Abstract
Background: Cardiomyopathies are a significant cause of heart failure, arrhythmia, and cardiac morbidity in the general population. Cardiovascular magnetic resonance (CMR) is a valuable tool for the diagnostic work-up of patients with acute cardiac events. Objectives: This study evaluated the diagnostic value of CMR and the yield of cardiomyopathies in hospitalized cardiac patients with acute presentation. Methods: A retrospective analysis was conducted with 535 consecutive hospitalized patients who underwent CMR at Hippokration Hospital, Athens, Greece, to identify a subset of scans performed on an urgent basis of hospitalized patients. Demographic data, causes of admission, CMR findings, and plasma cardiac biomarkers (hs-Troponin I, NT-proBNP, and CRP) were systematically recorded. Results: Out of the initial 535 CMR scans evaluated, a further analysis was conducted with 104 patients who were in hospital and underwent CMR on an urgent basis. From the total population of hospitalized patients, 33% had CMR findings indicative of underlying cardiomyopathy, with dilated cardiomyopathy being the most common subtype (36%), followed by arrhythmogenic cardiomyopathy (27%), hypertrophic cardiomyopathy (15%), or other subtypes (e.g., cardiac amyloidosis, sarcoidosis, endomyocardial fibrosis, EGPA, or unclassified). CMR led to the reclassification of the initial diagnosis into that of underlying cardiomyopathy in 32% of cases. The highest reclassification rate was observed within the subgroup with heart failure (71%), followed by that of acute myocardial infarction/ischemic heart disease (24%) and myocarditis (22%). Conclusions: CMR imaging effectively contributed to the differential diagnosis of hospitalized patients with acute cardiac events that remained without a definitive diagnosis after their initial work-up and uncovered underlying cardiomyopathy in almost one-third of this cohort.
1. Introduction
Cardiomyopathies are a significant cause of morbidity and mortality [1] and among the major causes of heart failure and sudden cardiac death in young individuals [2]. Risk stratification and prognosis is linked with the underlying cardiomyopathy subtype, and accurate diagnosis is essential for optimal treatment and patient management [3,4]. Cardiomyopathies may lead not only to symptoms of chronic heart failure (HF) [5] but also to acute symptoms including chest pain, new-onset ventricular arrhythmias (VAs), or the decompensation of HF [6,7,8]. Although once considered rare, cardiomyopathies collectively account for a significant number of patients presenting with acute cardiac symptomatology [9]; increased vigilance is crucial to prevent misdiagnosis.
Cardiovascular magnetic resonance (CMR) is a valuable noninvasive diagnostic technique for assessing cardiomyopathy phenotypes [10,11,12]. CMR not only provides information on biventricular volumes and systolic function but also offers characterization of myocardial tissue, aiding in the differential diagnosis [13,14]. Multiparametric CMR imaging enables the detection of fibrotic tissue and myocardial edema [15,16,17], or even myocardial infiltration with amyloid, iron, or fat [13]. This is crucial for raising the suspicion of rare cardiomyopathies that often remain undetected [18,19]. The value of CMR in diagnosing cardiomyopathies is highlighted in the recent relevant clinical recommendations for the management of patients with cardiomyopathy, where CMR has a class I indication for all cardiomyopathy patients at initial evaluation [20].
In the acute setting, CMR plays a role in the differential diagnosis of patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) [21,22]. It is estimated that CMR can lead to reclassification and determine the specific diagnosis in approximately 70% of such cases [23]. CMR contributes to the etiological diagnosis of patients with acute HF [24] and in patients with VAs to assess for the presence of arrhythmic substrate, i.e., scarring, which may be associated with their clinical arrhythmias [25]. Therapeutic interventions can, therefore, also be guided by CMR findings.
Since the diagnostic yield of CMR imaging for cardiomyopathies in the acute hospital setting has not been systematically evaluated, we aimed to assess the value of CMR in reclassifying diagnoses and establishing a cardiomyopathy diagnosis in acutely ill hospitalized cardiac patients. These patients remained undiagnosed after the initial in-hospital work-up, posing both a diagnostic and therapeutic challenge.
2. Methods
2.1. Study Design
The inclusion criterion for this study was the performance of an in-hospital CMR scan at Hippokration Hospital, Athens, Greece, from the start of CMR clinical service implementation in January 2020 up to March 2023. The study included only hospitalized patients who underwent an urgent CMR scan, excluding outpatients. No additional inclusion criteria were applied.
Among the 535 CMR scans conducted during this period, 103 were performed on an urgent basis for hospitalized patients with acute cardiac symptomatology as part of their diagnostic algorithm. These scans were selected for further analysis. Demographic characteristics such as age, sex, and ethnicity, along with the CMR date, admission cause, diagnosis, and relevant electrocardiographic findings, were documented. Functional CMR parameters and laboratory markers, e.g., high-sensitivity Troponin I (hs-Troponin I), B-type Natriuretic Peptide (BNP)/N-terminal pro-B-type Natriuretic Peptide (NT-proBNP), and high-sensitivity C-reactive Protein (hs-CRP), were also recorded when available.
The objective of the study was to assess the diagnostic and reclassification value of CMR in acutely ill cardiac patients, with a specific focus to explore its yield for cardiomyopathies in this special population of hospitalized patients with acute clinical presentation that remained without a definitive diagnosis after their initial diagnostic work-up.
