Phototrophs in Unique Habitats of Thermomineral Springs in Central Serbia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Analysis of Ecological and Water Parameters
2.3. Sampling Sites and Sampling Procedure
2.4. Analysis of Phototrophic Microorganisms
2.5. Analysis of Chlorophyll a/Primary Production
2.6. Cultivation of Cyanobacteria
2.7. Molecular Analyses
2.8. Statistical Analysis
3. Results
3.1. Physical and Chemical Parameters of Thermomineral Springs
3.2. Sampling Sites Characteristics
3.3. Results on Analysis of Phototrophic Microorganisms
3.4. Chlorophyll a
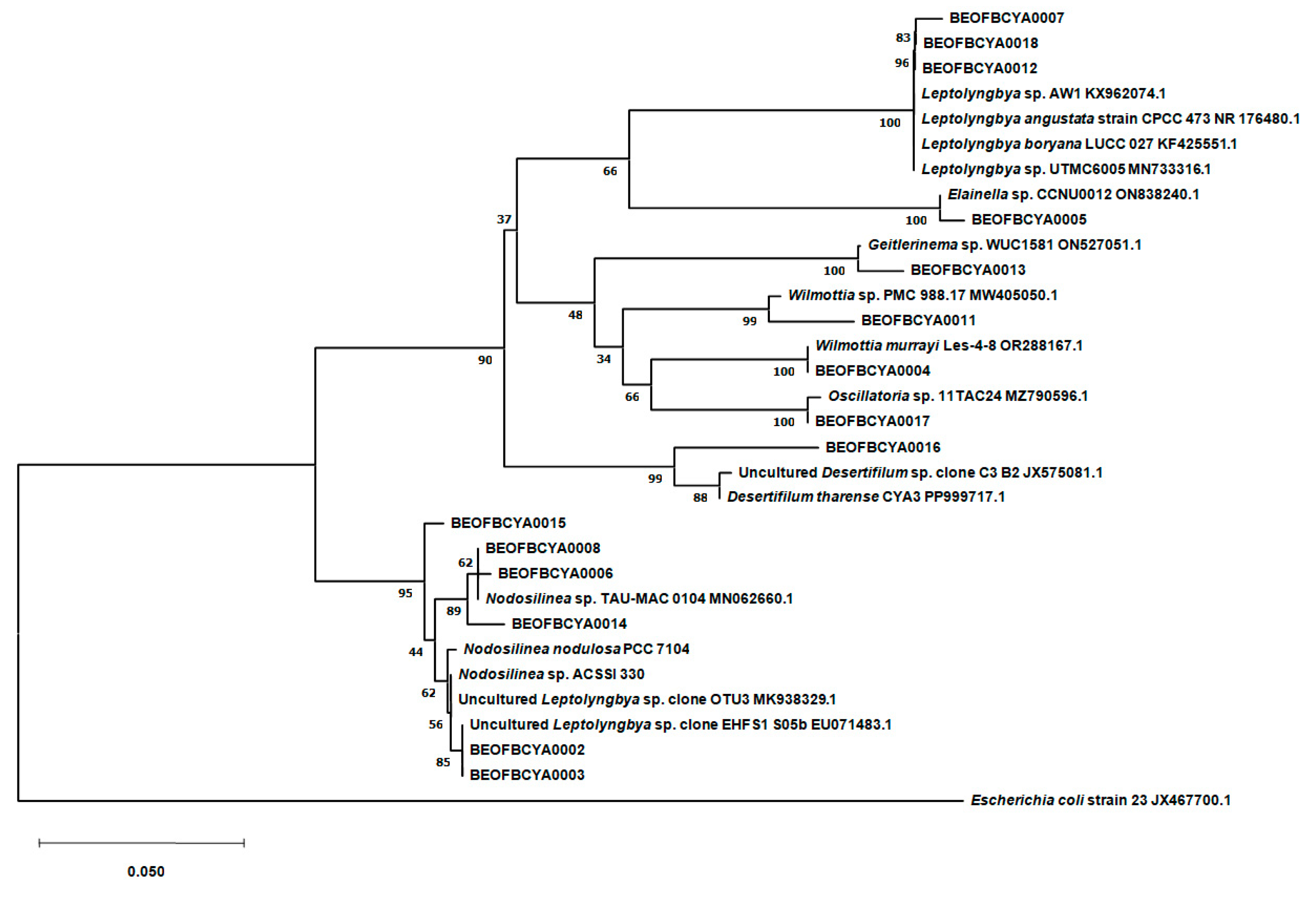
3.5. Molecular Analysis of Cultured Cyanobacteria
3.6. Statistical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Z. Thermal Springs and Geothermal Energy in the Qinghai-Tibetan Plateau and the Surroundings; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Reiss, M.; Chifflard, P. Hydromorphology and biodiversity in headwaters—An eco-faunistic substrate preference assessment in forest springs of the German Subdued Mountains. Biodivers. Ecosyst. Link. Struct. Funct. 2015, 205, 205–240. [Google Scholar] [CrossRef]
- Gosseaume, P.; Beauger, A.; Voldoire, O.; Allain, E.; Wetzel, C.E.; Jamoneau, A. Diatom metacommunity processes in thermo-mineral springs in the Auvergne Region, France. Hydrobiologia 2024, 851, 3855–3868. [Google Scholar] [CrossRef]
- Dinka, M.O.; Loiskandl, W.; Ndambuki, J.M. Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J. Hydrol. Reg. Stud. 2015, 3, 444–456. [Google Scholar] [CrossRef]
- Cantonati, M.; Gerecke, R.; Bertuzzi, E. Springs of the Alps–sensitive ecosystems to environmental change: From biodiversity assessments to long-term studies. Hydrobiologia 2006, 562, 59–96. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, D.D.; Bansal, D.D.; Mishra, S.; Mohammed, A.; Arora, R.; Sharma, A.; Sharma, R.K.; Tripathi, R.P. Extremophiles: Sustainable resource of natural compounds—Extremolytes. In Sustainable Biotechnology; Singh, O.V., Harvey, S.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 279–294. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Wojciechowski, M.F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 2009, 4, e7801. [Google Scholar] [CrossRef] [PubMed]
- Warren-Rhodes, K.A.; Rhodes, K.L.; Boyle, L.N.; Pointing, S.B.; Chen, Y.; Liu, S.; McKay, C.P. Cyanobacterial ecology across environmental gradients and spatial scales in China’s hot and cold deserts. FEMS Microbiol. Ecol. 2007, 61, 470–482. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Gómez-Ortiz, D.; Malmberg, P.; Huang, T.; Shen, Y.; Anglés, A.; Amils, R. Preservation of Underground Microbial Diversity in Ancient Subsurface Deposits (>6 Ma) of the Rio Tinto Basement. Microorganisms 2021, 9, 1592. [Google Scholar] [CrossRef]
