Biomarkers in Atopic Dermatitis in Children: A Comprehensive Review
Abstract
1. Introduction
1.1. Atopic Dermatitis and the Atopic March
1.2. Pathogenesis and Immune Mechanisms
1.3. Clinical Manifestations, Diagnosis, and Treatment
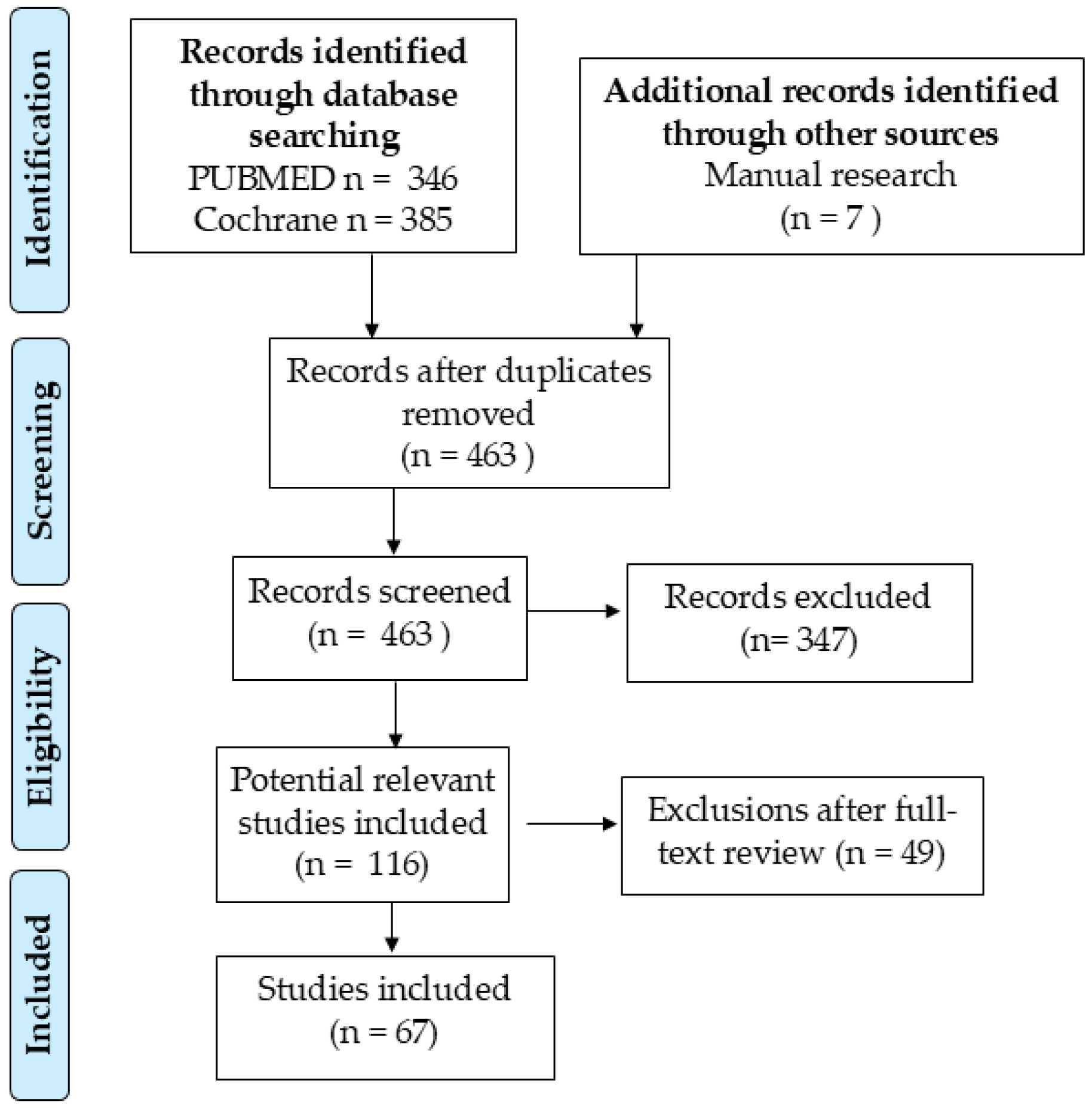
2. Materials and Methods
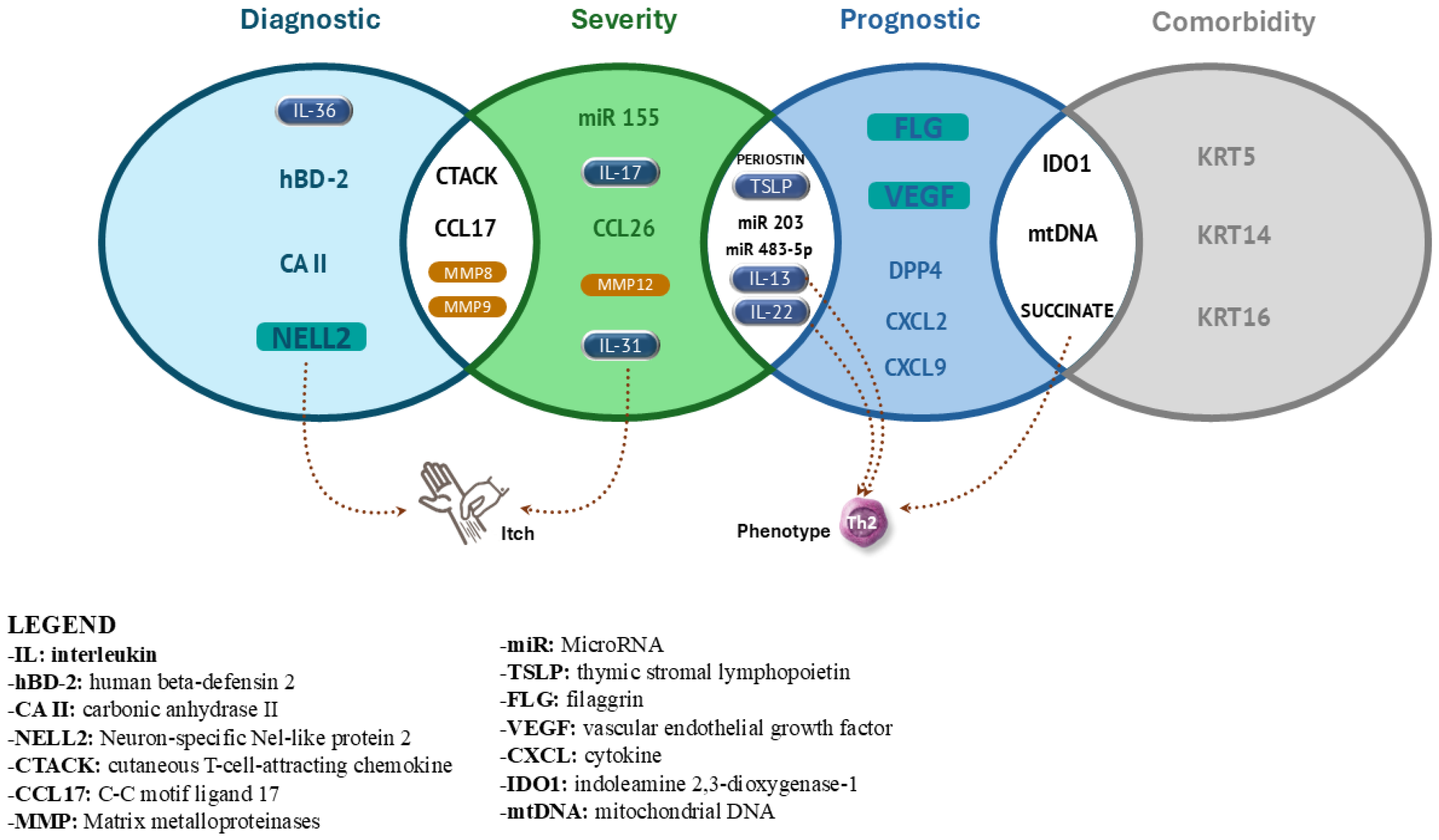
3. Results
3.1. Diagnostic Biomarkers
3.1.1. Carbonic Anhydrase II (CA II) and Neuron-Specific Nel-like Protein 2 (NELL2)
3.1.2. Urinary Lipid Profile Analysis
3.1.3. NOS2 and CCL27 (CTACK)
3.1.4. IL-36γ and Human Beta-Defensin 2 (hBD-2)
3.1.5. Matrix Metalloproteinases (MMP-8 e MMP-9)
3.2. Prognostic and Screening Biomarkers
3.2.1. Filaggrin Mutations
3.2.2. Natural Moisturizing Factor (NMF) and Transepidermal Water Loss (TEWL)
3.2.3. Vascular Endothelial Growth Factor (VEGF) and Indoleamine 2,3-Dioxygenase-1 (IDO1)
3.2.4. MicroRNAs (miR-155, miR-203, and miR-483-5p) and Succinate mtDNA
3.3. Biomarkers for Severity Assessment in Atopic Dermatitis
3.3.1. Th2/Th22 Immune Mediators
3.3.2. IL-8 and CTACK
3.3.3. IL-1β and Thymic Stromal Lymphopoietin (TSLP)
3.3.4. Th17/Th22 Cytokines, DPP-4, and Other Immune Markers
3.3.5. Role of the Skin Microflora
3.4. Predictive Biomarkers
3.4.1. Periostin and DPP-4
3.4.2. IL-22, CXCL9, CXCL2, and MDC/CCL22
3.5. Biomarkers for Comorbidities in Patients with Atopic Dermatitis
3.5.1. VEGF and Indoleamine 2,3-Dioxygenase-1 (IDO1)
3.5.2. KRT5, KRT14, KRT16, and FLG
3.5.3. Succinate and Mitochondrial DNA (mtDNA)
| Biomarker | Category | Function | Reference | Technique for Measuring Biomarkers in Blood |
|---|---|---|---|---|
| IL-13, IL-22 | Serum-Specific, Severity, Predictive | Key cytokines in the Th2/Th22 pathways; correlate with disease severity and predict responses to IL-13/IL-22 inhibitors. | Thijs et al. [55], Brunner et al. [75] | ELISA, Multiplex Immunoassay (Luminex), qPCR |
| IL-36 | Skin-Specific, Severity | Associated with neutrophilic inflammation and disease severity in AD. | Otobe et al. [30] | ELISA, qPCR |
| TARC/CCL17 | Serum-Specific, Severity, Diagnostic | Biomarker for inflammation and treatment response; correlates with the SCORAD score and disease activity. | Mastraftsi et al. [2], Angelova-Fischer et al. [69] | ELISA, Luminex |
| FLG mutations | Skin-Specific, Prognostic | Associated with early-onset AD, barrier dysfunction, and progression to the atopic march. | Paternoster et al. [35], Irvine et al. [84] | PCR, NGS (Next-Generation Sequencing), Sanger Sequencing |
| VEGF | Serum-Specific, Prognostic | Low levels predict persistent AD in infants; involved in skin vascularization. | Lauffer et al. [44] | ELISA, Luminex, Western Blot |