2.2. CMR Imaging
All CMR scans of hospitalized patients were retrospectively reviewed by Level 3 CMR experts blinded to the initial or final clinical diagnosis. Studies that were not eligible for analysis for technical reasons (i.e., missing images or bad image quality) were omitted, and the corresponding patients were excluded from the analysis (n = 4). The analysis sought to identify potential underlying cardiomyopathies and classify the specific type for each patient. The diagnoses were determined solely based on the findings from the CMR images, as evaluated by a Level 3 CMR specialist. No additional data from the patients’ laboratory studies or medical history were utilized to establish the final diagnosis. Reclassification was defined by comparing the initial cause of admission with the diagnosis derived from the CMR findings.
2.3. CVI42 Measurements
A quantitative analysis of ventricular volumes, systolic function, and myocardial mass was performed using the CVI42 v6.1 software. Standard CMR-derived parameters included left and right ventricular end-diastolic and end-systolic volumes, stroke volumes, ejection fractions, and cardiac output. Myocardial mass was assessed in both the systole and diastole. The presence of Late gadolinium enhancement (LGE) in PSIR images was also evaluated to identify the presence of myocardial fibrosis. The use of this software ensured reproducibility in the assessment of cardiac function and tissue characterization.
2.4. Statistical Analysis
All statistical analyses were performed using the GraphPad Prism 10.1.1 Software. The frequencies of all variables were calculated, and data were categorized accordingly. Continuous variables, due to their skewed and asymmetric distribution, are presented as medians, along with interquartile ranges (IQRs), to provide a summary of the central tendency and variability. Categorical variables are expressed as percentages, offering a depiction of the distribution across groups. Comparisons between groups were carried out using statistical tests based on data type and distribution. Non-parametric methods were employed for continuous variables due to the lack of normality, while chi-square or Fisher’s exact tests were used for categorical data. Statistical significance was defined as a p-value < 0.05.
3. Results
3.1. Patient Demographics and Causes of Hospital Admission
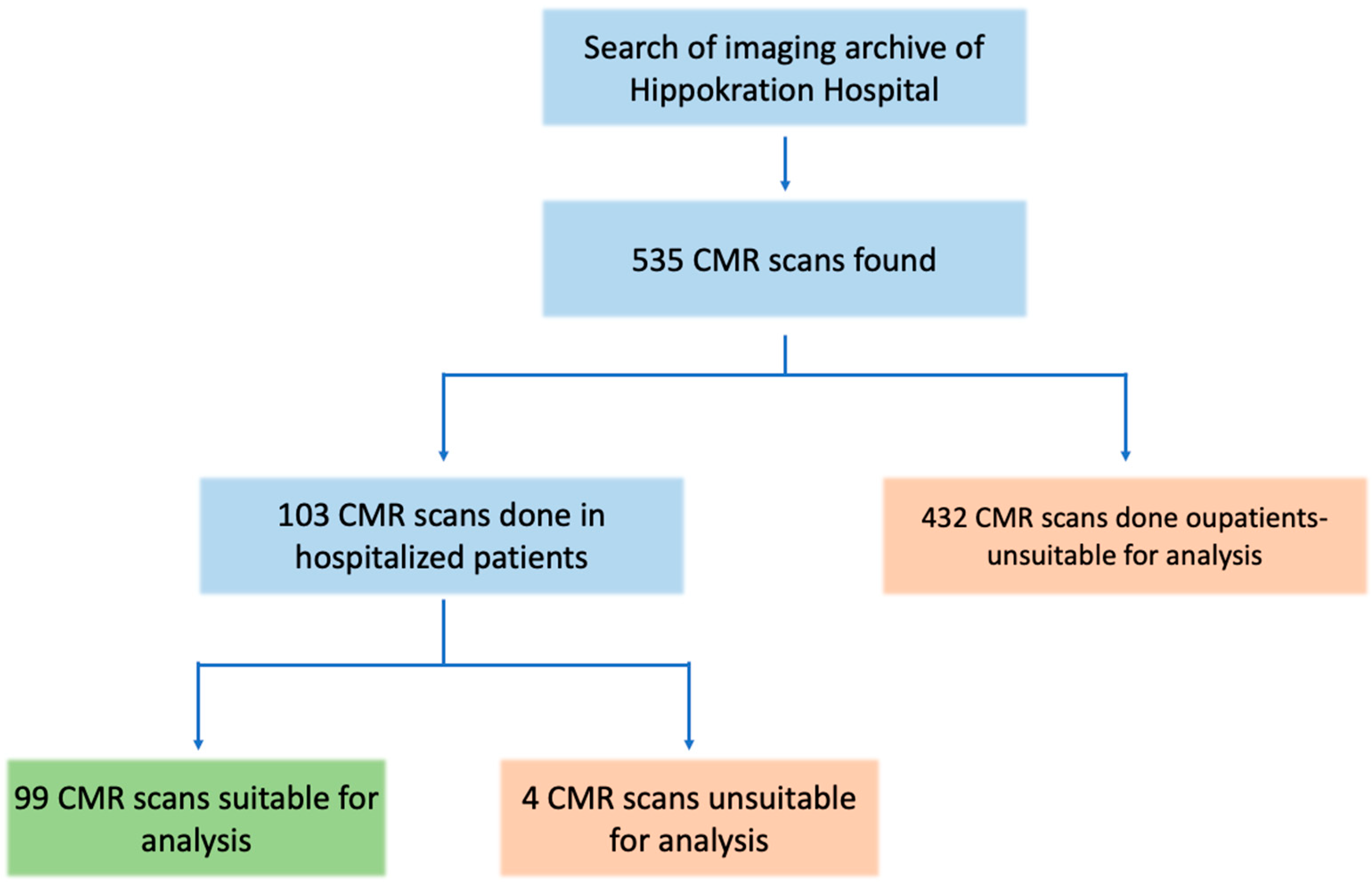
A total of 535 CMR scans were retrieved from the hospital’s archive search. Of these, 103 scans were performed with hospitalized patients. Four scans were excluded from the analysis due to the lack of gadolinium contrast or missing LGE images. The remaining 99 scans, all from unique inpatients, were included in the study for further analysis (Figure 1). The median age of this in-patient sample was 47 years (IQR: 31–63), and 35% of the patients were female. Detailed demographic information, along with data on CMR findings and biomarkers for the study population, are presented in Table 1.