- Oren, A. Thermodynamic limits to microbial life at high salt concentrations. Environ. Microbiol. 2011, 13, 1908–1923. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. Introduction to the cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Plescia, J.B.; Johnson, J.R.; Ferris, M. Visible-near infrared spectroscopy of hyperthermophile organisms, Yellowstone National Park. In Proceedings of the General Meeting of the NASA Astrobiology Institute (2001); Carnegie Institution of Washington: Washington, DC, USA, 2001; pp. 129–130. [Google Scholar]
- Izagiurre, I.; Allende, L.; Tell, G. Algal communities of a geothermally heated lagoon on Deception Island (South Shetlands Islands). Polar Biol. 2006, 29, 364–371. [Google Scholar] [CrossRef]
- Hindak, F. On Chlorogloeopsis fritschii (Cyanophyta/Cyanobacteria) from thermal springs in Slovakia and from saline lake in Tunisia. Algol. Stud. 2008, 126, 47–64. [Google Scholar] [CrossRef]
- Abdelwahab, H.E.; Amin, A.S. Diatoms Diversity of Thermal Springs in the Southwest Region, Saudi Arabia. Egypt. Acad. J. Biol. Sci. H Bot. 2017, 8, 59–74. [Google Scholar] [CrossRef]
- Serôdio, J.; Lavaud, J. Diatoms and their ecological importance. In Life Below Water; Springer International Publishing: Cham, Switzerland, 2022; pp. 304–312. [Google Scholar]
- Quintela, A.; Almeida, S.; Terroso, D.; Ferreira da Silva, E.; Forjaz, V.; Rocha, F. Diatom assemblages of thermal and mineral waters from volcanic environments in São Miguel Island, Azores. Diatom Res. 2013, 28, 407–417. [Google Scholar] [CrossRef]
- Souffreau, C.; Vanormelingen, P.; Verleyen, E.; Sabbe, K.; Vyverman, W. Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 2010, 49, 309–324. [Google Scholar] [CrossRef]
- Round, F.E. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; Volume 747. [Google Scholar]
- Stoyneva, M.P. Survey on green algae of Bulgarian thermal springs. Biol. Bratisl. 2003, 58, 563–574. [Google Scholar]
- Kochhar, N.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Blaženčić, J.; Cvijan, M. Alge u mineralnim vodama Ribarske, Brestovačke i Jošaničke banje. Biosistematika 1980, 6, 117–134. [Google Scholar]
- Cvijan, M. Algae in thermomineral waters of Niška banja spa (Serbia). Glas. Instituta Za Bot. I Bot. Bašte Univ. U Beogr. 1994, 26–27, 87–96. [Google Scholar]
- Šaraba, V.; Popović, S.; Krunić, O.; Subakov Simić, G.; Kljajić, Ţ.; Lazić, M. Mineral waters of Serbia and development of phototrophic microbial communities near points of emergence and on wellheads. Acta Carsologica 2017, 46, 295–316. [Google Scholar] [CrossRef]
- Šaraba, V.; Popović, S.; Obradović, V.; Štrbački, J.; Gajić, V.; Vulić, P.; Subakov Simić, G.; Krunić, O. Macroscopic, optical and diffraction assessment of encrustations and SEM analyses of phototrophic microbial mats from wellheads and select zones of emergence of mineral water in Serbia. Geol. Croat. 2019, 72, 145–162. [Google Scholar] [CrossRef]
- Šovran, S.; Jakovljević, O.; Milićević, A.; Knežević, A.; Krizmanić, J. Diversity of phototrophic microbial organisms in Gornja Trepča Spa, Serbia. In Proceedings of the 3rd International Conference “Conference on Advances in Science and Technology” COAST 2024, Herceg Novi, Montenegro, 29 May–1 June 2024; Book of Abstracts. p. 56. [Google Scholar]
