Protective Effect of Exogenous Adenosine Triphosphate Against Ocular Toxicity of Linezolid in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals
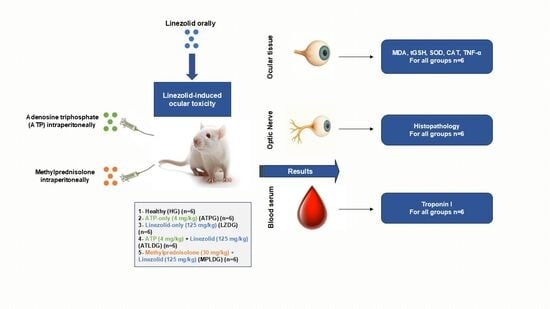
2.3. Experimental Groups
2.4. Experimental Procedure
2.5. Biochemical Analysis
2.5.1. Preparation of Samples
2.5.2. Determination of MDA, GSH Levels, SOD and CAT Activities in Ocular Tissue
2.5.3. Ocular Tissue TNF-α Determination
2.5.4. Blood Serum TP-I Determination
2.6. Histopathological Method
2.7. Statistical Analysis
3. Results
3.1. Biochemical Results
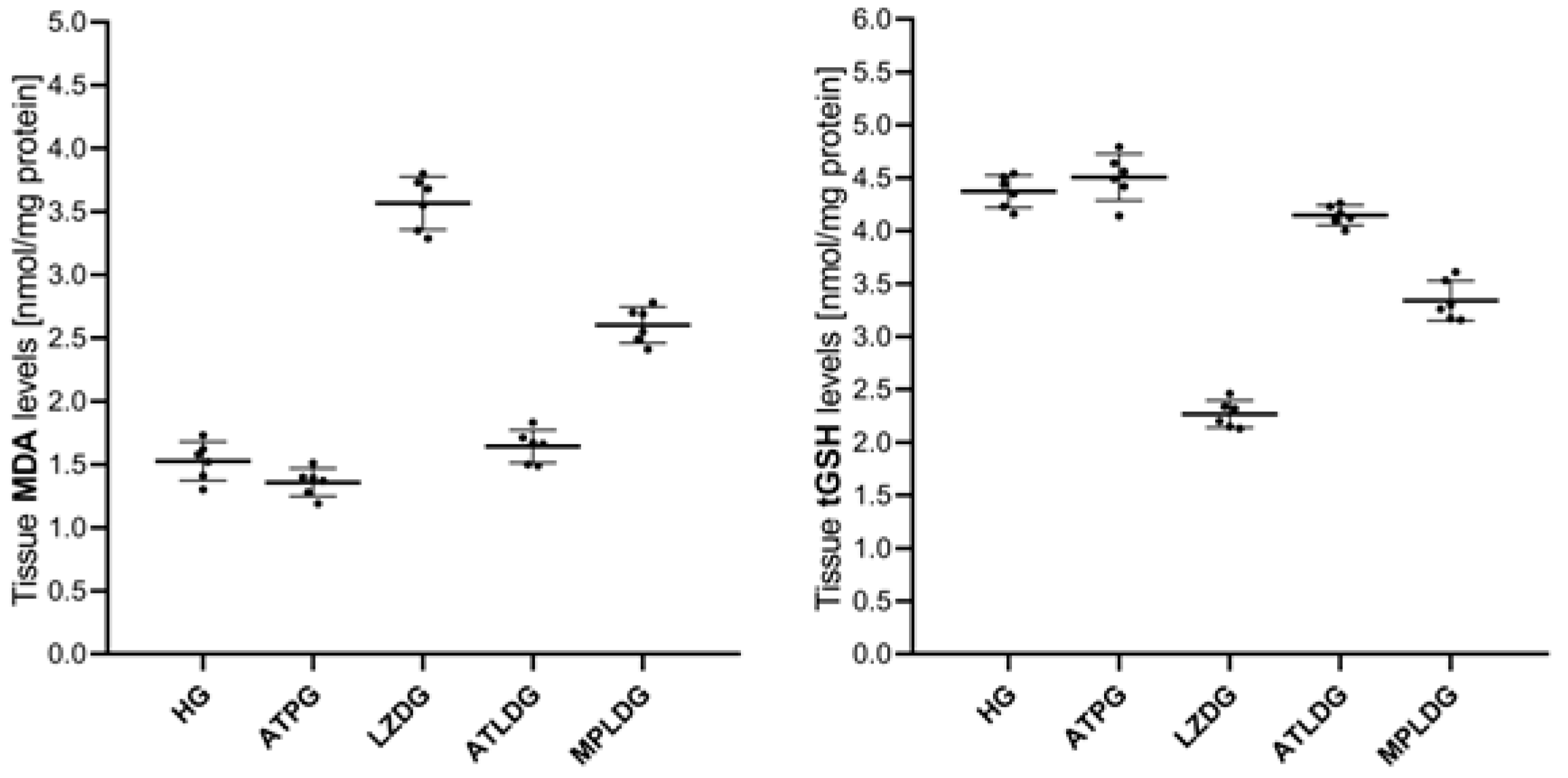
3.1.1. MDA and tGSH Level Analysis Results
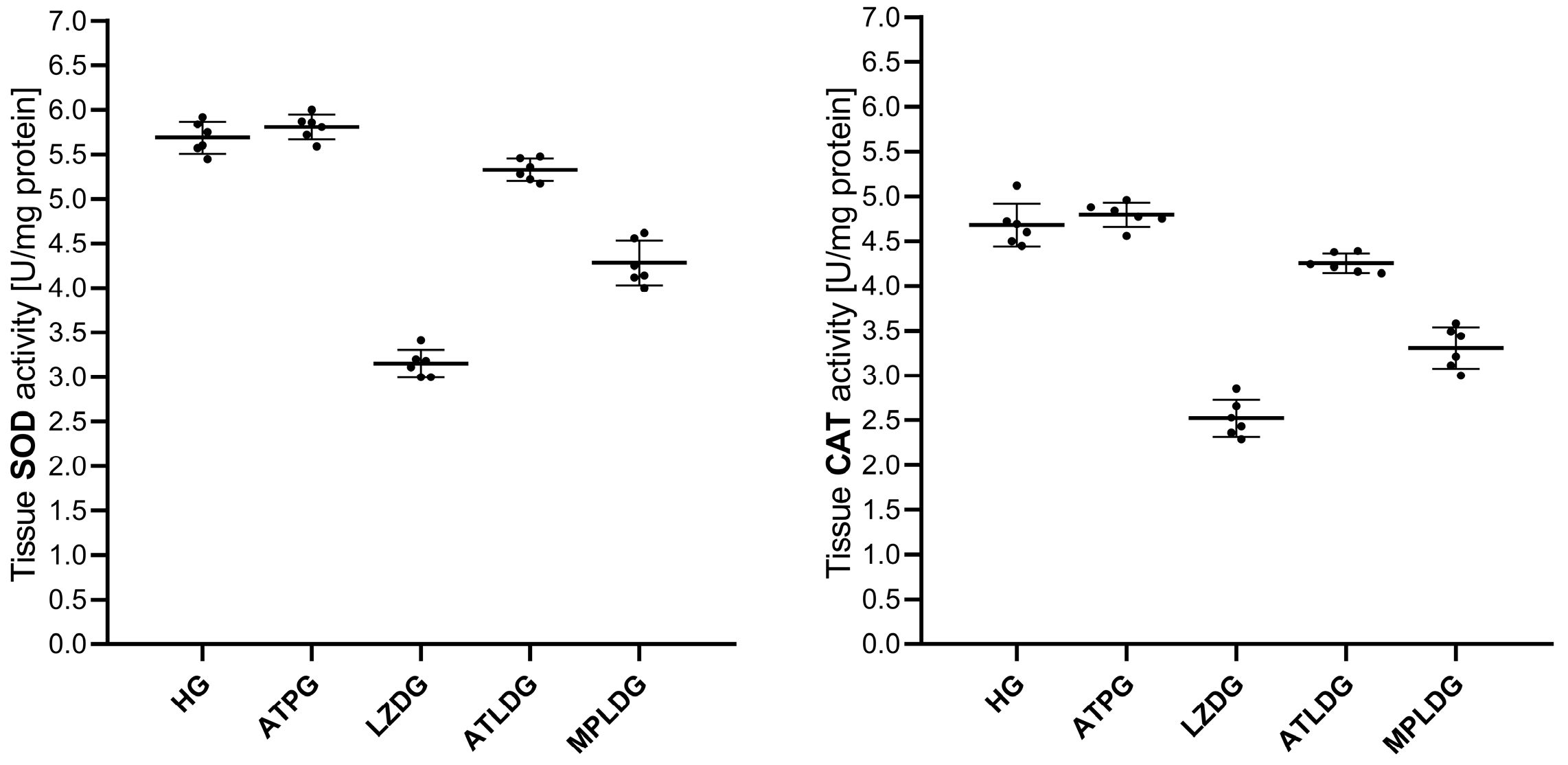
3.1.2. SOD and CAT Analysis Results
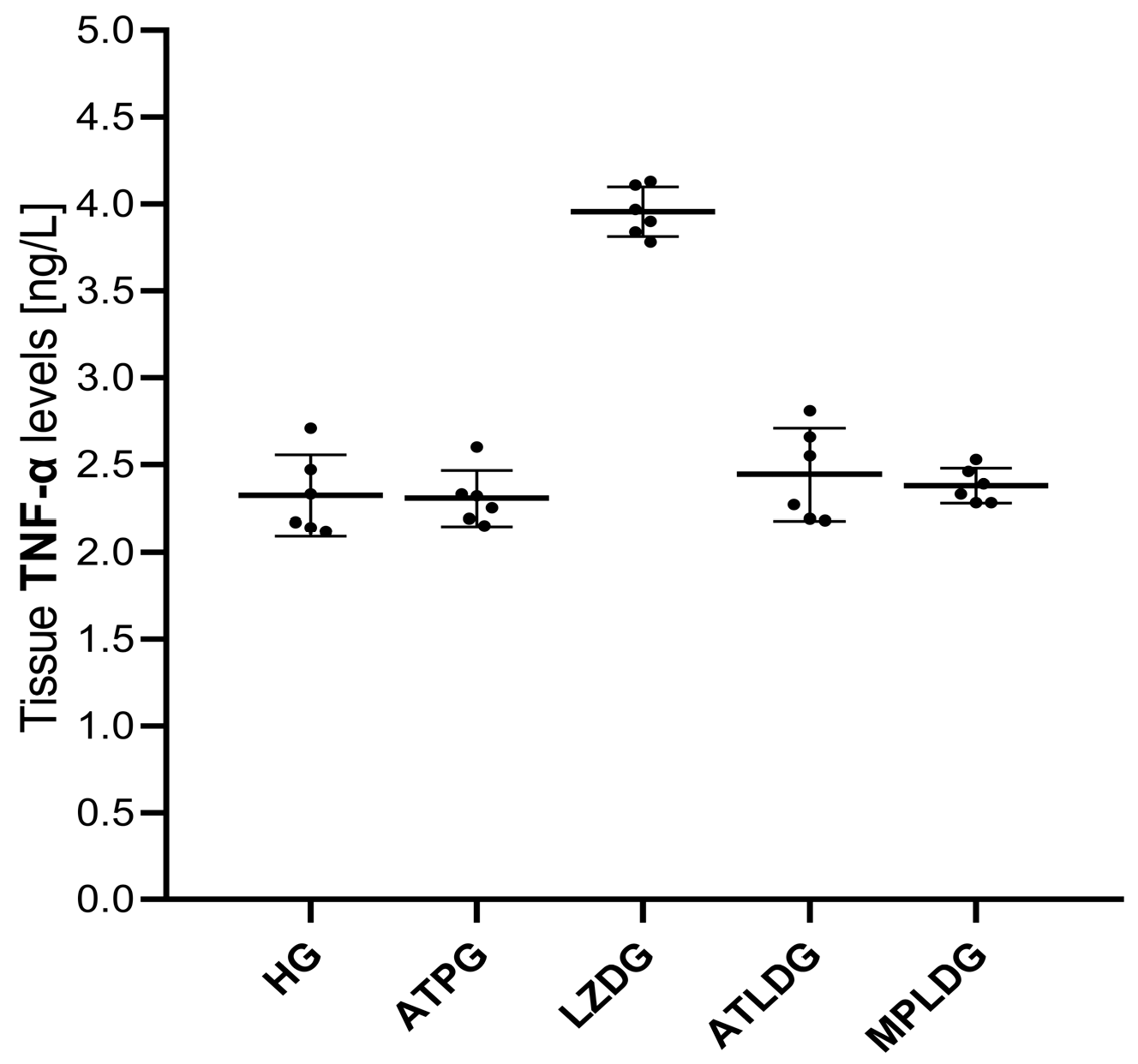
3.1.3. Ocular Tissue TNF-α Assay Results