| NELL2 | Skin-Specific, Diagnostic | Linked to pruritus and overexpressed in AD epidermis, aiding in the differentiation from psoriasis. | Kamsteeg et al. [25] | ELISA, Western Blot, qPCR |
| MMP-8, MMP-9 | Skin-Specific, Diagnostic, Severity | Reflect tissue remodeling and inflammation; correlate with AD lesion activity. | Harper et al. [32] | ELISA, Luminex, Zymography |
| MMP-12 | Skin-Specific, Severity | Reflects tissue remodeling and correlates with AD lesion severity. | Yu et al. [65] | ELISA, Zymography |
| Succinate, mtDNA | Serum-Specific, Prognostic, Comorbidity | Drive systemic inflammation; promote progression to atopic march via gut–immune interactions. | Wang et al. [50], Mills et al. [85] | Succinate: LC-MS/MS (Liquid Chromatography–Mass Spectrometry), GC-MS mtDNA: qPCR, ddPCR (Droplet Digital PCR) |
| DPP-4 | Serum-Specific, Endotype-Specific, Predictive | Linked to specific AD phenotypes; predicts response to IL-13-targeted therapies like tralokinumab. | Maintz et al. [10], Wollenberg et al. [87] | ELISA, Activity Assay (Fluorometric/Colorimetric) |
| IL-31 | Serum-Specific, Severity | The “itch cytokine”, associated with pruritus but shows inconsistent correlation with disease severity. | Ozceker et al. [67] | ELISA, Luminex |
| Periostin | Serum-Specific, Endotype-Specific, Severity | Reflects Th2-driven inflammation; associated with eosinophilia and moderate AD severity. | Kou et al. [66] | ELISA, Western Blot |
| TSLP | Skin-Specific, Prognostic, Severity | Strongly correlates with SCORAD and TEWL scores; predicts barrier dysfunction. | Kim et al. [62], Nygaard et al. [63] | ELISA, Luminex, qPCR |
| hBD-2 | Skin-Specific, Diagnostic | Elevated in psoriasis and minimally expressed in AD, aiding in differential diagnosis. | Jansen et al. [31] | ELISA, Western Blot |
| CXCL2, CXCL9 | Serum-Specific, Predictive | CXCL9 predicts the response to cyclosporine; CXCL2 indicates the efficacy of dupilumab in high Th17 profiles. | Glickman et al. [76] | ELISA, Luminex, qPCR |
| IDO1 | Serum-Specific, Prognostic, Comorbidity | Indicates risk of eczema herpeticum; associated with antiviral immune responses. | Staudacher et al. [82] | qPCR (for mRNA), ELISA, LC-MS/MS (for metabolites such as tryptophan and kynurenine) |
| CA II | Skin-Specific, Severity | Associated with skin barrier dysfunction and inflammation. | Kamsteeg et al. [24,25] | ELISA, qPCR |
| miR 155 | Serum-Specific, Severity | Elevated levels correlate with AD severity in children. | Sonkoly et al. [45] | qPCR, Microarray |
