Candidate Genetic Modifiers in Alport Syndrome: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Design
2.3. Clinical Data
2.4. Genetic Testing
3. Results
3.1. Clinical Onset
3.2. Pathological Characteristics
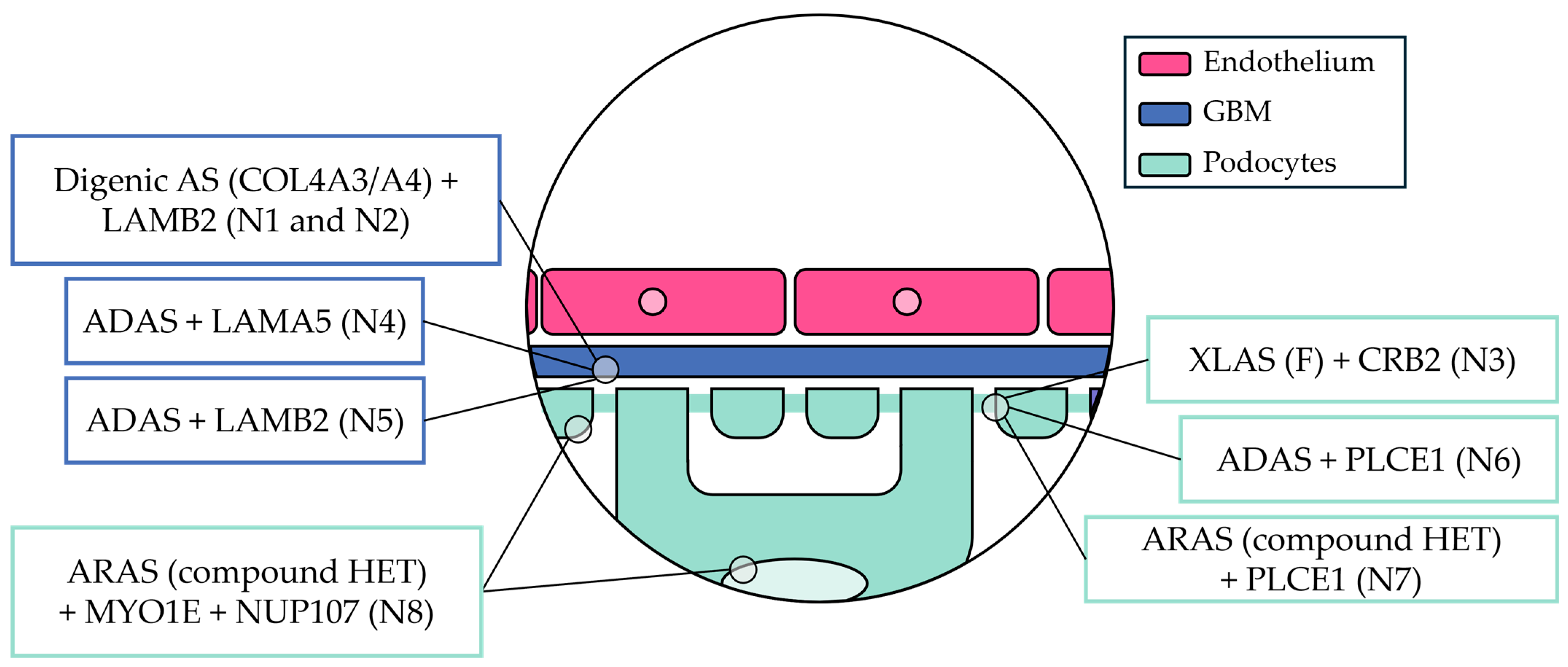
3.3. Genetic Characteristics
3.4. Treatment Record
3.5. Follow-Up
4. Discussion
4.1. Non-Collagenous Extracellular Matrix Proteins
4.1.1. Laminin Subunit β2—LAMB2
4.1.2. Laminin Subunit α5—LAMA5
4.2. Slit Diaphragm Proteins
4.2.1. Crumbs Cell Polarity Complex Component 2—CRB2
4.2.2. Phospholipase C ε1—PLCE1
4.3. Podocyte Cytoskeletal Proteins
4.4. Podocyte Nuclear Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Gregorio, V.; Caparali, E.B.; Shojaei, A.; Ricardo, S.; Barua, M. Alport Syndrome: Clinical Spectrum and Therapeutic Advances. Kidney Med. 2023, 5, 100631. [Google Scholar] [CrossRef] [PubMed]
- Savige, J.; Gregory, M.; Gross, O.; Kashtan, C.; Ding, J.; Flinter, F. Expert Guidelines for the Management of Alport Syndrome and Thin Basement Membrane Nephropathy. J. Am. Soc. Nephrol. 2013, 24, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Savige, J.; Lipska-Zietkiewicz, B.S.; Watson, E.; Hertz, J.M.; Deltas, C.; Mari, F.; Hilbert, P.; Plevova, P.; Byers, P.; Cerkauskaite, A.; et al. Guidelines for Genetic Testing and Management of Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2022, 17, 143–154. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 301050 (Last Edited on 10 October 2023). Available online: https://www.omim.org/entry/301050?search=301050&highlight=301050 (accessed on 25 January 2025).