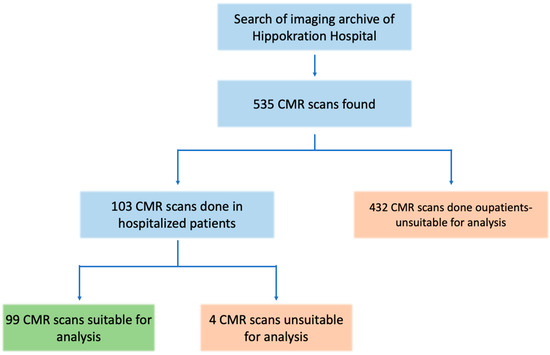
Figure 1.
Study flowchart. Out of the initial 535 CMR scans, 99 scans were included in the analysis of hospitalized patients with acute cardiac events.

Table 1.
Demographic and clinical characteristics of hospitalized patients included in the analysis.
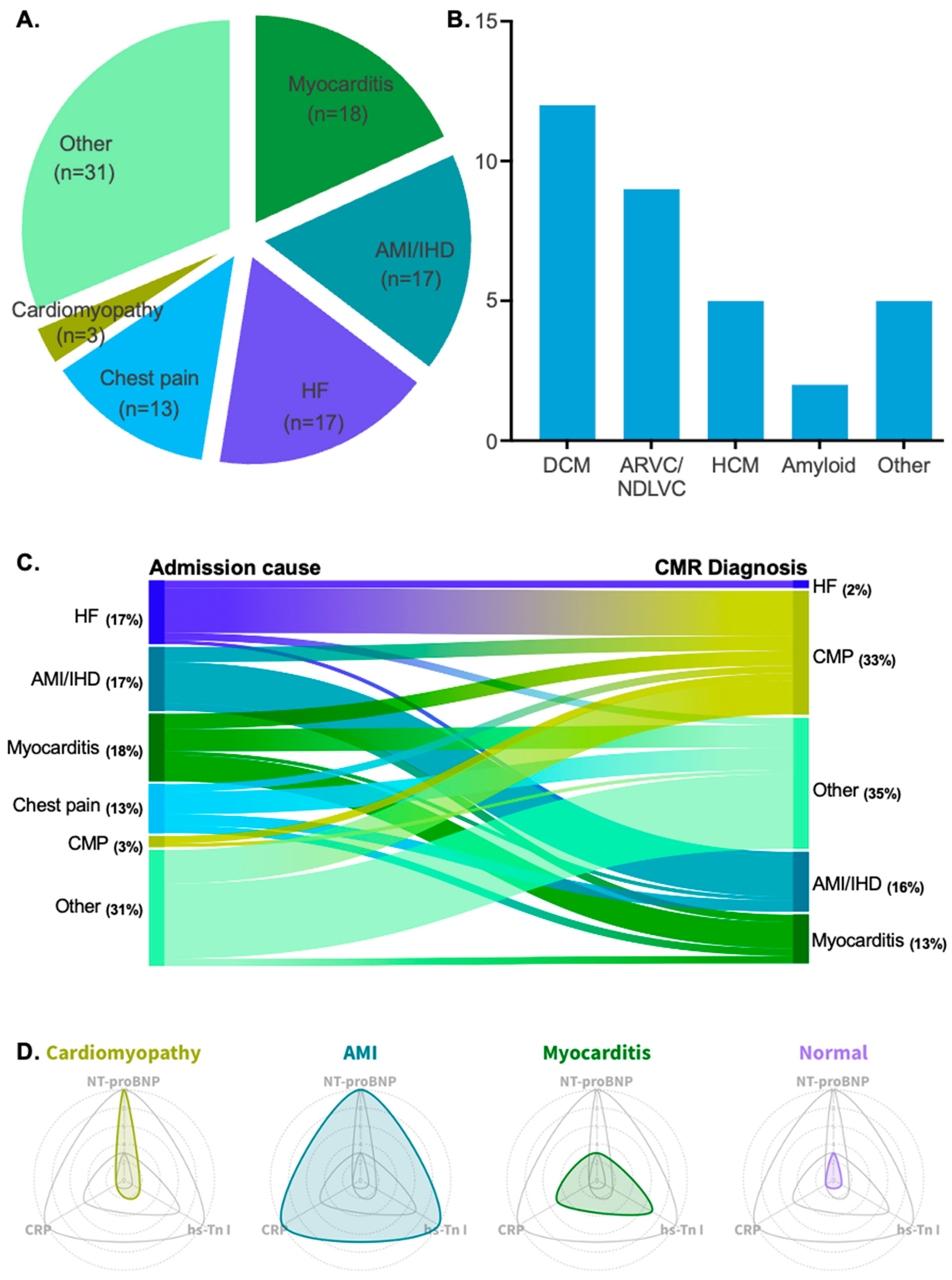
Among the clinical indications for hospitalization, possible myocarditis was the most common cause of admission, accounting for 18% of cases. Acute myocardial infarction (AMI)/ischemic heart disease (IHD) and heart failure HF were the second most common causes of admission, each accounting for 17%. Chest pain followed, with a frequency of 13%. Lastly, cardiomyopathy as the primary cause of admission was reported in 3% of cases. All patient admission causes are depicted in Figure 2A.