| miR 203 | Serum-Specific, Diagnostic | Potential non-invasive biomarker for monitoring inflammation and disease progression. | Chen et al. [46] | qPCR, Microarray |
| miR 483-5p | Serum/Urine-Specific, Diagnostic | Promising biomarker for non-invasive disease monitoring. | Gilad et al. [47] | qPCR, Microarray |
| CCL26 | Serum-Specific, Severity | Linked to eosinophilic inflammation and AD severity. | Thijs et al. [55] | ELISA, Luminex |
| IL-17 | Serum-Specific, Severity | Th17 cytokine, elevated in severe AD cases. | Yang et al. [64] | ELISA, Luminex, qPCR |
| KRT5 | Skin-Specific, Diagnostic | Upregulated in AD; indicates keratinocyte dysfunction. | Thijs et al. [55] | qPCR, Western Blot |
| KRT14 | Marker of basal keratinocytes; upregulated in AD lesions. | |||
| KRT16 | Overexpressed in hyperproliferative epidermis; associated with AD severity. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Galli, E.; Cinicola, B.; Carello, R.; Caimmi, S.; Brindisi, G.; De Castro, G.; Zicari, A.M.; Tosca, M.A.; Manti, S.; Martelli, A.; et al. Atopic dermatitis. Acta Biomed. 2020, 91, e2020011. [Google Scholar] [CrossRef]
- Mastraftsi, S.; Vrioni, G.; Bakakis, M.; Nicolaidou, E.; Rigopoulos, D.; Stratigos, A.J.; Gregoriou, S. Atopic Dermatitis: Striving for Reliable Biomarkers. J. Clin. Med. 2022, 11, 4639. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Ziehfreund, S.; Tizek, L.; Hangel, N.; Fritzsche, M.C.; Weidinger, S.; Smith, C.; Bryce, P.J.; Greco, D.; van den Bogaard, E.H.; Flohr, C.; et al. Requirements and expectations of high-quality biomarkers for atopic dermatitis and psoriasis in 2021-a two-round Delphi survey among international experts. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Maiello, N.; Giannetti, A.; Ricci, G.; Cinicola, B.; Carello, R.; Indolfi, C.; Caffarelli, C.; Marseglia, A.; Calvani, M.; Del Giudice, M.M.; et al. Atopic dermatitis and atopic march: Which link? Acta Biomed. 2021, 92, e2021525. [Google Scholar] [CrossRef]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A.; et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.B.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef]
- Maintz, L.; Welchowski, T.; Herrmann, N.; Brauer, J.; Traidl-Hoffmann, C.; Havenith, R.; Müller, S.; Rhyner, C.; Dreher, A.; Schmid, M.; et al. IL-13, periostin and dipeptidyl-peptidase-4 reveal endotype-phenotype associations in atopic dermatitis. Allergy 2023, 78, 1554–1569. [Google Scholar] [CrossRef]