- Risler, J.J. Description et classification géologique des sources minérales et thermales du Massif Central. BRGM Rep. 1974, 74, 24. [Google Scholar]
- Popović, S.; Subakov Simić, G.; Stupar, M.; Unković, N.; Krunić, O.; Savić, N.; Ljaljević Grbić, M. Cave biofilms: Characterization of phototrophic cyanobacteria and algae and chemotrophic fungi from three caves in Serbia. J. Cave Karst Stud. 2017, 79, 10–23. [Google Scholar] [CrossRef]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G.M. A Methods Manual for the Collection, Preparation and Analysis of Diatom Samples, Version 1.0; WRC Report TT 281/07; Water Research Commission: Pretoria, South Africa, 2007. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil/1st Part: Chroococcales. In Süsswasserflora von Mitteleuropa, 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm: Ulm, Germany, 1998; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales; Elsevier GmbH: Munich, Germany, 2005; p. 759. [Google Scholar]
- Komárek, J. Cyanoprokaryota: 3rd Part: Heterocystous Genera; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Süßwasserflora von Mitteleuropa Bd. 19 (3); Springer Spektrum: Berlin/Heidelberg, Germany, 2013; pp. 1–1130. [Google Scholar]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017. [Google Scholar]
- Karthick, B.; Taylor, J.C.; Mahesh, M.K.; Ramachandra, T.V. Protocols for collection, preservation and enumeration of diatoms from aquatic habitats for water quality monitoring in India. Soil Water Sci. 2010, 3, 25. [Google Scholar]
- Popović, S.; Subakov Simić, G.; Stupar, M.; Unković, N.; Predojević, D.; Jovanović, J.; Ljaljević Grbić, M. Cyanobacteria, algae, and microfungi present in biofilm from Božana Cave (Serbia). Int. J. Speleol. 2015, 44, 141–149. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Nikolić, N.; Subakov Simić, G.; Golić, I.; Popović, S. The effects of biocides on the growth of aerophytic green algae (Chlorella sp.) isolated from a cave environment. Arch. Biol. Sci. 2021, 73, 341–351. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Kaštovský, J.; Komárek, J. Phototrophic microvegetation of thermal springs in Karlovy Vary, Czech Republic. In Algae and Extreme Environments; Nova Hedwigia Beih, 2001; Volume 123, pp. 107–119. [Google Scholar]
- Öztürk, S.; Kurt, O. The taxonomy and distribution of algae in the thermal springs of Türkiye. Acta Bot. Croat. 2024, 83, 92–99. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Tang, J. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 2007, 33, 183–209. [Google Scholar] [CrossRef]
