Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature
Abstract
1. Introduction
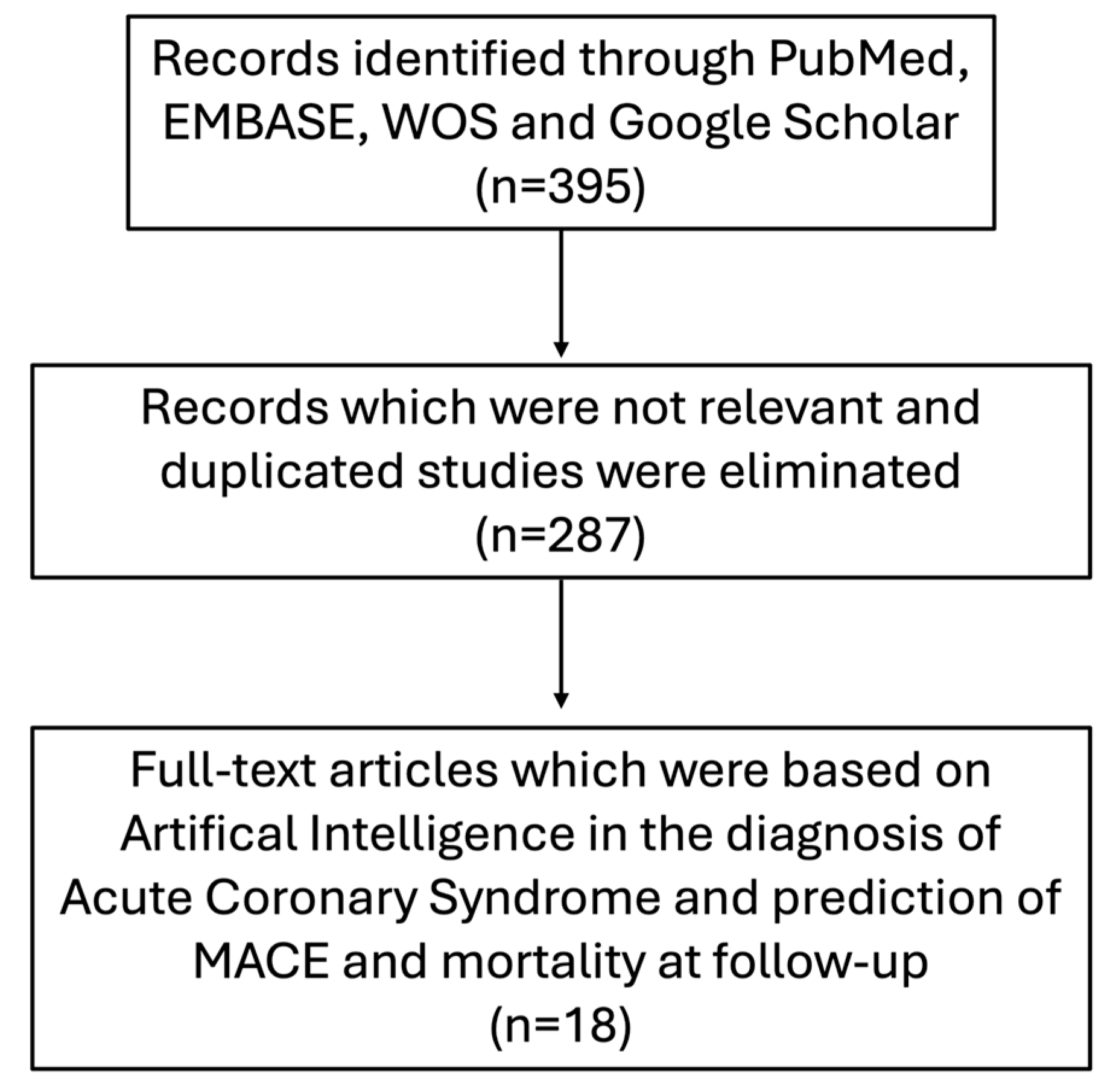
2. Materials and Methods
3. Diagnosis of ACS and Pitfalls
4. AI and Its Role in the Diagnosis of ACS
| First Author, Year of Publication, Reference No. | No. of Patients | Machine Learning Models | Data | Application | Accuracy/ AUROC/ F1-Score * |
|---|---|---|---|---|---|
| Al Zaiti et al., 2020 [31] | n = 1244 | LR GBM ANN | 12-lead ECG | Diagnosis of ACS Diagnosis of NSTE-ACS | 0.76/0.82/- 0.74/0.78/- (ML-fusion) |
| Chen et al., 2022 [32] | n = 275 | CNN-LSTM | 12-lead ECG | Diagnosis of STEMI | 0.99/0.99/0.91 |
| Zhao et al., 2020 [33] | n = 8238 | Res-Net | 12-lead ECG | Diagnosis of STEMI | 0.94/0.97/0.93 |
| Choi et al., 2022 [34] | n = 187 | CNN | 12-lead ECG | Diagnosis of STEMI | 0.86/0.91/- |
| Herman et al., 2024, [35] | n = 12,765 | DNN | 12-lead ECG | Diagnosis of OMI | 0.91/0.94/- |
| Liu et al., 2021 [36] | n = 77,799 | DLM | 12-lead ECG 12-lead ECG + hs-cTnI | Diagnosis of STEMI Diagnosis of NSTEMI | 0.96/0.97/- 0.95/0.98/- |
| Wu et al., 2019 [37] | n = 268 | ANN | Clinical, laboratory and 12-lead ECG data | Diagnosis of NSTEMI | 0.93/0.98/- |
| Qin et al., 2023 [38] | n = 2878 | SVM XGBoost RF NB LR GBM | Clinical, laboratory and 12-lead ECG data | Diagnosis of NSTEMI | 0.95/0.97/0.96 (XGBoost) |
| Berikol et al., 2016 [39] | n = 228 | SVM NB LR | Clinical, laboratory, echocardiogra-phic and 12-lead ECG data | Diagnosis of ACS | 0.99/-/1 (SVM) |
| Than et al., 2019 [40] | n = 11,011 | GBM | Clinical data and hs-cTnI | Diagnosis of type 1 MI | 0.97/0.96/- |
| Doudedis et al., 2023 [41] | n = 20,324 | XGBoost | Clinical, laboratory data and hs-cTnI | Diagnosis of MI | 0.94/0.95/- |
| Kayvanpour et al., 2021 [42] | n = 148 | ANN | Micro-RNAs (10 miRNA analyzed) | Diagnosis of ACS | 0.96/0.99/- |
5. AI and Its Role in Predicting Major Adverse Cardiac Events (MACE) in Patients with ACS
| First Author, Year of Publication, Reference No. | No. of Patients | ML Models | Outcome Predicted | Performance (AUROC/F1-Score) * |
|---|---|---|---|---|
| Khera et al., 2021 [44] | n = 755,402 | XGBoost ANN Meta-classifier | In-hospital mortality | XGBoost: 0.90/0.43 ANN: 0.88/0.41 Meta-classifier: 0.89/0.43 |
| Hadanny et al., 2021 [45] | n = 25,709 | RF | 30-day mortality | RF: 0.80/- |
| Sherazi et al., 2020 [46] | n = 8227 | GBM DNN RF GLM | 1-year mortality | GBM: 0.90/0.97 DNN: 0.90/0.95 RF: 0.89/0.96 GLM: 0.87/0.96 |