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 620536 (Last Edited on 6 October 2023). Available online: https://www.omim.org/entry/620536?search=620536&highlight=620536 (accessed on 25 January 2025).
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 203780 (Last Edited on 6 October 2023). Available online: https://www.omim.org/entry/203780?search=203780&highlight=203780 (accessed on 25 January 2025).
- Oka, M.; Nozu, K.; Kaito, H.; Fu, X.J.; Nakanishi, K.; Hashimura, Y.; Morisada, N.; Yan, K.; Matsuo, M.; Yoshikawa, N.; et al. Natural History of Genetically Proven Autosomal Recessive Alport Syndrome. Pediatr. Nephrol. 2014, 29, 1535–1544. [Google Scholar] [CrossRef]
- Savige, J.; Storey, H.; Cheong, H.I.; Kang, H.G.; Park, E.; Hilbert, P.; Persikov, A.; Torres-Fernandez, C.; Ars, E.; Torra, R.; et al. X-Linked and Autosomal Recessive Alport Syndrome: Pathogenic Variant Features and Further Genotype-Phenotype Correlations. PLoS ONE 2016, 11, e0161802. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 104200 (Last Edited on 6 October 2023). Available online: https://www.omim.org/entry/104200?search=104200&highlight=104200 (accessed on 25 January 2025).
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 141200 (Last Edited on 28 April 2023). Available online: https://www.omim.org/entry/141200?search=141200&highlight=141200 (accessed on 25 January 2025).