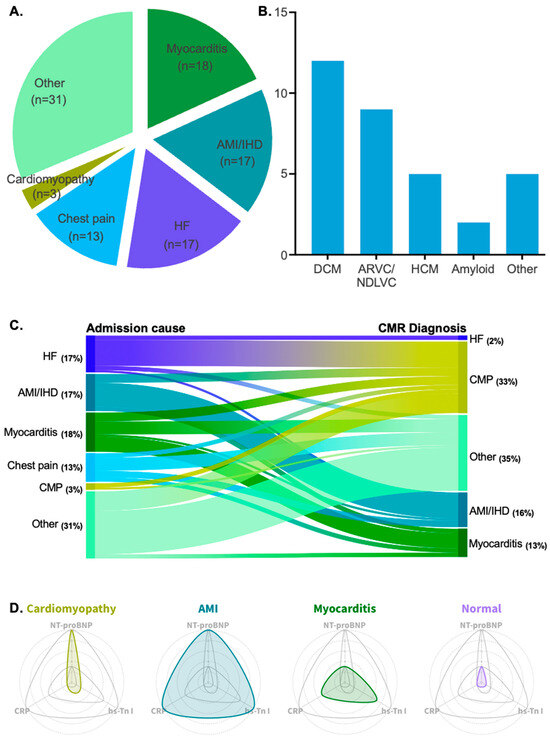
Figure 2.
(A) Pie chart showing the patient cohort divided by admission cause. (B) Bar chart showing all diagnosed cardiomyopathy types with their corresponding patient count. (C) Alluvial plot showing the reclassification of diagnoses after the implementation of CMR imaging. (D) Radar plots showing the fluctuation of hs-Tn I, NT-proBNP, and CRP in four different patient groups: cardiomyopathy patients, AMI/IHD patients, myocarditis patients, and patients with normal CMR scans.
3.2. Clinical Diagnosis via CMR
Retrospective analysis via CMR scans revealed that 33% (n = 33) of patients exhibited findings indicative of underlying cardiomyopathy. Among these patients, dilated cardiomyopathy was the most prevalent clinical entity (36%, n = 12). Arrhythmogenic cardiomyopathy (arrhythmogenic right ventricular cardiomyopathy or left-dominant forms in the non-dilated left ventricular cardiomyopathy spectrum) and hypertrophic cardiomyopathy were the second and third most frequent types, with frequencies of 27% (n = 9) and 15% (n = 5), respectively. Other rarer forms of cardiomyopathy included cardiac sarcoidosis, eosinophilic granulomatosis with polyangiitis, and endomyocardial fibrosis. All types of diagnosed cardiomyopathies are listed in Table 2 and illustrated in Figure 2B.

Table 2.
Types of cardiomyopathies diagnosed with CMR.
Additionally, myocarditis was the most probable diagnosis in 13% (n = 13) of the cases, while AMI/IHD accounted for 8% (n = 8) of diagnoses. The absence of any abnormal CMR findings was recorded in 26% (n = 26) of the scans, which were considered normal scans. Lastly, 19% (n = 19) of the CMR scans were suggestive of other diagnoses (e.g., pericarditis, valvular heart disease, or vasculitis with cardiac involvement).
3.3. Reclassification of Clinical Diagnosis via CMR
Interestingly, patients from all main admission categories were significantly reclassified into different diagnostic categories after the implementation of CMR (chi2 = 104.1, p < 0.0001). In total, 32% (n = 31) of patients with reported admission causes of heart failure, chest pain, AMI/IHD, acute myocarditis, or other cardiac events (e.g., arrhythmia, pericarditis, or conduction disorders) were reclassified as having an acute presentation of cardiomyopathy.
The highest reclassification rate was observed among patients admitted for HF decompensation. In 71% (n = 12) of these patients, underlying probable cardiomyopathy was identified. Patients with an initial diagnosis of AMI/IHD were reclassified into a cardiomyopathy diagnosis at an estimated frequency of 24% (n = 4). Patients who were admitted to the hospital for a myocarditis episode were found to have underlying cardiomyopathy at a frequency of 22% (n = 4). Lastly, a substantial percentage of patients admitted for chest pain were reclassified after CMR towards an acute presentation of underlying cardiomyopathy (15%, n = 2), and an equal percentage was reclassified as having an acute myocarditis episode (15%, n = 2). The reclassification rate achieved via CMR is depicted in Figure 2C. Examples of CMR images with underlying non-ischemic cardiomyopathy in the studied population are depicted in Figure 3.