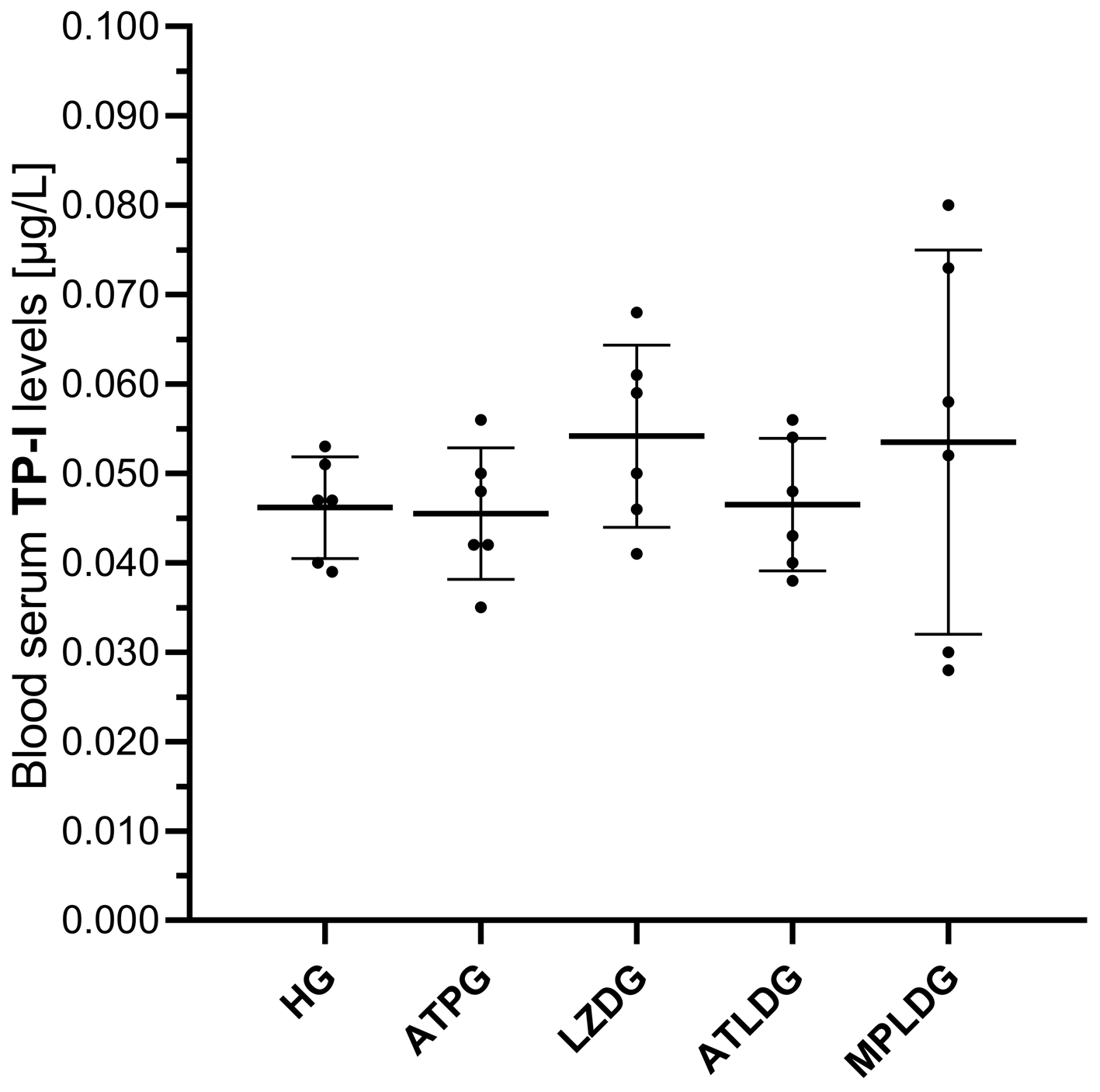
3.1.4. Evaluation of TP-I Levels in Blood Serum
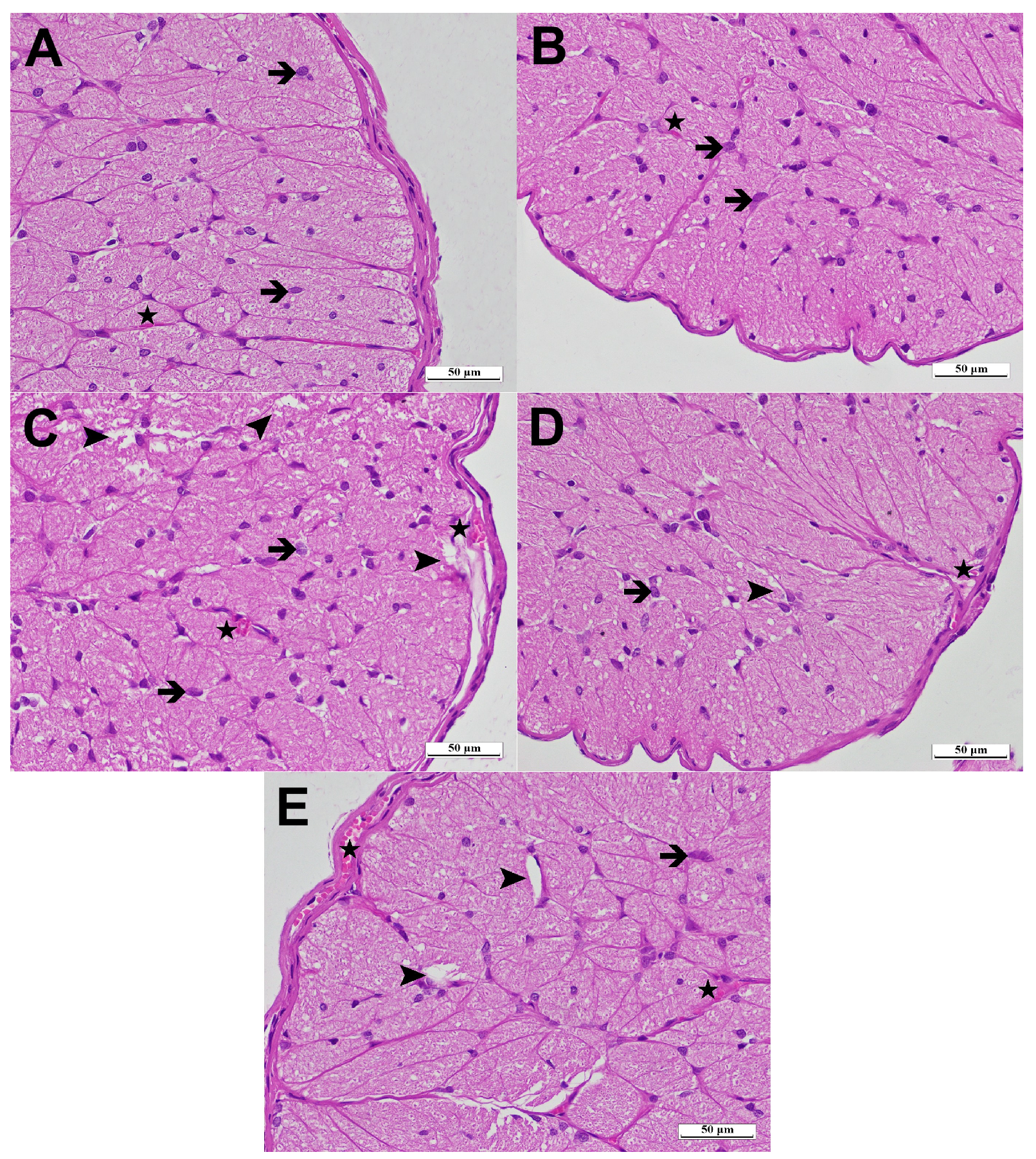
3.2. Histopathological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| tGSH | Total Glutathione |
| SOD | Superoxid |
| CAT | Catalase |
| ATP | Adenosine Triphosphate |
| TNF-α | Tumor Necrosis Factor-alpha |
| TP-I | Troponin I |
| ROS | Reactive Oxygen Species |
| ELISA | Enzyme-linked Immunosorbent Assay |
| LPO | Lipid Peroxidation |
References
- Azzouz, A.; Preuss, C.V. Linezolid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535428/ (accessed on 7 August 2025).
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Devel. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Diekema, D.I.; Jones, R.N. Oxazolidinones: A review. Drugs 2000, 59, 7–16. [Google Scholar] [CrossRef]