- López-Rodríguez, M.C.; Asencio, A.D.; Meijide, R.M.; Torres, E. Extremophilic cyanobacteria from thermo-mineral springs of spas in Atlantic environments. Syst. Biodivers. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Cantonati, M.; Bilous, O.; Spitale, D.; Angeli, N.; Segadelli, S.; Bernabè, D.; Lichtenwöhrer, K.; Gerecke, R.; Saber, A.A. Diatoms from the spring ecosystems selected for the long-term monitoring of climate-change effects in the Berchtesgaden National Park (Germany). Water 2022, 14, 381. [Google Scholar] [CrossRef]
- Leira, M.; Meijide-Failde, R.; Torres, E. Diatom communities in thermo-mineral springs of Galicia (NW Spain). Diatom Res. 2017, 32, 29–42. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Iliopoulos, I.; Kokkaliari, M.; Moforis, L.; Economou-Amilli, A. Microbial Mat Stratification in Travertine Depositions of Greek Hot Springs and Biomineralization Processes. Minerals 2022, 12, 1408. [Google Scholar] [CrossRef]
- Ghahraman, Z.; Riahi, H.; Shariatmadari, Z.; Heidari, F. Diversity of thermophilic cyanobacteria in Maragheh Mineral Springs and variable environmental factors. J. Phycol. Res. 2020, 4, 572–581. [Google Scholar]
- Albertano, P.; Bruno, L.; D’Ottavi, D.; Moscone, D.; Palleschi, G. Effect of photosynthesis on pH variation in cyanobacterial biofilms from Roman catacombs. J. Appl. Phycol. 2000, 12, 279–384. [Google Scholar] [CrossRef]
- Thapa, S.; Bharti, A.; Prasanna, R. Algal biofilms and their biotechnological significance. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–303. [Google Scholar]
- Cantonati, M.; Komárek, J.; Montejano, G. Cyanobacteria in ambient springs. Biodivers. Conserv. 2015, 24, 865–888. [Google Scholar] [CrossRef]
- Melo, X.F.; de la Rosa, I.N.; Baffico, G.; Temporetti, P.; Wenzel, M.T.; Cabrera, J.; Diaz, M. Limnological characterization of the water, algae and mud resources used in Copahue Thermal Complex (Neuquén, Argentina). Austral Ecol. 2021, 31, 400–412. [Google Scholar]
- Ward, D.M.; Castenholz, R.W.; Miller, S.R. Cyanobacteria in geothermal habitats. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 39–63. [Google Scholar]
- Stoyneva-Gärtner, M.; Androv, M.; Uzunov, B.; Ivanov, K.; Gärtner, G. Algal Biodiversity of Nine Megaliths in South-East Bulgaria. Life 2024, 14, 948. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Bolatkhan, K.; Sidorov, R.A.; Mironov, K.S.; Skrypnik, A.N.; Kupriyanova, E.V.; Los, D.A. Polyphasic characterization of the thermotolerant cyanobacterium Desertifilum sp. strain IPPAS B-1220. FEMS Microbiol. Lett. 2017, 364, fnx027. [Google Scholar] [CrossRef]
- Strunecký, O.; Elster, J.; Komárek, J. Taxonomic revision of the freshwater cyanobacterium “Phormidium murrayi” = Wilmottia murrayi. Fottea 2011, 11, 57–71. [Google Scholar] [CrossRef]
- Stal, L.J.; Caumette, P. (Eds.) Microbial Mats: Structure, Development and Environmental Significance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 35. [Google Scholar]
- Jahodářová, E.; Dvořák, P.; Hašler, P.; Holušová, K.; Poulíčková, A. Elainella gen. nov.: A new tropical cyanobacterium characterized using a complex genomic approach. Eur. J. Phycol. 2018, 53, 39–51. [Google Scholar] [CrossRef]