- Savva, M.; Papadopoulos, N.G.; Gregoriou, S.; Katsarou, S.; Papapostolou, N.; Makris, M.; Xepapadaki, P. Recent Advancements in the Atopic Dermatitis Mechanism. Front. Biosci. (Landmark Ed.) 2024, 29, 84. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innov. Ski. Sci. Mol. Popul. Health 2022, 2, 100131. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef] [PubMed]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021, 17, 835–852. [Google Scholar] [CrossRef]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Tham, E.H.; Chia, M.; Riggioni, C.; Nagarajan, N.; Common, J.E.A.; Kong, H.H. The skin microbiome in pediatric atopic dermatitis and food allergy. Allergy 2024, 79, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Canter, T.; Han, J.; Lefferdink, R.; Erickson, T.; Rangel, S.; Kameyama, N.; Kim, H.J.; Pavel, A.B.; et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J. Allergy Clin. Immunol. 2020, 145, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Knipping, K.; Knippels, L.M.J.; Dupont, C.; Garssen, J. Serum biomarkers for allergy in children. Pediatr. Allergy Immunol. 2017, 28, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R. Biomarkers for Atopic Dermatitis in Children. Pediatr. Allergy Immunol. Pulmonol. 2016, 29, 164–169. [Google Scholar] [CrossRef]
- D’Auria, E.; Indolfi, C.; Acunzo, M.; Dinardo, G.; Comberiati, P.; Peroni, D.; Zuccotti, G.V.; Miraglia Del Giudice, M. Biologics and small molecules: The re-evolution in the treatment of atopic dermatitis in children and adolescents. Current state of the art and future perspectives. Expert Rev. Clin. Immunol. 2025, 20, 1–13. [Google Scholar] [CrossRef]
- Kamsteeg, M.; Zeeuwen, P.L.J.M.; Jongh, G.J.D.; Rodijk-Olthuis, D.; Zeeuwen-Franssen, M.E.J.; Van Erp, P.E.J.; Schalkwijk, J. Increased expression of carbonic anhydrase II (CA II) in lesional skin of atopic dermatitis: Regulation by Th2 cytokines. J. Investig. Dermatol. 2007, 127, 1786–1789. [Google Scholar] [CrossRef]
- Kamsteeg, M.; Jansen, P.A.M.; Van Vlijmen-Willems, I.M.J.J.; Van Erp, P.E.J.; Rodijk-Olthuis, D.; Van Der Valk, P.G.; Feuth, T.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Molecular diagnostics of psoriasis, atopic dermatitis, allergic contact dermatitis and irritant contact dermatitis. Br. J. Dermatol. 2010, 162, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Hamasaki, Y.; Inagaki, S.; Nakamura, T.; Horikami, D.; Yamamoto-Hanada, K.; Inuzuka, Y.; Shimosawa, T.; Kobayashi, K.; Narita, M.; et al. Urinary lipid profile of atopic dermatitis in murine model and human patients. FASEB J. 2021, 35, e21949. [Google Scholar] [CrossRef]