- Antal, E.J.; Hendershot, P.E.; Batts, D.H.; Sheu, W.P.; Hopkins, N.K.; Donaldson, K.M. Linezolid, a novel oxazolidinone antibiotic: Assessment of monoamine oxidase inhibition using pressor response to oral tyramine. J. Clin. Pharmacol. 2001, 41, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.V.; Cao, A.A.; McClelland, C.M.; Lee, M.S. Linezolid optic neuropathy. Curr. Opin. Ophthalmol. 2023, 34, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Veerman, K.; Goosen, J.; Spijkers, K.; Jager, N.; Heesterbeek, P.; Telgt, D. Prolonged use of linezolid in bone and joint infections: A retrospective analysis of adverse effects. J. Antimicrob. Chemother. 2023, 78, 2660–2666. [Google Scholar] [CrossRef]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.W.; Marriott, D.J.E.; Neely, M.; IATDMCT Antimicrobial Scientific Committee. Therapeutic drug monitoring can improve linezolid dosing regimens in current clinical practice: A review of linezolid pharmacokinetics and pharmacodynamics. Ther. Drug Monit. 2020, 42, 83–92. [Google Scholar] [CrossRef]
- Javaheri, M.; Khurana, R.N.; O’Hearn, T.M.; Lai, M.M.; Sadun, A.A. Linezolid-induced optic neuropathy: A mitochondrial disorder? Br. J. Ophthalmol. 2007, 91, 111–115. [Google Scholar] [CrossRef]
- Kendir-Demirkol, Y.; Jenny, L.A.; Demirkol, A.; Ozen, M.; Ayata, A.; Canatan, D. The protective effects of pyridoxine on linezolid-induced hematological toxicity, hepatotoxicity, and oxidative stress in rats. Turk. Arch. Pediatr. 2023, 58, 298–301. [Google Scholar] [CrossRef]
- Vivekanandan, L.; Sheik, H.; Singaravel, S.; Thangavel, S. Ameliorative effect of silymarin against linezolid-induced hepatotoxicity in methicillin-resistant Staphylococcus aureus (MRSA) infected Wistar rats. Biomed. Pharmacother. 2018, 108, 1303–1312. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, L.; Liu, T.; Zhang, R.; Yang, P.; Wang, X.; Dai, L. Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Front. Pharmacol. 2022, 13, 1037814. [Google Scholar] [CrossRef]
- Ye, X.; Huang, A.; Wang, X.; Wen, C.; Hu, L.; Lin, G. Linezolid inhibited synthesis of ATP in mitochondria: Based on GC-MS metabolomics and HPLC method. Biomed. Res. Int. 2018, 2018, 3128270. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, adenosine triphosphate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538317 (accessed on 7 August 2025).