- Amarouche-Yala, S.; Benouadah, A.; El Ouahab Bentabet, A.; López-García, P. Morphological and phylogenetic diversity of thermophilic cyanobacteria in Algerian hot springs. Extremophiles 2014, 18, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, A.; Garcia-Pichel, F.; Nübel, U.; Vázquez-Juárez, R. Cyanobacterial diversity in extreme environments in Baja California, Mexico: A polyphasic study. Int. Microbiol. 2001, 4, 227–236. [Google Scholar] [CrossRef]
- Kaštovský, J.; Johansen, J.R.; Hauerová, R.; Akagha, M.U. Hot is rich—An enormous diversity of simple trichal cyanobacteria from Yellowstone hot springs. Diversity 2023, 15, 975. [Google Scholar] [CrossRef]
- Perkerson, R.B., III; Johansen, J.R.; Kovacik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique pseudanabaenalean (cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
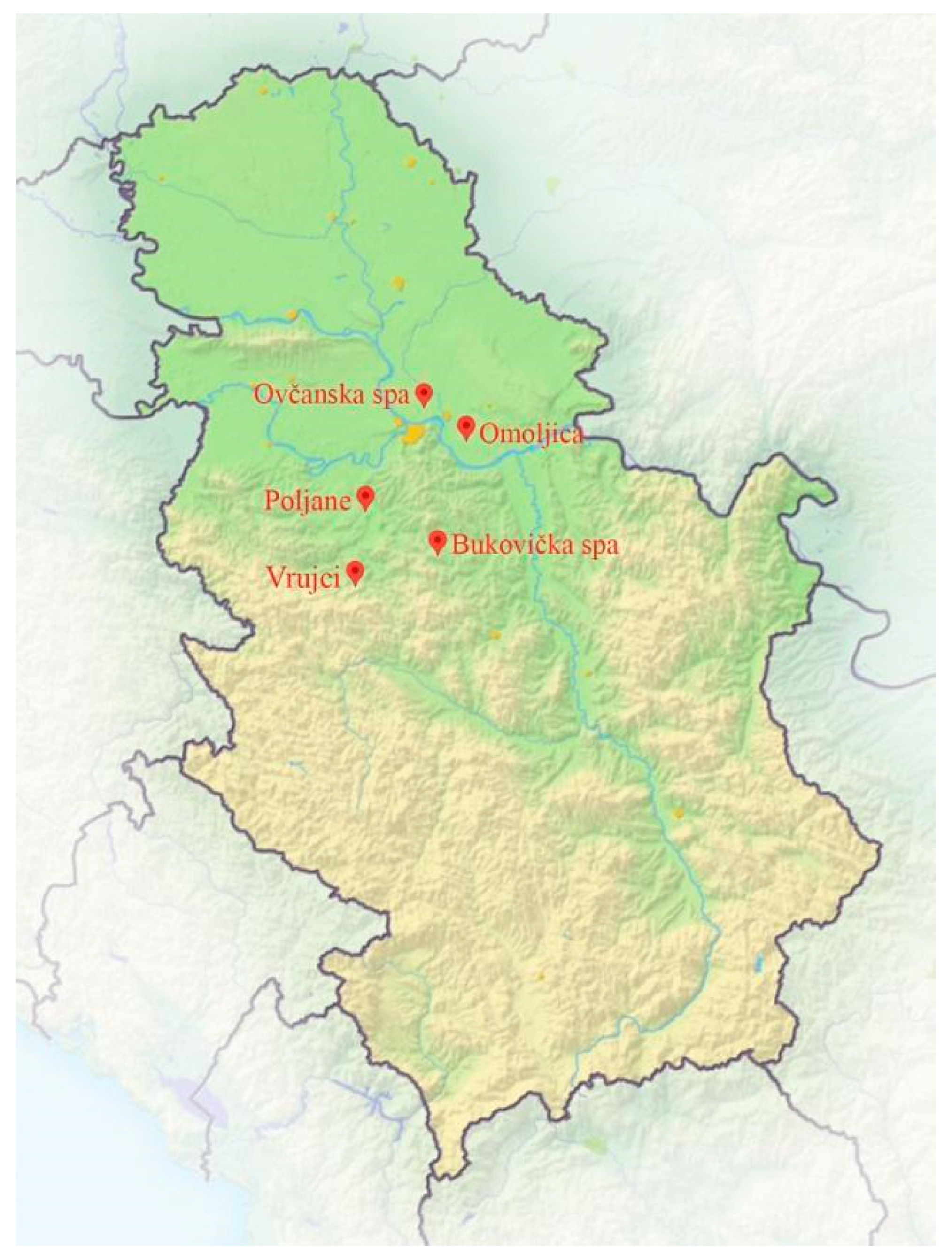




| Locality | Site | Coordinates | Source |
|---|---|---|---|
| Bukovička Spa | Bu T1 | 44.3086948 N, 20.5525070 E | Topla česma tap |
| Bu T2 | |||
| Bu T3 | |||
| Bu T4 | 44.3079347 N, 20.5510747 E | Knjaz Mihailo tap | |
| Bu M | Mud | ||
| Omoljica | Om T1 | 44.3079347 N, 20.5510747 E | Natural pool |
| Om T2 | Natural pool | ||
| Om S | Sediment | ||
| Ovčanska Spa | Ov T1 | 44.76168396 N, 20.75283817 E | Natural pool |
| Ov S | Sediment | ||
| Vrujci | Vr S | 44.2204950 N, 20.1521023 E | Sediment |
| Vr T1 | Natural pool | ||
| Vr T2 | 44.2203266 N, 20.1524234 E | Vrujci tap | |
| Vr T3 | Vrujci tap | ||
| Vr M | Mud | ||
| Poljane | Po T1 | 44.52263555 N, 20.2366812 E | Pipe where water barely drips |
| Parameters | Bukovička Spa | Omoljica | Ovčanska Spa | Vrujci | Poljane | |
|---|---|---|---|---|---|---|
| Bukovička Spa–Topli Izvor | Bukovička Spa–Knjaz Mihailo | |||||
| Water Temperature (°C) | 17.5 | 8.5 | 22 | 20.3 | 25.1 | 17.4 |
| Turbidity (NTU) | 9.46 | 0.87 | 0.82 | 4.24 | 0.04 | 0.02 |
| pH Value | 6.86 | 7.63 | 8.1 | 7.59 | 7.45 | 8.19 |
| TDS (ppt) | 3.2 | 0.1 | 1.2 | 17.6 | 0.382 | 0.9 |
| EC (ms) | 4.6 | 0.2 | 1.8 | / | 0.5 | 1.3 |
| O2 (mg/L) | 2.9 | 10.94 | 2.46 | 9.38 | 6.6 | 2.1 |
| O2 (% saturation) | 16.6 | 92.5 | 27.5 | 91 | 62.2 | 17.6 |
| Conductivity at 20 °C (µS/cm) | 4220 | 233 | 1744 | 2550 | 461 | 1187 |
| Ammonia, NH4-N (mg/L) | 0.05 | 0.06 | 2.52 | 19 | <0.05 | 7.89 |
| Nitrites, NO2-N (mg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Nitrates, NO3-N (mg/L) | <0.5 | 0.9 | <0.5 | 20 | 2.01 | <0.5 |
| Chlorides, Cl− (mg/L) | 27.6 | 7.9 | 31.7 | 995 | 3.61 | 25.44 |
| Sulfates, SO42− (mg/L) | 1.2 | 42.2 | 9.8 | <0.5 | 10 | 3.25 |
| Fluorides, F (mg/L) | 5 | 0.1 | 1.3 | <0.05 | 0.75 | 3.19 |
| Bromides, Br (mg/L) | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Silicates, SiO2 (mg/L) | 81.59 | 7.67 | 17.97 | 28 | 13.179 | 22.355 |
| Orthophosphates, PO4-P (mg/L) | 0.019 | <0.01 | 0.087 | 0.011 | 0.054 | 0.043 |
| Total Phosphorus (mg/L) | 0.044 | 0.008 | 0.114 | 0.051 | 0.154 | 0.085 |