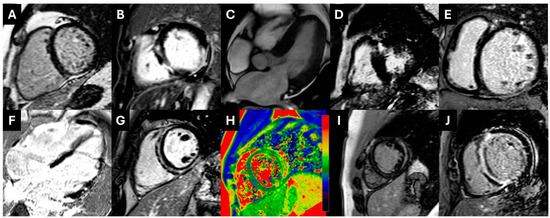
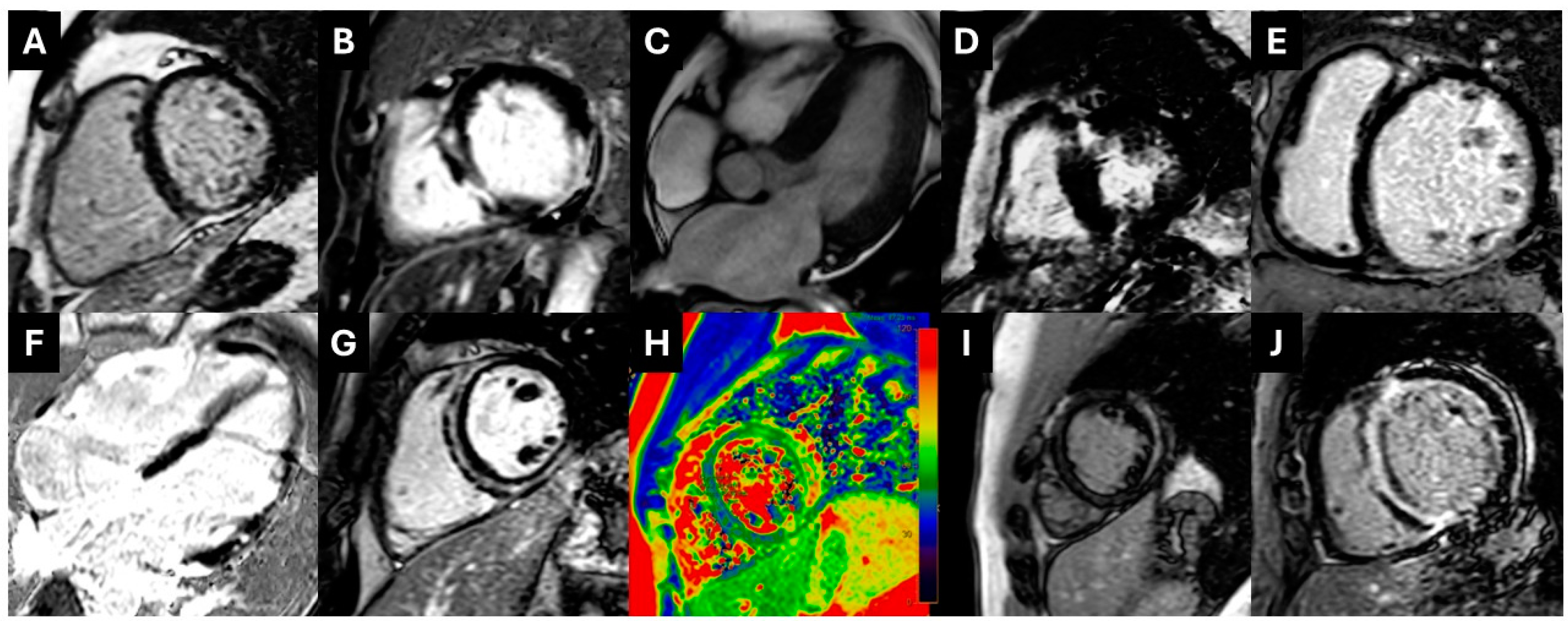
Figure 3.
(A). PSIR images showing subepicardial inferior wall LGE extending to the right ventricle compatible with ARVC with biventricular involvement. (B). Transmural lateral wall plus midwall LGE in the interventricular septum in the setting of NDLVC. (C,D). Patient with CMR findings compatible with HOCM; symmetrical concentric LV hypertrophy (C) and presence of extensive LGE in the lower interventricular insertion point (D). (Ε). Full-blown DCM with midwall LGE in the IVS. (F). Extensive–diffuse LGE in multiple myocardial walls compatible with sarcoid cardiomyopathy. (G,H). Patient with ring-like subepicardial and midwall LGE compatible with left-dominant ACM (NDLVC) (G) and myocardial edema in T2-Mapping (H). (I). Subepicardial LGE in the mid-lateral wall and intramural LGE in the interventricular septum compatible with myocarditis and possible underlying cardiomyopathy. (J). Diffuse subendocardial to full-thickness LGE in a patient with cardiac amyloidosis. ACM: arrhythmogenic cardiomyopathy; ARVC: arrhythmogenic right ventricular cardiomyopathy; DCM: dilated cardiomyopathy; HOCM: hypertrophic obstructive cardiomyopathy; IVS: interventricular septum; NDLVC: non-dilated left ventricular cardiomyopathy; LGE: late gadolinium enhancement; LV: left ventricular.
3.4. Biomarker Values Among Different Patient Groups
A distinct biomarker pattern was identified among the different patient groups based on CMR findings. Patients exhibited varying levels of hs-Troponin I, NT-proBNP, and CRP, with significant differences observed between those with cardiomyopathy, myocarditis, AMI/IHD, and normal CMR scans. Cardiomyopathy patients had significantly lower hs-Troponin I levels than those with myocarditis and AMI/IHD (p < 0.01), highlighting a clear differentiation based on troponin levels. NT-proBNP levels were higher in cardiomyopathy patients compared to those with myocarditis, even though the difference did not reach statistical significance (p = 0.21). CRP levels were also lower in cardiomyopathy patients compared to myocarditis and AMI/IHD groups, with both differences reaching statistical significance (p < 0.05). These biomarker variations suggest a distinct inflammatory and myocardial injury profile in cardiomyopathy. A detailed comparison of biomarker values is presented in Table 3, while Figure 2D illustrates the observed pattern and its diagnostic implications.

Table 3.
Comparison of cardiac biomarkers among patient groups based on CMR findings.
4. Discussion
The findings of the present study suggest that a significant proportion of acutely ill cardiac patients with an indication to undergo a CMR to aid in the diagnostic assessment had findings indicative of underlying cardiomyopathy. Referral for a CMR scan at an urgent in-hospital setting frequently led to a reclassification of a diagnosis among hospitalized patients with an acute cardiac presentation, and almost one-third of these patients were diagnosed with probable/definite cardiomyopathy.