- Saquet, A.A.; Streif, J.; Bangerth, F. Changes in ATP, ADP, and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’ pears and ‘Jonagold’ apples during controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.M.; Shi, J.; Qu, H.X.; Xue, S.; Duan, X.W.; Shi, J.; Prasad, N.K. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem. 2010, 118, 42–47. [Google Scholar] [CrossRef]
- Erdem, K.T.O.; Bedir, Z.; Ates, I.; Kuyrukluyildiz, U.; Coban, T.A.; Yazici, G.N.; Arslan, Y.K.; Suleyman, Z.; Suleyman, H. The effect of adenosine triphosphate on propofol-induced myopathy in rats: A biochemical and histopathological evaluation. Korean J. Physiol. Pharmacol. 2021, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Huang, S.P.; Chang, C.H.; Lin, K.H.; Chang, S.W.; Tsai, R.K. Protective effects of systemic treatment with methylprednisolone in a rodent model of non-arteritic anterior ischemic optic neuropathy (rAION). Exp. Eye Res. 2015, 131, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E255–E266. [Google Scholar] [CrossRef]
- Icel, E.; Suleyman, H.; Yazici, G.N.; Bakan, N.; Sunar, M. Effects of adenosine triphosphate on methanol-induced experimental optic nerve damage in rats: Biochemical and histopathological evaluation. Cutaneous Ocul. Toxicol. 2020, 39, 244–248. [Google Scholar] [CrossRef]
- Wang, T.; Guo, D.; Dong, X.; Mu, L. Effect of linezolid on hematological and oxidative parameters in rats. J. Antibiot. 2014, 67, 433–437. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Dinc, K.; Ozyurt, R.; Coban, T.A.; Yazici, G.N.; Suleyman, Z.; Yavuzer, B.; Suleyman, H. The effect of carvacrol on the proinflammatory cytokines, histology, and fertility outcome of cisplatin-related ovarian change in a rat model. Taiwan J. Obstet. Gynecol. 2023, 62, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.J.; Kim, S.J.; Han, K.E.; Choi, E.J.; Lee, J.E. Case report: Linezolid-associated toxic optic neuropathy in patients with pulmonary tuberculosis: Case series and mini review. Front. Pharmacol. 2025, 16, 1547236. [Google Scholar] [CrossRef]
- Ishii, N.; Kinouchi, R.; Inoue, M.; Yoshida, A. Linezolid-induced optic neuropathy with a rare pathological change in the inner retina. Int. Ophthalmol. 2016, 36, 761–766. [Google Scholar] [CrossRef]
- Brandariz-Nunez, D.; Hernandez-Corredoira, V.; Guarc-Prades, E.; Garcia-Navarro, B. Optic neuropathy associated with linezolid: Systematic review of cases. Farm. Hosp. 2019, 43, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, J.; Tang, K.; Zhao, Z.; Ding, Y.; Zhang, J. Cataract with linezolid-induced toxic optic neuropathy: Case report and mini review. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Duke, S.L.; Kump, L.I.; Yuan, Y.; West, W.W.; Sachs, A.J.; Haider, N.B.; Margalit, E. The safety of intraocular linezolid in rabbits. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3115–3119. [Google Scholar] [CrossRef]
- Kulkarni, K.; Del Priore, L.V. Linezolid induced toxic optic neuropathy. Br. J. Ophthalmol. 2005, 89, 1664–1665. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Coster, R.V.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.-J.; Groote, C.C.-D.; Vandecasteele, S.; et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- Bano, S.; Nawaz, A.; Numan, A.; Hassan, M.A.; Shafique, M.B.A. A case report and literature review of the outcome of linezolid-induced optic and peripheral neuropathy in patients with multidrug-resistant pulmonary TB. Front. Neurol. 2022, 13, 908584. [Google Scholar] [CrossRef]
- Pilania, R.K.; Arora, A.; Agarwal, A.; Jindal, A.K.; Aggarwal, K.; Krishnan, G.; Suri, D.; Gupta, A.; Singh, S.; Gupta, V. Linezolid-induced mitochondrial toxicity presenting as retinal nerve fiber layer microcysts and optic and peripheral neuropathy in a patient with chronic granulomatous disease. Retina Cases Brief Rep. 2021, 15, 224–229. [Google Scholar] [CrossRef]