| Calcium, Ca2+ (mg/L) | 86.1 | 36.8 | 4 | 44 | 75.3 | 6 |
| Magnesium, Mg2+ (mg/L) | 16.3 | 7.8 | 10.7 | 86.5 | 17.5 | 6.8 |
| Total Hardness (dH) | 15.7 | 6.9 | 3 | 26 | 15 | 2.4 |
| Alkalinity (n/10HCl) | 601 | 22 | 197 | 104 | 55 | 143 |
| Bicarbonates (mg/L) | 3665 | 134 | 1202 | 634 | 336 | 872 |
| Total Organic Carbon (TOC) (mg/L) | 2.94 | 1.41 | 5.64 | 1.61 | 2.33 | 3.47 |
| Total Nitrogen (TN) (mg/L) | <0.5 | <0.5 | 2 | 19 | 0.8 | 3.36 |
| Identified Phototrophicmicroorganisms | Bukovička Spa | Omoljica | Ovčanska Spa | Vrujci | Poljane |
|---|---|---|---|---|---|
| Cyanobacteria | |||||
| Anagnostidinema amphibium (Gomont) Strunecký, Bohunická, J.R.Johansen and Komárek | + | ||||
| Aphanocapsa fusco-lutea Hansgirg | + | + | + | ||
| Aphanocapsa Nägeli sp. | + | ||||
| Aphanothece saxicola Nägeli | + | ||||
| Arthrospira Stizenberger ex Gomont sp. | + | ||||
| Chroococcidiopsis Geitler sp. | + | ||||
| Chroococcus thermalis (Meneghini) Nägeli | + | ||||
| Chroococcus varius A.Braun | + | + | |||
| Cyanosarcina Kovácik sp. | + | ||||
| Desertifilum tharense * Dadheech and Krienitz | + | ||||
| Elainella Jahodárová, Dvorák and Hasler sp. * | + | ||||
| Geitlerinema (Anagnostidis and Komárek) Anagnostidis sp. * | + | ||||
| Gloeocapsa atrata Kützing | + | + | |||
| Gloeocapsa fusco-lutea Nägeli ex Kützing/Ercegović | + | ||||
| Gloeocapsa punctata Nägeli | + | + | |||
| Gloeocapsa violacea Kützing | + | + | |||
| Jaaginema geminatum (Schwabe ex Gomont) Anagnostidis and Komárek | + | ||||
| Kamptonema jasorvense (Vouk) Strunecký, Komárek and J.Smarda | + | ||||
| Leptolyngbya boryana * (Gomont) Anagnostidis and Komárek | + | + | + | ||
| Leptolyngbya foveolarum (Gomont) Anagnostidis and Komárek | + | + | + | ||
| Leptolyngbya perforans (Geitler) Anagnostidis and Komárek | + | ||||
| Leptolyngbya Anagnostidis and Komárek sp. * | + | + | |||
| Leptolyngbya thermalis Anagnostidis | + | ||||
| Nodosilinea R.B.Perkerson and D.A.Casamatta sp. * | + | + | + | ||
| Oscillatoria cf. nitida Vaucher ex Gomont | + | ||||
| Oscillatoria Vaucher ex Gomont sp. * | + | ||||
| Oscillatoria subbrevis Schmidle | + | ||||
| Phormidium cf. terebriforme Kützing ex Gomont | + | ||||
| Phormidium corium Gomont | + | ||||
| Phormidium kuetzingianum (Kirchner ex Hansgirg) Anagnostidis and Komárek | + | ||||
| Phormidium laetevirens (P.Crouan and H.Crouan ex Gomont) Anagnostidis and Komárek | + | + | |||
| Phormidium Kützing ex Gomont sp.1 | + | ||||
| Phormidium Kützing ex Gomont sp.2 | + | ||||
| Phormidium Kützing ex Gomont sp.3 | + | ||||
| Phormidium Kützing ex Gomont sp.4 | + | ||||
| Phormidium Kützing ex Gomont sp.5 | + | ||||
| Phormidium tergestinum (Rabenhorst ex Gomont) Anagnostidis and Komárek | + | ||||
| Porphyrosiphon notarisii Kützing ex Gomont | + | ||||
| Pseudanabaena minima (G.S.An) Anagnostidis | + | ||||
| Pseudanabaena thermalis Anagnostidis | + | ||||
| Synechococcus bigranulatus Skuja | + | + | |||
| Synechococcus elongatus (Nägeli) Nägeli | + | ||||