- Garzorz-Stark, N.; Krause, L.; Lauffer, F.; Atenhan, A.; Thomas, J.; Stark, S.P.; Franz, R.; Weidinger, S.; Balato, A.; Mueller, N.S.; et al. A novel molecular disease classifier for psoriasis and eczema. Exp. Dermatol. 2016, 25, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Knapp, B.; Garzorz, N.; Mattii, M.; Pullabhatla, V.; Pennino, D.; Andres, C.; Traidl-Hoffmann, C.; Cavani, A.; Theis, F.J.; et al. Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci. Transl. Med. 2014, 6, 244ra90. [Google Scholar] [CrossRef] [PubMed]
- D’Erme, A.M.; Wilsmann-Theis, D.; Wagenpfeil, J.; Hölzel, M.; Ferring-Schmitt, S.; Sternberg, S.; Wittmann, M.; Peters, B.; Bosio, A.; Bieber, T.; et al. IL-36γ (IL-1F9) is a biomarker for psoriasis skin lesions. J. Investig. Dermatol. 2015, 135, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Otobe, S.; Sugaya, M.; Nakajima, R.; Oka, T.; Takahashi, N.; Kabasawa, M.; Miyagaki, T.; Asano, Y.; Sato, S. Increased interleukin-36γ expression in skin and sera of patients with atopic dermatitis and mycosis fungoides/Sézary syndrome. J. Dermatol. 2018, 45, 468–471. [Google Scholar] [CrossRef]
- Jansen, P.A.M.; Rodijk-Olthuis, D.; Hollox, E.J.; Kamsteeg, M.; Tjabringa, G.S.; de Jongh, G.J.; van Vlijmen-Willems, I.M.J.J.; Bergboer, J.G.M.; van Rossum, M.M.; de Jong, E.M.G.J.; et al. β-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS ONE 2009, 4, e4725. [Google Scholar] [CrossRef]
- Harper, J.I.; Godwin, H.; Green, A.; Wilkes, L.E.; Holden, N.J.; Moffatt, M.; Cookson, W.O.; Layton, G.; Chandler, S. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br. J. Dermatol. 2010, 162, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.F.; Leung, D.Y.M.; Beck, L.A.; Berin, C.M.; Boguniewicz, M.; Busse, W.W.; Chatila, T.A.; Geha, R.S.; Gern, J.E.; Guttman-Yassky, E.; et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J. Allergy Clin. Immunol. 2019, 143, 894–913. [Google Scholar] [CrossRef]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.P.; Curtin, J.A.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.J.; Guttman-Yassky, E. Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Leung, D.Y.M. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy. Asthma Immunol. Res. 2019, 11, 4–15. [Google Scholar] [CrossRef]
- Izuhara, K.; Yamaguchi, Y.; Ohta, S.; Nunomura, S.; Nanri, Y.; Azuma, Y.; Nomura, N.; Noguchi, Y.; Aihara, M. Squamous Cell Carcinoma Antigen 2 (SCCA2, SERPINB4): An Emerging Biomarker for Skin Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 1102. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Onozuka, D.; Nunomura, S.; Saeki, H.; Takenaka, M.; Matsumoto, M.; Kataoka, Y.; Fujimoto, R.; Kaneko, S.; Morita, E.; et al. The ability of biomarkers to assess the severity of atopic dermatitis. J. Allergy Clin. Immunol. Glob. 2024, 3, 100175. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.J.H.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039.e1. [Google Scholar] [CrossRef]