- Pescucci, C.; Mari, F.; Longo, I.; Vogiatzi, P.; Caselli, R.; Scala, E.; Abaterusso, C.; Gusmano, R.; Seri, M.; Miglietti, N.; et al. Autosomal-Dominant Alport Syndrome: Natural History of a Disease Due to COL4A3 or COL4A4 Gene. Kidney Int. 2004, 65, 1598–1603. [Google Scholar] [CrossRef]
- Jais, J.P.; Knebelmann, B.; Giatras, I.; De Marchi, M.; Rizzoni, G.; Renieri, A.; Weber, M.; Gross, O.; Netzer, K.O.; Flinter, F.; et al. X-Linked Alport Syndrome: Natural History and Genotype-Phenotype Correlations in Girls and Women Belonging to 195 Families: A “European Community Alport Syndrome Concerted Action” Study. J. Am. Soc. Nephrol. 2003, 14, 2603–2610. [Google Scholar] [CrossRef]
- Kamiyoshi, N.; Nozu, K.; Fu, X.J.; Morisada, N.; Nozu, Y.; Ye, M.J.; Imafuku, A.; Miura, K.; Yamamura, T.; Minamikawa, S.; et al. Genetic, Clinical, and Pathologic Backgrounds of Patients with Autosomal Dominant Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2016, 11, 1441–1449. [Google Scholar] [CrossRef]
- Furlano, M.; Martínez, V.; Pybus, M.; Arce, Y.; Crespí, J.; del Venegas, M.P.; Bullich, G.; Domingo, A.; Ayasreh, N.; Benito, S.; et al. Clinical and Genetic Features of Autosomal Dominant Alport Syndrome: A Cohort Study. Am. J. Kidney Dis. 2021, 78, 560–570.e1. [Google Scholar] [CrossRef]
- Groopman, E.E.; Marasa, M.; Cameron-Christie, S.; Petrovski, S.; Aggarwal, V.S.; Milo-Rasouly, H.; Li, Y.; Zhang, J.; Nestor, J.; Krithivasan, P.; et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N. Engl. J. Med. 2019, 380, 142–151. [Google Scholar] [CrossRef]
- Blasco, M.; Quiroga, B.; García-Aznar, J.M.; Castro-Alonso, C.; Fernández-Granados, S.J.; Luna, E.; Fernández Fresnedo, G.; Ossorio, M.; Izquierdo, M.J.; Sanchez-Ospina, D.; et al. Genetic Characterization of Kidney Failure of Unknown Etiology in Spain: Findings From the GENSEN Study. Am. J. Kidney Dis. 2024, 84, 719–730.e1. [Google Scholar] [CrossRef] [PubMed]
- Uzzo, M.; Moroni, G.; Ponticelli, C. Thin Basement Membrane: An Underrated Cause of End-Stage Renal Disease. Nephron 2023, 147, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, C.E.; Ding, J.; Garosi, G.; Heidet, L.; Massella, L.; Nakanishi, K.; Nozu, K.; Renieri, A.; Rheault, M.; Wang, F.; et al. Alport Syndrome: A Unified Classification of Genetic Disorders of Collagen IV α345: A Position Paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018, 93, 1045–1051. [Google Scholar] [CrossRef]
- Malone, A.F.; Phelan, P.J.; Hall, G.; Cetincelik, U.; Homstad, A.; Alonso, A.S.; Jiang, R.; Lindsey, T.B.; Wu, G.; Sparks, M.A.; et al. Rare Hereditary COL4A3/COL4A4 Variants May Be Mistaken for Familial Focal Segmental Glomerulosclerosis. Kidney Int. 2014, 86, 1253–1259. [Google Scholar] [CrossRef]
- Xie, J.; Wu, X.; Ren, H.; Wang, W.; Wang, Z.; Pan, X.; Hao, X.; Tong, J.; Ma, J.; Ye, Z.; et al. COL4A3 Mutations Cause Focal Segmental Glomerulosclerosis. J. Mol. Cell Biol. 2014, 6, 498–505. [Google Scholar] [CrossRef]