| Synechococcus Nägeli sp. | + | + | |||
| Synechocystis Sauvageau sp. | + | ||||
| Wilmottia murrayi * (West & G.S.West) Strunecký, Elster, and Komárek | + | ||||
| Wilmottia O.Strunecky, J.Elster, and J.Komárek sp. * | + | ||||
| Wolskyella cf. floridana Claus | + | + | |||
| Bacillariophyta | |||||
| Achnanthes coarctata (Brébisson ex W.Smith) Grunow | + | ||||
| Achnanthidium exiguum (Grunow) Czarnecki | + | + | + | + | + |
| Achnanthidium gracillimum (F.Meister) Lange-Bertalot | + | ||||
| Achnanthidium microcephalum Kützing | + | + | + | ||
| Achnanthidium straubianum (Lange-Bertalot) Lange-Bertalot | + | ||||
| Adlafia bryophila (J.B.Petersen) Lange-Bertalot | + | ||||
| Adlafia muralis (Grunow) Monnier and Ector | + | ||||
| Amphora pediculus (Kützing) Grunow | + | + | |||
| Anomoeoneis cf. capitata E.Pfitzer | + | ||||
| Anomoeoneis sphaerophora Pfitzer | + | ||||
| Brachysira vitrea (Grunow) R.Ross | + | ||||
| Caloneis fontinalis (Grunow) A.Cleve | + | ||||
| Caloneis silicula (Ehrenberg) Cleve | + | ||||
| Caloneis Cleve sp. | + | ||||
| Cocconeis euglypta Ehrenberg | + | ||||
| Cocconeis euglyptoides (Geitler) Lange-Bertalot | + | ||||
| Craticula ambigua (Ehrenberg) D.G.Mann | + | + | |||
| Craticula Grunow sp. | + | ||||
| Cymbella affinis Kützing | + | ||||
| Denticula kuetzingii Grunow | + | ||||
| Encyonopsis minuta Krammer and E.Reichardt | + | ||||
| Gomphonema acidoclinatum Lange-Bertalot and E.Reichardt | + | ||||
| Gomphonema cymbelliclinum E.Reichardt and Lange-Bertalot | + | ||||
| Gomphonema parvulum (Kützing) Kützing | + | + | + | + | + |
| Gomphonema pumilum var rigidum E.Reichardt and Lange-Bertalot | + | ||||
| Gomphonema Ehrenberg sp.1 | + | ||||
| Gomphonema Ehrenberg sp.2 | + | ||||
| Grunowia solgensis (A.Cleve) Aboal | + | ||||
| Halamphora coffeiformis (C.Agardh) Mereschkowsky | + | ||||
| Halamphora (Cleve) Mereschkowsky sp. | + | ||||
| Hantzschia abundans Lange-Bertalot | + | ||||
| Hantzschia amphyoxis (Ehrenberg) Grunow | + | ||||
| Hantzschia calcifuga E.Reichardt and Lange-Bertalot | + | ||||
| Hantzschia Grunow sp. | + | ||||
| Humidophila contenta (Grunow) Lowe, Kociolek, Johansen, Van de Vijver, Lange-Bertalot and Kopalová | + | ||||
| Humidophila perpusilla (Grunow) R.L.Lowe, Kociolek, J.R.Johansen, Van de Vijver, Lange-Bertalot and Kopalová | + | ||||
| Lemnicola hungarica (Grunow) Round and Basson | + | ||||
| Luticola mutica (Kützing) D.G.Mann | + | ||||
| Melosira varians C.Agardh | + | + | |||
| Navicula cincta Pantocsek | + | + | + | ||
| Navicula cryptocephala Kützing | + | ||||
| Navicula cryptotenella Lange-Bertalot | + | ||||
| Navicula reichardtiana Lange-Bertalot | + | ||||
| Navicula rostellata Kützing | + | ||||
| Navicula salinarum Grunow | + | ||||
| Navicula Bory sp.1 | + | + | + | + | |
| Navicula Bory sp.2 | + | ||||
| Navicula vandamii Schoeman and R.E.M.Archibald | + | ||||
| Neidium dubium (Ehenberg) Cleve | + | ||||