Dilated cardiomyopathy was identified as the most commonly diagnosed type among cardiomyopathies, consistent with the existing literature that identifies it as the most prevalent form [26,27,28]. Interestingly, the second most commonly diagnosed cardiomyopathy in this group of patients was arrhythmogenic cardiomyopathy (right- or left-dominant forms), a disease whose prevalence in the general population is considered significantly lower [29,30]. This is especially important, as myocarditis episodes can frequently serve as the initial clinical presentation of underlying arrhythmogenic cardiomyopathy [31,32,33]. Therefore, heightened awareness regarding the early use of CMR imaging is crucial for the timely diagnosis of such patients [34,35].
Cardiac biomarkers such as hs-troponin I, NT-proBNP, and CRP are not disease-specific biomarkers, but they can aid in the diagnostic work-up of myocardial diseases [36]. In this study, a specific pattern pointing to a cardiomyopathy diagnosis was identified. Acutely ill cardiac patients with underlying cardiomyopathy exhibit significantly elevated NT-proBNP levels, accompanied by only mildly elevated hs-Troponin I and CRP levels [37]. This combination can increase the clinician’s suspicion for the existence of underlying cardiomyopathy, as the main alternative diagnoses show different elevation patterns for these three biomarkers [38,39,40].
CMR can also inform treatment decisions, thereby improving patient prognosis. In the absence of CMR implementation, patients admitted for heart failure decompensation would likely be treated symptomatically, without the underlying condition being addressed [41]. Latent cardiomyopathies would remain undiagnosed, meaning that no risk stratification for these patients would be performed, and life-saving interventions, like the implantation of an implantable cardioverter–defibrillator, would not be considered [20].
MINOCA is a heterogeneous group of diseases, making specific diagnoses challenging [42]. Definitive diagnoses of patients presenting with MINOCA are often unachievable without the use of CMR, and individualized treatment, like dual-antiplatelet therapy and angiotensin-converting enzyme inhibitors in patients with evidence of an ischemic scar/NSTEMI, as indicated through CMR, would not be initiated on time [43]. Conversely, in patients presenting with MINOCA or those initially diagnosed with myocardial infarction, CMR can reveal non-ischemic cardiac events (e.g., myocarditis or an acute presentation of cardiomyopathy). In such cases, CMR prevents unnecessary empirical treatment with anti-ischemic agents, which would provide no benefit [23]. These adjustments in medical management, along with the frequent need for additional diagnostic testing prompted by CMR findings, highlight the crucial role of the modality in managing hospitalized patients [44].
Lastly, without the implementation of CMR, patients presenting with myocarditis episodes linked to underlying cardiomyopathy may remain undiagnosed and receive treatment solely as a myocarditis episode, with a failure to address the underlying cause. Identifying cardiomyopathies through CMR can guide therapeutic interventions, thereby enhancing prognoses and reducing the risk of acute cardiac events such as sudden cardiac death or heart failure decompensation [45].
5. Limitations
This study involved limitations that should be considered when interpreting the results. First, it was a single-center, retrospective analysis conducted at Hippokration Hospital, Athens, Greece. This limits the generalizability of the findings to other healthcare settings and populations. The study was performed at a single tertiary care center, which may have inherent biases related to the referral patterns and patient characteristics specific to this institution. As such, the results may not fully reflect the diagnostic yield of CMR in other hospital settings, particularly in non-tertiary hospitals or community-based care environments. Furthermore, the patient cohort in this study was relatively small. While this number provides valuable insights, larger multicenter studies with a more diverse sample population are needed to confirm the findings and assess the applicability of CMR in broader patient populations. A larger cohort would also help address any statistical power issues and allow for more robust subgroup analyses. Lastly, as part of a retrospective study, the data were collected from existing medical records, which may be subject to documentation errors or incomplete information. The reliance on archived clinical data may have limited the ability to account for all relevant factors, such as variations in clinical management, patient comorbidities, or the potential influence of unmeasured variables. Additionally, patients with poor image quality or missing data were excluded from the analysis, which could have resulted in selection bias, potentially affecting the study’s findings. Lastly, electrocardiographic data were inadequately documented, and echocardiographic data were absent from the available patient records. As a result, these data were not included in the analysis.
6. Conclusions
In hospitalized patients with acute cardiac presentation who require urgent CMR imaging as part of their diagnostic work-up, the diagnostic yield for cardiomyopathies is substantial. The reclassification rate of CMR is significant, with almost one-third of patients with acute cardiac presentation having findings of underlying cardiomyopathy in this cohort. In-hospital CMR enables diagnostic reclassification, as well as the timely identification of underlying cardiomyopathy as the cause of acute cardiac presentation, and it informs therapeutic decisions. The setting up of a clinical CMR service for cardiology inpatients at a tertiary hospital center helps with the diagnosis of common and rarer forms of cardiomyopathy before hospital discharge.
Author Contributions
Conceptualization, A.A., A.K. (Alexandros Kasiakogias) and C.V.; Methodology, T.T.; Investigation, T.T.; Resources, T.T., A.M., A.K. (Antonia Kolovou) and E.P.; Data curation, T.T.; Writing—original draft, T.T.; Supervision, A.A., A.K. (Alexandros Kasiakogias), G.L., K.T. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hippokration Hospital (40323, 02/05/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AMI: acute myocardial infarction; BNP: B-type Natriuretic Peptide; CMR: cardiovascular magnetic resonance; CRP: C-reactive Protein; ESC: European Society of Cardiology; HF: heart failure; hs-Troponin I: High-sensitivity Troponin I; ICD: implantable cardioverter–defibrillator; IHD: ischemic heart disease; IQR: interquartile range; MINOCA: myocardial infarction with non-obstructive coronary arteries; NSTEMI: non-ST-elevation myocardial infarction; NT-proBNP: N-terminal pro-B-type Natriuretic Peptide.