- Gast, C.; Pengelly, R.J.; Lyon, M.; Bunyan, D.J.; Seaby, E.G.; Graham, N.; Venkat-Raman, G.; Ennis, S. Collagen (COL4A) Mutations Are the Most Frequent Mutations Underlying Adult Focal Segmental Glomerulosclerosis. Nephrol. Dial. Transplant. 2016, 31, 961–970. [Google Scholar] [CrossRef]
- Yao, T.; Udwan, K.; John, R.; Rana, A.; Haghighi, A.; Xu, L.; Hack, S.; Reich, H.N.; Hladunewich, M.A.; Cattran, D.C.; et al. Integration of Genetic Testing and Pathology for the Diagnosis of Adults with FSGS. Clin. J. Am. Soc. Nephrol. 2019, 14, 213–223. [Google Scholar] [CrossRef]
- Daga, S.; Ding, J.; Deltas, C.; Savige, J.; Lipska-Ziętkiewicz, B.S.; Hoefele, J.; Flinter, F.; Gale, D.P.; Aksenova, M.; Kai, H.; et al. The 2019 and 2021 International Workshops on Alport Syndrome. Eur. J. Hum. Genet. 2022, 30, 507–516. [Google Scholar] [CrossRef]
- Quinlan, C.; Rheault, M.N. Genetic Basis of Type Iv Collagen Disorders of the Kidney. Clin. J. Am. Soc. Nephrol. 2021, 16, 1101–1109. [Google Scholar] [CrossRef]
- Stokman, M.F.; Renkema, K.Y.; Giles, R.H.; Schaefer, F.; Knoers, N.V.A.M.; Van Eerde, A.M. The Expanding Phenotypic Spectra of Kidney Diseases: Insights from Genetic Studies. Nat. Rev. Nephrol. 2016, 12, 472–483. [Google Scholar] [CrossRef]
- Storey, H.; Savige, J.; Sivakumar, V.; Abbs, S.; Flinter, F.A. COL4A3/COL4A4 Mutations and Features in Individuals with Autosomal Recessive Alport Syndrome. J. Am. Soc. Nephrol. 2013, 24, 1945–1954. [Google Scholar] [CrossRef]
- Matthaiou, A.; Poulli, T.; Deltas, C. Prevalence of Clinical, Pathological and Molecular Features of Glomerular Basement Membrane Nephropathy Caused by COL4A3 or COL4A4 Mutations: A Systematic Review. Clin. Kidney J. 2020, 13, 1025–1036. [Google Scholar] [CrossRef]
- Bekheirnia, M.R.; Reed, B.; Gregory, M.C.; McFann, K.; Shamshirsaz, A.A.; Masoumi, A.; Schrier, R.W. Genotype-Phenotype Correlation in X-Linked Alport Syndrome. J. Am. Soc. Nephrol. 2010, 21, 876–883. [Google Scholar] [CrossRef]
- Gibson, J.T.; Huang, M.; Shenelli Croos Dabrera, M.; Shukla, K.; Rothe, H.; Hilbert, P.; Deltas, C.; Storey, H.; Lipska-Ziętkiewicz, B.S.; Chan, M.M.Y.; et al. Genotype-Phenotype Correlations for COL4A3-COL4A5 Variants Resulting in Gly Substitutions in Alport Syndrome. Sci. Rep. 2022, 12, 2722. [Google Scholar] [CrossRef]
- Takemon, Y.; Wright, V.; Davenport, B.; Gatti, D.M.; Sheehan, S.M.; Letson, K.; Savage, H.S.; Lennon, R.; Korstanje, R. Uncovering Modifier Genes of X-Linked Alport Syndrome Using a Novel Multiparent Mouse Model. J. Am. Soc. Nephrol. 2021, 32, 1961–1973. [Google Scholar] [CrossRef]
- Deltas, C.; Papagregoriou, G.; Louka, S.F.; Malatras, A.; Flinter, F.; Gale, D.P.; Gear, S.; Gross, O.; Hoefele, J.; Lennon, R.; et al. Genetic Modifiers of Mendelian Monogenic Collagen IV Nephropathies in Humans and Mice. Genes 2023, 14, 1686. [Google Scholar] [CrossRef]