- Ní Chaoimh, C.; Nico, C.; Puppels, G.J.; Caspers, P.J.; Wong, X.F.C.C.; Common, J.E.; Irvine, A.D.; Hourihane, J.O.B. In vivo Raman spectroscopy discriminates between FLG loss-of-function carriers vs wild-type in day 1-4 neonates. Ann. Allergy Asthma Immunol. 2020, 124, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kabashima, S.; Inoue, E.; Sasaki, T.; Niizeki, H.; Saito, H.; Matsumoto, K.; et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol. Int. 2016, 65, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209.e1. [Google Scholar] [CrossRef] [PubMed]
- Lauffer, F.; Baghin, V.; Standl, M.; Stark, S.P.; Jargosch, M.; Wehrle, J.; Thomas, J.; Schmidt-Weber, C.B.; Biedermann, T.; Eyerich, S.; et al. Predicting persistence of atopic dermatitis in children using clinical attributes and serum proteins. Allergy 2021, 76, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Janson, P.; Majuri, M.L.; Savinko, T.; Fyhrquist, N.; Eidsmo, L.; Xu, N.; Meisgen, F.; Wei, T.; Bradley, M.; et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J. Allergy Clin. Immunol. 2010, 126, 581–589.e20. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed]
- Jungersted, J.M.; Scheer, H.; Mempel, M.; Baurecht, H.; Cifuentes, L.; Høgh, J.K.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T.; Weidinger, S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 2010, 65, 911–918. [Google Scholar] [CrossRef]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid Res. 2022, 63, 100235. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Huang, J.; He, H.; Zhou, L.; He, Y.; Yan, J.; Tao, A. Succinate and mitochondrial DNA trigger atopic march from atopic dermatitis to intestinal inflammation. J. Allergy Clin. Immunol. 2023, 151, 1050–1066.e7. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Arkwright, P.D.; Koplin, J.J. Impact of a Decade of Research Into Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2023, 11, 63–71. [Google Scholar] [CrossRef]
- Miraglia del Giudice, M.; Klain, A.; Indolfi, C.; Dinardo, G.; Bifulco, D.; Quaranta, G.; Lucia Bencivenga, C.; Decimo, F. MiniReview Marcia Atopica: Ci sono nuove evidenze? Riv. Di Immunol. E Allergol. Pediatr. 2021, 35, 17–22. Available online: https://old.riaponline.it/article/marcia-atopica-ci-sono-nuove-evidenze/ (accessed on 25 February 2025).