References
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef]
- Tsatsopoulou, A.; Protonotarios, I.; Xylouri, Z.; Papagiannis, I.; Anastasakis, A.; Germanakis, I.; Patrianakos, A.; Nyktari, E.; Gavras, C.; Papadopoulos, G.; et al. Cardiomyopathies in children: An overview. Hellenic J. Cardiol. 2023, 72, 43–56. [Google Scholar] [CrossRef]
- Sinagra, G.; Carriere, C.; Clemenza, F.; Minà, C.; Bandera, F.; Zaffalon, D.; Gugliandolo, P.; Merlo, M.; Guazzi, M.; Agostoni, P. Risk stratification in cardiomyopathy. Eur. J. Prev. Cardiol. 2020, 27 (Suppl. 2), 52–58. [Google Scholar] [CrossRef]
- Zegkos, T.; Tziomalos, G.; Parcharidou, D.; Ntelios, D.; Papanastasiou, C.A.; Karagiannidis, E.; Gossios, T.; Rouskas, P.; Katranas, S.; Paraskevaidis, S.; et al. Validation of the new American College of Cardiology/American Heart Association Guidelines for the risk stratification of sudden cardiac death in a large Mediterranean cohort with Hypertrophic Cardiomyopathy. Hellenic J. Cardiol. 2022, 63, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Towbin, J.A. Dilated cardiomyopathy. Lancet 2010, 375, 752–762. [Google Scholar] [CrossRef]
- Lukas Laws, J.; Lancaster, M.C.; Ben Shoemaker, M.; Stevenson, W.G.; Hung, R.R.; Wells, Q.; Brinkley, D.M.; Hughes, S.; Anderson, K.; Roden, D.; et al. Arrhythmias as Presentation of Genetic Cardiomyopathy. Circ. Res. 2022, 130, 1698–1722. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Andrews, L.; Chokshi, N.; Owens, A.T.; Munson, T.; Mehr, L.; Chowns, J.; Deo, R. Desmoplakin-Related Cardiomyopathy Presenting with Syncope And Elevated Troponins. J. Am. Coll. Cardiol. 2024, 83 (Suppl. 13), 2724. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Xintarakou, A.; Protonotarios, A.; Lazaros, G.; Miliou, A.; Tsioufis, K.; Vlachopoulos, C. Imagenetics for Precision Medicine in Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2024, 17, e004301. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Almogheer, B.; Azzu, A.; Alati, E.; Papagkikas, P.; Cheong, J.; Clague, J.; Wechalekar, K.; Baksi, J.; Alpendurada, F. Typical and atypical imaging features of cardiac amyloidosis. Hellenic J. Cardiol. 2021, 62, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Kallifatidis, A.; Kourtidou, S.; Lama, N.; Christidi, A.; Detorakis, E.; Chatzantonis, G.; Vrachliotis, T.; Karamitsos, T.; Kouskouras, K.; et al. Cardiovascular magnetic resonance for the evaluation of patients with cardiovascular disease: An overview of current indications, limitations, and procedures. Hellenic J. Cardiol. 2023, 70, 53–64. [Google Scholar] [CrossRef]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Bazmpani, M.A.; Tziatzios, I.; Toumpourleka, M.; Karvounis, C.; Karamitsos, T.D. Double-chambered left ventricle characterized by CMR. Hellenic J. Cardiol. 2017, 58, 459–460. [Google Scholar] [CrossRef]
- Ugander, M.; Bagi, P.S.; Oki, A.J.; Chen, B.; Hsu, L.Y.; Aletras, A.H.; Shah, S.; Greiser, A.; Kellman, P.; Arai, A.E. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Antoniou, C.K.; Stillman, A.; Lalude, O.; Henry, T.; Lerakis, S. Myocardial fibrosis detected with Gadolinium Delayed Enhancement in Cardiac Magnetic Resonance Imaging and Ventriculoarterial Coupling alterations in patients with Acute Myocarditis. Hellenic J. Cardiol. 2016, 57, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Tsampras, T.; Lazaros, G.; Tsioufis, K.; Vlachopoulos, C. A phenomap of TTR amyloidosis to aid diagnostic screening. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Antonopoulos, A.; Tsampras, T.; Ioannides, M.; Eftychiou, C.; Panagiotopoulos, I.; Naka, K.; Zacharoulis, A.; Ktenopoulos, N.; Katsimaglis, G.; Vavouranakis, E.; et al. Insights into cardiac amyloidosis screening in TAVI from a Greek/Cypriot Cohort: The GRECA-TAVI registry. Eur. Heart J. 2024, 45 (Suppl. 1), ehae666. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Liang, K.; Nakou, E.; Del Buono, M.G.; Montone, R.A.; D’Amario, D.; Bucciarelli-Ducci, C. The Role of Cardiac Magnetic Resonance in Myocardial Infarction and Non-obstructive Coronary Arteries. Front Cardiovasc. Med. 2021, 8, 821067. [Google Scholar] [CrossRef] [PubMed]