- Daga, S.; Fallerini, C.; Furini, S.; Pecoraro, C.; Scolari, F.; Ariani, F.; Bruttini, M.; Mencarelli, M.A.; Mari, F.; Renieri, A.; et al. Non-Collagen Genes Role in Digenic Alport Syndrome. BMC Nephrol. 2019, 20, 70. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Li, Q.; Wang, K. InterVar: Clinical Interpretation of Genetic Variants by the 2015 ACMG-AMP Guidelines. Am. J. Hum. Genet. 2017, 100, 267–280. [Google Scholar] [CrossRef]
- Gross, O.; Netzer, K.O.; Lambrecht, R.; Seibold, S.; Weber, M. Meta-analysis of Genotype–Phenotype Correlation in X-linked Alport Syndrome: Impact on Clinical Counselling. Nephrol. Dial. Transplant. 2002, 17, 1218–1227. [Google Scholar] [CrossRef]
- Jais, J.P.; Knebelmann, B.; Giatras, I.; De Marchi, M.; Rizzoni, G.; Renieri, A.; Weber, M.; Gross, O.; Netzer, K.-O.; Flinter, F.; et al. X-Linked Alport Syndrome. J. Am. Soc. Nephrol. 2000, 11, 649–657. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Rana, K.; Tonna, S.; Lin, T.; Sin, L.; Savige, J. COL4A3 Mutations and Their Clinical Consequences in Thin Basement Membrane Nephropathy (TBMN). Kidney Int. 2004, 65, 786–790. [Google Scholar] [CrossRef]
- Morinière, V.; Dahan, K.; Hilbert, P.; Lison, M.; Lebbah, S.; Topa, A.; Bole-Feysot, C.; Pruvost, S.; Nitschke, P.; Plaisier, E.; et al. Improving Mutation Screening in Familial Hematuric Nephropathies through next Generation Sequencing. J. Am. Soc. Nephrol. 2014, 25, 2740–2751. [Google Scholar] [CrossRef]
- Weber, S.; Strasser, K.; Rath, S.; Kittke, A.; Beicht, S.; Alberer, M.; Lange-Sperandio, B.; Hoyer, P.F.; Benz, M.R.; Ponsel, S.; et al. Identification of 47 Novel Mutations in Patients with Alport Syndrome and Thin Basement Membrane Nephropathy. Pediatr. Nephrol. 2016, 31, 941–955. [Google Scholar] [CrossRef]
- Machuca, E.; Benoit, G.; Nevo, F.; Tête, M.J.; Gribouval, O.; Pawtowski, A.; Brandström, P.; Loirat, C.; Niaudet, P.; Gubler, M.C.; et al. Genotype-Phenotype Correlations in Non-Finnish Congenital Nephrotic Syndrome. J. Am. Soc. Nephrol. 2010, 21, 1209–1217. [Google Scholar] [CrossRef]
- Funk, S.D.; Lin, M.H.; Miner, J.H. Alport Syndrome and Pierson Syndrome: Diseases of the Glomerular Basement Membrane. Matrix Biol. 2018, 71–72, 250–261. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Hashimoto, T.; Takizawa, K.; Urae, S.; Masuda, H.; Matsunuma, M.; Yamada, Y.; Hamada, K.; Nomizu, M.; Liapis, H.; et al. Laminin Β2 Variants Associated with Isolated Nephropathy That Impact Matrix Regulation. JCI Insight 2021, 6, e145908. [Google Scholar] [CrossRef]
- Funk, S.D.; Bayer, R.H.; Malone, A.F.; McKee, K.K.; Yurchenco, P.D.; Miner, J.H. Pathogenicity of a Human Laminin Β2 Mutation Revealed in Models of Alport Syndrome. J. Am. Soc. Nephrol. 2018, 29, 949–960. [Google Scholar] [CrossRef]
- Gubler, M.; Levy, M.; Broyer, M.; Naizot, C.; Gonzales, G.; Perrin, D.; Habib, R. Alport’s Syndrome: A Report of 58 Cases and a Review of the Literature. Am. J. Med. 1981, 70, 493–505. [Google Scholar] [CrossRef]
