2025; 15(1):60. https://doi.org/10.3390/life15010060
Chicago/Turabian StyleHong, SeokHyun, Da-Been Lee, Dae-Wui Yoon, Seung-Lim Yoo, and Jinkwan Kim. 2025. "The Effect of Sleep Disruption on Cardiometabolic Health" Life 15, no. 1: 60. https://doi.org/10.3390/life15010060
APA StyleHong, S., Lee, D.-B., Yoon, D.-W., Yoo, S.-L., & Kim, J. (2025). The Effect of Sleep Disruption on Cardiometabolic Health. Life, 15(1), 60. https://doi.org/10.3390/life15010060