- Daneshrad, J.A.; Ordovas, K.; Sierra-Galan, L.M.; Hays, A.G.; Mamas, M.A.; Bucciarelli-Ducci, C.; Parwani, P. Role of Cardiac Magnetic Resonance Imaging in the Evaluation of MINOCA. J. Clin. Med. 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ferrari, V.A.; Han, Y. Cardiovascular Magnetic Resonance Imaging and Heart Failure. Curr. Cardiol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Andreini, D.; Dello Russo, A.; Pontone, G.; Mushtaq, S.; Conte, E.; Perchinunno, M.; Guglielmo, M.; Santos, A.C.; Magatelli, M.; Baggiano, A.; et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients With Normal Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 410–421. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers. 2019, 5, 32. [Google Scholar] [CrossRef]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: From epidemiologic to genetic phenotypes: A translational review of current literature. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef]
- Huggins, G.S.; Kinnamon, D.D.; Haas, G.J.; Jordan, E.; Hofmeyer, M.; Kransdorf, E.; Ewald, G.A.; Morris, A.A.; Owens, A.; Lowes, B.; et al. Prevalence and Cumulative Risk of Familial Idiopathic Dilated Cardiomyopathy. JAMA 2022, 327, 454–463. [Google Scholar] [CrossRef]
- Pilichou, K.; Thiene, G.; Bauce, B.; Rigato, I.; Lazzarini, E.; Migliore, F.; Marra, M.P.; Rizzo, S.; Zorzi, A.; Daliento, L.; et al. Arrhythmogenic cardiomyopathy. Orphanet J. Rare Dis. 2016, 11, 33. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef]
- Tschöpe, C.; Cooper, L.T.; Torre-Amione, G.; Van Linthout, S. Management of Myocarditis-Related Cardiomyopathy in Adults. Circ. Res. 2019, 124, 1568–1583. [Google Scholar] [CrossRef]
- Bariani, R.; Cipriani, A.; Rizzo, S.; Celeghin, R.; Bueno Marinas, M.; Giorgi, B.; Gaspari, M.D.; Ritago, I.; Leoni, L.; Zorzi, A.; et al. ‘Hot phase’ clinical presentation in arrhythmogenic cardiomyopathy. EP Eur. 2020, 23, 907–917. [Google Scholar] [CrossRef]
- Bariani, R.; Rigato, I.; Cipriani, A.; Bueno Marinas, M.; Celeghin, R.; Basso, C.; Corrado, D.; Pilichou, K.; Bauce, B. Myocarditis-like Episodes in Patients with Arrhythmogenic Cardiomyopathy: A Systematic Review on the So-Called Hot-Phase of the Disease. Biomolecules 2022, 12, 1324. [Google Scholar] [CrossRef]
- Radunski, U.K.; Lund, G.K.; Stehning, C.; Schnackenburg, B.; Bohnen, S.; Adam, G.; Blankenberg, S.; Muellerleile, K. CMR in Patients With Severe Myocarditis: Diagnostic Value of Quantitative Tissue Markers Including Extracellular Volume Imaging. JACC Cardiovasc. Imaging 2014, 7, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Biesbroek, P.S.; Hirsch, A.; Zweerink, A.; van de Ven, P.M.; Beek, A.M.; Groenink, M.; Windhausen, F.; Planken, R.N.; van Rossum, A.C.; Nijveldt, R. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur. Heart J.-Cardiovasc. Imaging 2017, 19, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Pregenzer-Wenzler, A.; Abraham, J.; Barrell, K.; Kovacsovics, T.; Nativi-Nicolau, J. Utility of Biomarkers in Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; Gallagher, M.J.; Foley, M.; O’Mahony, C.; Critoph, C.; Gimeno, J.; Dawnay, A.; Mckenna, W.J.; Elliott, P.M. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2013, 34, 2529–2537. [Google Scholar] [CrossRef]
- Martens, P.; Cooper, L.T.; Tang, W.H.W. Diagnostic Approach for Suspected Acute Myocarditis: Considerations for Standardization and Broadening Clinical Spectrum. J. Am. Heart Assoc. 2023, 12, e031454. [Google Scholar] [CrossRef]
- James, S.K.; Armstrong, P.; Barnathan, E.; Califf, R.; Lindahl, B.; Siegbahn, A.; Simoons, M.L.; Topol, E.J.; Venge, P.; Wallentin, L.; et al. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: A GUSTO-IV substudy. J. Am. Coll. Cardiol. 2003, 41, 916–924. [Google Scholar] [CrossRef]
- Galvani, M.; Ottani, F.; Oltrona, L.; Ardissino, D.; Gensini, G.F.; Maggioni, A.P.; Mannucci, P.M.; Mininni, N.; Prando, M.D.; Tubaro, M.; et al. N-Terminal Pro-Brain Natriuretic Peptide on Admission Has Prognostic Value Across the Whole Spectrum of Acute Coronary Syndromes. Circulation 2004, 110, 128–134. [Google Scholar] [CrossRef]
- Roifman, I.; Hammer, M.; Sparkes, J.; Dall’Armellina, E.; Kwong, R.Y.; Wright, G. Utilization and impact of cardiovascular magnetic resonance on patient management in heart failure: Insights from the SCMR Registry. J. Cardiovasc. Magn. Reson. 2022, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claey, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Hampal, R.; Knott, K.D.; Plastiras, A.; Bunce, N.H. CMR is vital in the management of cardiology inpatients: A tertiary centre experience. Br. J. Cardiol. 2023, 30, 41. [Google Scholar]
- Bosman, L.P.; Te Riele, A. Arrhythmogenic right ventricular cardiomyopathy: A focused update on diagnosis and risk stratification. Heart 2022, 108, 90–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).