- Furlano, M.; Pilco-Teran, M.; Pybus, M.; Martínez, V.; Aza-Carmona, M.; Rius Peris, A.; Pérez-Gomez, V.; Berná, G.; Mazon, J.; Hernández, J.; et al. Increased Prevalence of Kidney Cysts in Individuals Carrying Heterozygous COL4A3 or COL4A4 Pathogenic Variants. Nephrol. Dial. Transplant. 2024, 39, 1442–1448. [Google Scholar] [CrossRef]
- Abrahamson, D.R.; Isom, K.; Roach, E.; Stroganova, L.; Zelenchuk, A.; Miner, J.H.; St. John, P.L. Laminin Compensation in Collagen A3(IV) Knockout (Alport) Glomeruli Contributes to Permeability Defects. J. Am. Soc. Nephrol. 2007, 18, 2465–2472. [Google Scholar] [CrossRef]
- Jones, L.K.; Lam, R.; McKee, K.K.; Aleksandrova, M.; Dowling, J.; Alexander, S.I.; Mallawaarachchi, A.; Cottle, D.L.; Short, K.M.; Pais, L.; et al. A Mutation Affecting Laminin Alpha 5 Polymerisation Gives Rise to a Syndromic Developmental Disorder. Development 2020, 147, dev189183. [Google Scholar] [CrossRef]
- Harraka, P.; Savige, J. Pathogenic LAMA5 Variants and Kidney Disease. Kidney360 2021, 2, 1876–1879. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Nagano, C.; Sekiguchi, K.; Tashiro, A.; Sugawara, N.; Sakaguchi, H.; Umeda, C.; Aoto, Y.; Ishiko, S.; Rossanti, R.; et al. Clear Evidence of LAMA5 Gene Biallelic Truncating Variants Causing Infantile Nephrotic Syndrome. Kidney360 2021, 2, 1968–1978. [Google Scholar] [CrossRef]
- Shannon, M.B.; Patton, B.L.; Harvey, S.J.; Miner, J.H. A Hypomorphic Mutation in the Mouse Laminin A5 Gene Causes Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2006, 17, 1913–1922. [Google Scholar] [CrossRef]
- Sampaolo, S.; Napolitano, F.; Tirozzi, A.; Reccia, M.G.; Lombardi, L.; Farina, O.; Barra, A.; Cirillo, F.; Melone, M.A.B.; Gianfrancesco, F.; et al. Identification of the First Dominant Mutation of LAMA5 Gene Causing a Complex Multisystem Syndrome Due to Dysfunction of the Extracellular Matrix. J. Med. Genet. 2017, 54, 710–720. [Google Scholar] [CrossRef]
- Voskarides, K.; Papagregoriou, G.; Hadjipanagi, D.; Petrou, I.; Savva, I.; Elia, A.; Athanasiou, Y.; Pastelli, A.; Kkolou, M.; Hadjigavriel, M.; et al. COL4A5 and LAMA5 Variants Co-Inherited in Familial Hematuria: Digenic Inheritance or Genetic Modifier Effect? BMC Nephrol. 2018, 19, 114. [Google Scholar] [CrossRef]
- Thompson, B.J.; Pichaud, F.; Röper, K. Sticking Together the Crumbs—An Unexpected Function for an Old Friend. Nat. Rev. Mol. Cell Biol. 2013, 14, 307–314. [Google Scholar] [CrossRef]
- Möller-Kerutt, A.; Rodriguez-Gatica, J.E.; Wacker, K.; Bhatia, R.; Siebrasse, J.P.; Boon, N.; van Marck, V.; Boor, P.; Kubitscheck, U.; Wijnholds, J.; et al. Crumbs2 Is an Essential Slit Diaphragm Protein of the Renal Filtration Barrier. J. Am. Soc. Nephrol. 2021, 32, 1053–1070. [Google Scholar] [CrossRef]
- Lamont, R.E.; Tan, W.H.; Innes, A.M.; Parboosingh, J.S.; Schneidman-Duhovny, D.; Rajkovic, A.; Pappas, J.; Altschwager, P.; Deward, S.; Fulton, A.; et al. Expansion of Phenotype and Genotypic Data in CRB2-Related Syndrome. Eur. J. Hum. Genet. 2016, 24, 1436–1444. [Google Scholar] [CrossRef]