| Nitzschia acicularis (Kützing) W.Smith | + | ||||
| Nitzschia amphibia Grunow | + | ||||
| Nitzschia communis Rabenhorst | + | + | |||
| Nitzschia dissipata (Kützing) Rabenhorst | + | + | |||
| Nitzschia fonticola (Grunow) Grunow | + | ||||
| Nitzschia inconspicua Grunow | + | ||||
| Nitzschia liebetruthii Rabenhorst | + | + | |||
| Nitzschia linearis W.Smith | + | ||||
| Nitzschia palea (Kützing) W.Smith | + | + | + | + | + |
| Nitzschia Hassall sp.1 | + | ||||
| Nitzschia Hassall sp.2 | + | ||||
| Nitzschia thermaloides Hustedt | + | + | + | ||
| Nitzschia umbonata (Ehrenberg) Lange-Bertalot | + | ||||
| Pinnularia Ehrenberg sp. | + | ||||
| Placoneis placentula Heinzerling | + | ||||
| Planothidium dubium (Grunow) Round and Bukhtiyarova | + | ||||
| Planothidium frequentissimum (Lange-Bertalot) Lange-Bertalot | + | + | |||
| Planothidium reichardtii Lange-Bertalot and Werum | + | ||||
| Platessa Lange-Bertalot sp. | + | ||||
| Pseudostaurosira brevistriata (Grunow) D.M.Williams and Round | + | ||||
| Reimeria uniseriata Sala, Guerrero and Ferrario | + | ||||
| Sellaphora bacillum (Ehrenberg) D.G.Mann | + | ||||
| Sellaphora complex pupula Mereschowsky | + | ||||
| Sellaphora laevissima (Kützing) D.G.Mann | + | ||||
| Sellaphora Mereschowsky sp. | + | + | |||
| Sellaphora stroemii (Hustedt) H.Kobayasi | + | ||||
| Sellaphora ventraloides (Hustedt) Falasco and Ector | + | ||||
| Stephanocyclus meneghiniana (Kützing) Kulikovskiy, Genkal and Kociolek | + | ||||
| Staurosira Ehrenberg sp. | + | ||||
| Surirella ovalis Brébisson | + | + | |||
| Surirella splendida (Ehrenberg) Ehrenberg | + | ||||
| Ulnaria ulna (Nitzsch) Compère | + | ||||
| Chlorophyta | |||||
| Desmococcus olivaceus (Persoon ex Acharius) J.R.Laundon | + | ||||
| Scenedesmus Meyen sp. | + | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milićević, A.; Popović, S.; Milovanović, V.; Karadžić, V.; Savković, Ž.; Bjelica, V.; Krizmanić, J.; Subakov-Simić, G.; Jakovljević, O. Phototrophs in Unique Habitats of Thermomineral Springs in Central Serbia. Life 2025, 15, 169. https://doi.org/10.3390/life15020169
Milićević A, Popović S, Milovanović V, Karadžić V, Savković Ž, Bjelica V, Krizmanić J, Subakov-Simić G, Jakovljević O. Phototrophs in Unique Habitats of Thermomineral Springs in Central Serbia. Life. 2025; 15(2):169. https://doi.org/10.3390/life15020169
Chicago/Turabian StyleMilićević, Ana, Slađana Popović, Vanja Milovanović, Vesna Karadžić, Željko Savković, Vukašin Bjelica, Jelena Krizmanić, Gordana Subakov-Simić, and Olga Jakovljević. 2025. "Phototrophs in Unique Habitats of Thermomineral Springs in Central Serbia" Life 15, no. 2: 169. https://doi.org/10.3390/life15020169
APA StyleMilićević, A., Popović, S., Milovanović, V., Karadžić, V., Savković, Ž., Bjelica, V., Krizmanić, J., Subakov-Simić, G., & Jakovljević, O. (2025). Phototrophs in Unique Habitats of Thermomineral Springs in Central Serbia. Life, 15(2), 169. https://doi.org/10.3390/life15020169