- Garrabou, G.; Soriano, A.; Pinos, T.; Casanova-Molla, J.; Pacheu-Grau, D.; Moren, C.; García-Arumí, E.; Morales, M.; Ruiz-Pesini, E.; Catalán-Garcia, M.; et al. Influence of mitochondrial genetics on the mitochondrial toxicity of linezolid in blood cells and skin nerve fibers. Antimicrob. Agents Chemother. 2017, 61, e00542-17. [Google Scholar] [CrossRef]
- Bohm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-Sjögren syndrome: Potential biomarkers for dry eye disease. Curr. Eye Res. 2016, 41, 1143–1149. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants—Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ren, X.; Leveillard, T. Modulating antioxidant systems as a therapeutic approach to retinal degeneration. Redox Biol. 2022, 57, 102510. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione metabolism and the novel role of mitochondrial GSH in retinal degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, A.D.; Somuncu, A.M.; Cicek, I.; Yavuzer, B.; Bulut, S.; Huseynova, G.; Tastan, T.B.; Gulaboglu, M.; Suleyman, H. Effects of adenosine triphosphate and coenzyme Q10 on potential hydroxychloroquine-induced retinal damage in rats. Exp. Eye Res. 2025, 255, 110387. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J.; Balaratnasingam, C.; Morgan, W.H.; Yu, P.K.; Su, E.N. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog. Retin. Eye Res. 2013, 36, 217–246. [Google Scholar] [CrossRef]
- Kang, E.Y.; Liu, P.K.; Wen, Y.T.; Quinn, P.M.J.; Levi, S.R.; Wang, N.K.; Tsai, R.K. Role of oxidative stress in ocular diseases associated with retinal ganglion cells degeneration. Antioxidants 2021, 10, 1948. [Google Scholar] [CrossRef]
- Greiner, J.V.; Glonek, T. Adenosine triphosphate (ATP) and protein aggregation in age-related vision-threatening ocular diseases. Metabolites 2023, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Patrakeeva, V.P. The role of extracellular ATP in the regulation of functional cell activity. Cell Tissue Biol. 2025, 19, 206–213. [Google Scholar] [CrossRef]
- Ocejo, A.; Correa, R. Methylprednisolone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.statpearls.com (accessed on 10 February 2025).
- Agashe, P.; Doshi, A. Linezolid induced optic neuropathy in a child treated for extensively drug resistant tuberculosis: A case report and review of literature. Saudi J. Ophthalmol. 2019, 33, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Early methylprednisolone treatment can stabilize the blood-optic nerve barrier in a rat model of anterior ischemic optic neuropathy (rAION). Investig. Ophthalmol. Vis. Sci. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Arnold, A.C.; Swanson, R.N.; Biswas, P.; Bassetti, M. Safety of long-term use of linezolid: Results of an open-label study. Ther. Clin. Risk Manag. 2016, 12, 1347–1354. [Google Scholar] [CrossRef]
- Kardani, S.; Hadia, R.; Shah, A.; Parmar, G.; Maheshwari, R.; Seth, A.; Rathod, T.; Joshi, D. A case report on reversible toxic optic neuropathy on long-term treatment of linezolid in an extensively drug-resistant tuberculosis patient. J. Pharm. Res. Int. 2021, 33, 7–11. [Google Scholar] [CrossRef]
- Kawashima, H.; Ozawa, Y.; Toda, E.; Homma, K.; Osada, H.; Narimatsu, T.; Nagai, N.; Tsubota, K. Neuroprotective and vision-protective effect of preserving ATP levels by AMPK activator. FASEB J. 2020, 34, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bond, K.; Sarraf, P.; Esterberg, R.; Serpa, M.; Twarog, M.; Xu, Y.; MacLeod, H.; Huang, Q.; Saint-Geniez, M. Protective effect of P2Y receptors antagonism on stress-induced retinal degeneration. bioRxiv 2024. [Google Scholar] [CrossRef]
| Biochemical Variables | ||||||
|---|---|---|---|---|---|---|
| Groups | MDA * (nmol/mg Protein) | tGSH * (nmol/mg Protein) | SOD * (U/mg Protein) | CAT * (U/mg Protein) | TNF-α * (ng/L) | TP-I ** (µg/L) |