- Yu, S.; Choi, W.I.; Choi, Y.J.; Kim, H.Y.; Hildebrandt, F.; Gee, H.Y. PLCE1 Regulates the Migration, Proliferation, and Differentiation of Podocytes. Exp. Mol. Med. 2020, 52, 594–603. [Google Scholar] [CrossRef]
- Li, A.S.; Ingham, J.F.; Lennon, R. Genetic Disorders of the Glomerular Filtration Barrier. Clin. J. Am. Soc. Nephrol. 2020, 15, 1818–1828. [Google Scholar] [CrossRef]
- Gbadegesin, R.; Hinkes, B.G.; Hoskins, B.E.; Vlangos, C.N.; Heeringa, S.F.; Liu, J.; Loirat, C.; Ozaltin, F.; Hashmi, S.; Ulmer, F.; et al. Mutations in PLCE1 Are a Major Cause of Isolated Diffuse Mesangial Sclerosis (IDMS). Nephrol. Dial. Transplant. 2008, 23, 1291–1297. [Google Scholar] [CrossRef]
- Boyer, O.; Benoit, G.; Gribouval, O.; Nevo, F.; Pawtowski, A.; Bilge, I.; Bircan, Z.; Deschênes, G.; Guay-Woodford, L.M.; Hall, M.; et al. Mutational Analysis of the PLCE1 Gene in Steroid Resistant Nephrotic Syndrome. J. Med. Genet. 2010, 47, 445–452. [Google Scholar] [CrossRef]
- Nabet, B.; Tsai, A.; Tobias, J.W.; Carstens, R.P. Identification of a Putative Network of Actin-Associated Cytoskeletal Proteins in Glomerular Podocytes Defined by Co-Purified MRNAs. PLoS ONE 2009, 4, e6491. [Google Scholar] [CrossRef][Green Version]
- Mao, J.; Wang, D.; Mataleena, P.; He, B.; Niu, D.; Katayama, K.; Xu, X.; Ojala, J.R.M.; Wang, W.; Shu, Q.; et al. Myo1e Impairment Results in Actin Reorganization, Podocyte Dysfunction, and Proteinuria in Zebrafish and Cultured Podocytes. PLoS ONE 2013, 8, e72750. [Google Scholar] [CrossRef]
- Mele, C.; Iatropoulos, P.; Donadelli, R.; Calabria, A.; Maranta, R.; Cassis, P.; Buelli, S.; Tomasoni, S.; Piras, R.; Krendel, M.; et al. MYO1E Mutations and Childhood Familial Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2011, 365, 295–306. [Google Scholar] [CrossRef]
- Krendel, M.; Kim, S.V.; Willinger, T.; Wang, T.; Kashgarian, M.; Flavell, R.A.; Mooseker, M.S. Disruption of Myosin 1e Promotes Podocyte Injury. J. Am. Soc. Nephrol. 2009, 20, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lennon, R.; Stuart, H.M.; Bierzynska, A.; Randles, M.J.; Kerr, B.; Hillman, K.A.; Batra, G.; Campbell, J.; Storey, H.; Flinter, F.A.; et al. Coinheritance of COL4A5 and MYO1E Mutations Accentuate the Severity of Kidney Disease. Pediatr. Nephrol. 2015, 30, 1459–1465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyake, N.; Tsukaguchi, H.; Koshimizu, E.; Shono, A.; Matsunaga, S.; Shiina, M.; Mimura, Y.; Imamura, S.; Hirose, T.; Okudela, K.; et al. Biallelic Mutations in Nuclear Pore Complex Subunit NUP107 Cause Early-Childhood-Onset Steroid-Resistant Nephrotic Syndrome. Am. J. Hum. Genet. 2015, 97, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Lovric, S.; Schapiro, D.; Schneider, R.; Marquez, J.; Asif, M.; Hussain, M.S.; Daga, A.; Widmeier, E.; Rao, J.; et al. Mutations in Multiple Components of the Nuclear Pore Complex Cause Nephrotic Syndrome. J. Clin. Investig. 2018, 128, 4313–4328. [Google Scholar] [CrossRef] [PubMed]