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; De Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Pavel, A.B.; Renert-Yuval, Y.; Wu, J.; Del Duca, E.; Diaz, A.; Lefferdink, R.; Fang, M.M.; Canter, T.; Rangel, S.M.; Zhang, N.; et al. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy 2021, 76, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children with Early-Onset Atopic Dermatitis. JAMA Dermatol. 2019, 155, 1358–1370. [Google Scholar] [CrossRef]
- He, H.; Bissonnette, R.; Wu, J.; Diaz, A.; Saint-Cyr Proulx, E.; Maari, C.; Jack, C.; Louis, M.; Estrada, Y.; Krueger, J.G.; et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2021, 147, 199–212. [Google Scholar] [CrossRef]
- Goleva, E.; Calatroni, A.; LeBeau, P.; Berdyshev, E.; Taylor, P.; Kreimer, S.; Cole, R.N.; Leung, D.Y.M. Skin tape proteomics identifies pathways associated with transepidermal water loss and allergen polysensitization in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 146, 1367–1378. [Google Scholar] [CrossRef]
- Murata, S.; Kaneko, S.; Morita, E. Interleukin-8 Levels in the Stratum Corneum as a Biomarker for Monitoring Therapeutic Effect in Atopic Dermatitis Patients. Int. Arch. Allergy Immunol. 2021, 182, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Amarbayasgalan, T.; Takahashi, H.; Dekio, I.; Morita, E. Interleukin-8 content in the stratum corneum as an indicator of the severity of inflammation in the lesions of atopic dermatitis. Int. Arch. Allergy Immunol. 2013, 160, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.E.; Lee, J.; Han, Y.; Jun, H.Y.; Kim, H.; Choi, J.; Leung, D.Y.M.; Ahn, K. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J. Allergy Clin. Immunol. 2016, 137, 1282–1285.e4. [Google Scholar] [CrossRef]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Ozceker, D.; Bulut, M.; Ozbay, A.C.; Dilek, F.; Koser, M.; Tamay, Z.; Guler, N. Assessment of IL-31 levels and disease severity in children with atopic dermatitis. Allergol. Immunopathol. 2018, 46, 322–325. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Nastalek, M.; Okumura, K.; Wojas-Pelc, A.; Undas, A.; Nishiyama, C. An association of TLR2–16934A>T polymorphism and severity/phenotype of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 715–721. [Google Scholar] [CrossRef]
- Angelova-Fischer, I.; Hipler, U.C.; Bauer, A.; Fluhr, J.W.; Tsankov, N.; Fischer, T.W.; Elsner, P. Significance of interleukin-16, macrophage-derived chemokine, eosinophil cationic protein and soluble E-selectin in reflecting disease activity of atopic dermatitis—From laboratory parameters to clinical scores. Br. J. Dermatol. 2006, 154, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Kawashima, H.; Takekuma, K.; Hoshika, A. Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr. Int. 2010, 52, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Kim, S.M.; Lee, K.H.; Bieber, T. Biomarkers for phenotype-endotype relationship in atopic dermatitis: A critical review. eBioMedicine 2024, 103, 105121. [Google Scholar] [CrossRef]
- Kägi, M.K.; Joller-Jemelka, H.; Wüthrich, B. Correlation of eosinophils, eosinophil cationic protein and soluble interleukin-2 receptor with the clinical activity of atopic dermatitis. Dermatology 1992, 185, 88–92. [Google Scholar] [CrossRef]
- Chu, H.; Kim, S.M.; Zhang, K.L.; Wu, Z.; Lee, H.; Kim, J.H.; Kim, H.L.; Kim, Y.R.; Kim, S.H.; Kim, W.J.; et al. Head and neck dermatitis is exacerbated by Malassezia furfur colonization, skin barrier disruption, and immune dysregulation. Front. Immunol. 2023, 14, 1114321. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; Jim On, S.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019, 143, 142–154. [Google Scholar] [CrossRef]
- Glickman, J.W.; Han, J.; Garcet, S.; Krueger, J.G.; Pavel, A.B.; Guttman-Yassky, E. Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J. Allergy Clin. Immunol. Pract. 2020, 8, 3622–3625.e19. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L.; et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941–951.e3. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; De Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Nomura, T.; Wu, J.; Kabashima, K.; Guttman-Yassky, E. Endophenotypic Variations of Atopic Dermatitis by Age, Race, and Ethnicity. J. Allergy Clin. Immunol. Pract. 2020, 8, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef]
- Staudacher, A.; Hinz, T.; Novak, N.; Von Bubnoff, D.; Bieber, T. Exaggerated IDO1 expression and activity in Langerhans cells from patients with atopic dermatitis upon viral stimulation: A potential predictive biomarker for high risk of Eczema herpeticum. Allergy 2015, 70, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef]