| HG | 1.53 ± 0.15 | 4.37 ± 0.15 | 5.69 ± 0.18 | 4.68 ± 0.24 | 2.32 ± 0.23 | 0.046 ± 0.006 |
| ATPG | 1.36 ± 0.11 | 4.51 ± 0.22 | 5.81 ± 0.14 | 4.79 ± 0.14 | 2.31 ± 0.16 | 0.046 ± 0.007 |
| LZDG | 3.57 ± 0.21 | 2.27 ± 0.13 | 3.15 ± 0.15 | 2.52 ± 0.21 | 3.96 ± 0.14 | 0.054 ± 0.010 |
| ATLDG | 1.64 ± 0.13 | 4.15 ± 0.09 | 5.33 ± 0.13 | 4.25 ± 0.11 | 2.44 ± 0.27 | 0.047 ± 0.007 |
| MPLDG | 2.60 ± 0.14 | 3.34 ± 0.19 | 4.28 ± 0.25 | 3.31 ± 0.23 | 2.38 ± 0.10 | 0.054 ± 0.021 |
| Group comparisons | p-values | |||||
| HG vs. ATPG | 0.328 | 0.613 | 0.762 | 0.843 | 1.000 | 1.000 |
| HG vs. LZDG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.494 |
| HG vs. ATLDG | 0.679 | 0.152 | 0.013 | 0.006 | 0.809 | 1.000 |
| HG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 | 0.987 | 0.918 |
| ATPG vs. LZDG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.484 |
| ATPG vs. ATLDG | 0.025 | 0.006 | <0.001 | <0.001 | 0.727 | 0.999 |
| ATPG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 | 0.965 | 0.901 |
| LZDG vs. ATLDG | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | 0.592 |
| LZDG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 |
| ATLDG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 | 0.975 | 0.935 |
| F value | 224.853 | 196.137 | 245.195 | 153.945 | 84.290 | 0.836 a |
| df (df1/df2) | 4/25 | 4/25 | 4/25 | 4/25 | 4/25 | 4/12.216 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.527 |
| Groups | Histopathological Grading Data | |||
|---|---|---|---|---|
| Destruction | Increase in Astrocyte Cell Population | Edema/Vacuolization | Vascular Dilatation/Congestion | |
| HG | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| ATPG | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| LZDG | 3.00 (2.00–3.00) | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) | 2.50 (2.00–3.00) |
| ATLDG | 0.00 (0.00–1.00) | 0.00 (0.00–1.00) | 0.00 (0.00–2.00) | 0.00 (0.00–1.00) |
| MPLDG | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) |
| Group comparisons | p-values | |||
| HG vs. ATPG | 1.000 | 1.000 | 1.000 | 1.000 |
| HG vs. LZDG | <0.001 | <0.001 | <0.001 | <0.001 |
| HG vs. ATLDG | 0.151 | 0.496 | 0.078 | 0.846 |
| HG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 |
| ATPG vs. LZDG | <0.001 | <0.001 | <0.001 | <0.001 |
| ATPG vs. ATLDG | 0.151 | 0.496 | 0.078 | 0.846 |
| ATPG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 |
| LZDG vs. ATLDG | <0.001 | <0.001 | <0.001 | <0.001 |
| LZDG vs. MPLDG | 0.087 | 1.000 | 0.933 | 1.000 |
| ATLDG vs. MPLDG | <0.001 | <0.001 | <0.001 | <0.001 |
| K-W | 156.089 a | 150.219 a | 150.433 a | 157.559 a |
| df | 4 | 4 | 4 | 4 |
| Asymptotic Sig. | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esenulku, C.M.; Cicek, I.; Somuncu, A.M.; Yavuzer, B.; Sezgin, E.T.; Tastan, T.B.; Yücel, N.; Karatas, E.; Suleyman, H. Protective Effect of Exogenous Adenosine Triphosphate Against Ocular Toxicity of Linezolid in Rats. Life 2025, 15, 1587. https://doi.org/10.3390/life15101587
Esenulku CM, Cicek I, Somuncu AM, Yavuzer B, Sezgin ET, Tastan TB, Yücel N, Karatas E, Suleyman H. Protective Effect of Exogenous Adenosine Triphosphate Against Ocular Toxicity of Linezolid in Rats. Life. 2025; 15(10):1587. https://doi.org/10.3390/life15101587
Chicago/Turabian StyleEsenulku, Cenap Mahmut, Ibrahim Cicek, Ahmet Mehmet Somuncu, Bulent Yavuzer, Esra Tuba Sezgin, Tugba Bal Tastan, Nurinisa Yücel, Ezgi Karatas, and Halis Suleyman. 2025. "Protective Effect of Exogenous Adenosine Triphosphate Against Ocular Toxicity of Linezolid in Rats" Life 15, no. 10: 1587. https://doi.org/10.3390/life15101587
APA StyleEsenulku, C. M., Cicek, I., Somuncu, A. M., Yavuzer, B., Sezgin, E. T., Tastan, T. B., Yücel, N., Karatas, E., & Suleyman, H. (2025). Protective Effect of Exogenous Adenosine Triphosphate Against Ocular Toxicity of Linezolid in Rats. Life, 15(10), 1587. https://doi.org/10.3390/life15101587