| N. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Female | Male | Female | Female | Male | Male | |
| Clinical Picture | Age—Onset (y.) | 23 | 29 | 29 | 24 | 36 | 42 | 23 | 28 |
| Renal Syndrome | Nephritic syndrome | Nephritic syndrome | Nephritic syndrome | Nephritic-nephrotic syndrome | Nephrotic range proteinuria | Nephrotic syndrome | Nephritic-nephrotic syndrome | Nephritic syndrome | |
| eGFR—Onset (mL/min/1.73m2) | 111 | 101 | 101 | 72 | 75 | 48 | 111 | 70 | |
| Proteinuria—Onset (g/day) | 0.95 | 3 | 0.8 | 22.0 | 4.6 | 3.5 | 3.6 | NA | |
| Hematuria—Onset | Microscopic | Microscopic | Microscopic | Microscopic | Episodes of macroscopic hematuria | Absent | Microscopic | Microscopic | |
| Extrarenal Features | Absent | Absent | Absent | Absent | Hearing loss | Absent | Absent | Hearing loss | |
| Kidney Cysts | No | No | No | No | Yes | Yes | No | No | |
| Family History | Positive Family History | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Clinical Picture | Nephritic syndrome | Nephritic syndrome | CKD | Nephrotic syndrome | CKD/KRT | - | Isolated microscopic hematuria | KRT | |
| Genetic/ Histologic Diagnosis of AS | Yes | Yes | No | No | No | - | No | Yes | |
| Kidney Biopsy | Kidney Biopsy | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Age—Kidney Biopsy (y.) | 23 | - | 31 | 24 | 44 | - | 32 | - | |
| Diagnosis | FSGS | - | TBMD | FSGS | TBMD | - | TBMD | - | |
| GBM Alterations | No EM available | - | Thin GBM | Thick GBM | Thin GBM | - | Thin GBM | - | |
| GS | Yes (focal) | - | No | Yes (focal) | No | - | No | - | |
| IF | NA | - | NA | NA | Negative | - | Positive (+) staining for C3 (mesangial) | - | |
| Podocyte Foot Effacement | No EM available | - | Focal | Focal | Absent | - | Focal | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lujinschi, Ș.N.; Sorohan, B.M.; Obrișcă, B.; Vrabie, A.; Rusu, E.; Zilișteanu, D.; Achim, C.; Andronesi, A.G.; Ismail, G. Candidate Genetic Modifiers in Alport Syndrome: A Case Series. Life 2025, 15, 298. https://doi.org/10.3390/life15020298
Lujinschi ȘN, Sorohan BM, Obrișcă B, Vrabie A, Rusu E, Zilișteanu D, Achim C, Andronesi AG, Ismail G. Candidate Genetic Modifiers in Alport Syndrome: A Case Series. Life. 2025; 15(2):298. https://doi.org/10.3390/life15020298
Chicago/Turabian StyleLujinschi, Ștefan Nicolaie, Bogdan Marian Sorohan, Bogdan Obrișcă, Alexandra Vrabie, Elena Rusu, Diana Zilișteanu, Camelia Achim, Andreea Gabriella Andronesi, and Gener Ismail. 2025. "Candidate Genetic Modifiers in Alport Syndrome: A Case Series" Life 15, no. 2: 298. https://doi.org/10.3390/life15020298
APA StyleLujinschi, Ș. N., Sorohan, B. M., Obrișcă, B., Vrabie, A., Rusu, E., Zilișteanu, D., Achim, C., Andronesi, A. G., & Ismail, G. (2025). Candidate Genetic Modifiers in Alport Syndrome: A Case Series. Life, 15(2), 298. https://doi.org/10.3390/life15020298