- Irvine, A.D.; McLean, W.H.I.; Leung, D.Y.M. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-Mcdermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Claudio-Etienne, E.; Frischmeyer-Guerrerio, P.A. Atopic dermatitis and food allergy: More than sensitization. Mucosal Immunol. 2024, 17, 1128–1140. [Google Scholar] [CrossRef]
- Geba, G.P.; Li, D.; Xu, M.; Mohammadi, K.; Attre, R.; Ardeleanu, M.; Musser, B. Attenuating the atopic march: Meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J. Allergy Clin. Immunol. 2023, 151, 756–766. [Google Scholar] [CrossRef]
- Thijs, J.L.; Van Seggelen, W.; Bruijnzeel-Koomen, C.; De Bruin-Weller, M.; Hijnen, D. New Developments in Biomarkers for Atopic Dermatitis. J. Clin. Med. 2015, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Saito-Abe, M.; Shima, K.; Fukagawa, S.; Uehara, Y.; Ueda, Y.; Iwamura, M.; Murase, T.; Kuwano, T.; Inoue, T.; et al. mRNAs in skin surface lipids unveiled atopic dermatitis at 1 month. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1385–1395. [Google Scholar] [CrossRef]
- Bar, J.; Del Duca, E.; David, E.; Bose, S.; Chefitz, G.; Brunner, P.M.; Bissonnette, R.; Guttman-Yassky, E. Skin Tape Stripping Reveals Distinct Biomarker Profiles in Chronic Hand Eczema of Patients with and Without Comorbid Atopic Dermatitis. Allergy 2025, 1–15. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.D.; Jeong, D.H.; Kim, S.M.; Park, C.O.; Lee, K.H. Development of a novel microneedle platform for biomarker assessment of atopic dermatitis patients. Ski. Res. Technol. 2023, 29, e13413. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Skin Predictive Biomarkers for the Development of Atopic Dermatitis and Food Allergy in Infants. Allergy. Asthma Immunol. Res. 2024, 16, 323–337. [Google Scholar] [CrossRef]
- Campanati, A.; Martina, E.; Diotallevi, F.; Radi, G.; Marani, A.; Sartini, D.; Emanuelli, M.; Kontochristopoulos, G.; Rigopoulos, D.; Gregoriou, S.; et al. Saliva Proteomics as Fluid Signature of Inflammatory and Immune-Mediated Skin Diseases. Int. J. Mol. Sci. 2021, 22, 7018. [Google Scholar] [CrossRef]
- Hiremath, G.; Olive, A.; Shah, S.; Davis, C.M.; Shulman, R.J.; Devaraj, S. Comparing methods to collect saliva from children to analyze cytokines related to allergic inflammation. Ann. Allergy. Asthma Immunol. 2015, 114, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Pessemier, B.D.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.; Zheng, P.; Liu, E.; Bai, S.; Chen, S.; Pang, Y.; Xiao, X.; Yang, H.; Guo, J. Changes in oral, skin, and gut microbiota in children with atopic dermatitis: A case-control study. Front. Microbiol. 2024, 15, 1442126. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics Function in Preventing Atopic Dermatitis in Children. Int. J. Mol. Sci. 2022, 23, 5409. [Google Scholar] [CrossRef]
- D’Elios, S.; Trambusti, I.; Verduci, E.; Ferrante, G.; Rosati, S.; Marseglia, G.L.; Drago, L.; Peroni, D.G. Probiotics in the prevention and treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S26), 43–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ni, Y.; Wu, X.; Chen, G. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: A systematic review and Meta-analysis. J. Dermatol. Treat. 2022, 33, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. An. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.; de Bruin-Weller, M.; Drylewicz, J.; van Wijk, F.; Thijs, J. Biomarkers in atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1163–1168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indolfi, C.; Grella, C.; Klain, A.; Dinardo, G.; Colosimo, S.; Piatto, D.; Nespoli, C.; Perrotta, A.; Miraglia del Giudice, M. Biomarkers in Atopic Dermatitis in Children: A Comprehensive Review. Life 2025, 15, 375. https://doi.org/10.3390/life15030375
Indolfi C, Grella C, Klain A, Dinardo G, Colosimo S, Piatto D, Nespoli C, Perrotta A, Miraglia del Giudice M. Biomarkers in Atopic Dermatitis in Children: A Comprehensive Review. Life. 2025; 15(3):375. https://doi.org/10.3390/life15030375
Chicago/Turabian StyleIndolfi, Cristiana, Carolina Grella, Angela Klain, Giulio Dinardo, Simone Colosimo, Dario Piatto, Claudia Nespoli, Alessandra Perrotta, and Michele Miraglia del Giudice. 2025. "Biomarkers in Atopic Dermatitis in Children: A Comprehensive Review" Life 15, no. 3: 375. https://doi.org/10.3390/life15030375
APA StyleIndolfi, C., Grella, C., Klain, A., Dinardo, G., Colosimo, S., Piatto, D., Nespoli, C., Perrotta, A., & Miraglia del Giudice, M. (2025). Biomarkers in Atopic Dermatitis in Children: A Comprehensive Review. Life, 15(3), 375. https://doi.org/10.3390/life15030375