| Sherazi et al., 2020 [47] | n = 11,189 | SVE ET RF GBM | MACE | SVE: 0.99/0.91 ET: 0.99/0.90 RF: 0.98/0.90 GBM: 0.98/0.85 |
| D’Ascenzo et al., 2021 [48] | n = 19,826 | PRAISE score (AdaBoost) | 1-year mortality Recurrent MI Major bleeding | AdaBoost: 0.92/- AdaBoost: 0.81/- AdaBoost: 0.86/- |
| Mohammad et al., 2022 [49] | n = 139,288 | ANN | 1-year mortality 1-year HF hospitalization | ANN: 0.84/- ANN: 0.78/- |
| Lee et al., 2021 [50] | n = 14,183 | RF SVM XGBoost Lasso LR Ridge LR Elastic net LR | In-hospital mortality 3-month mortality 1-year mortality | STEMI—In-hospital mortality RF 0.92/0.09 SVM 0.87/0.07 XGBoost 0.94/0.11 Lasso LR 0.92/0.12 Ridge LR 0.92/0.08 Elastic net LR 0.92/0.12 |
| STEMI—1-year mortality RF 0.77/0.03 SVM 0.69/0.02 XGBoost 0.80/0.04 Lasso LR 0.79/0.05 Ridge LR 0.79/0.04 Elastic net LR 0.79/0.04 | ||||
| NSTEMI—In-hospital mortality RF 0.92/0.10 SVM 0.85/0.06 XGBoost 0.91/0.10 Lasso LR 0.92/0.01 Ridge LR 0.92/0.10 Elastic net LR 0.92/0.10 | ||||
| NSTEMI—1-year mortality RF 0.79/0.10 SVM 0.72/0.08 XGBoost 0.81/0.11 Lasso RF 0.82/0.10 Ridge RF 0.81/0.10 Elastic net RF 0.81/0.10 |
6. Challenges and Future Directions
- Technical challenges: First, training neural networks requires a large amount of data to be accessible, and, in this regard, lacking data remains a challenge. Data collection and storage remain challenging, requiring innovative tools and collaborations among multiple centers to acquire enough data to train high-performance models. Furthermore, deep learning allows deep relationships between data to be formulated. Still, this form of learning is highly dependent on the quality and reliability of the data to which it is exposed: any pre-existing systematic errors in the source could lead to a risk of perpetuating them. Neural networks are highly vulnerable to minimal perturbations in the data (black box nature). For example, the change of a few pixels in an input image, imperceptible to the human observer, could lead a well-validated CNN to make a radical error in data classification, resulting in an incorrect output. The quality of data obtainable in clinical practice on which an ML model is tested and validated is a further issue: the models are often derived from high-quality databases with meticulously obtained ECGs, so the application of such obtained models in the emergency setting, where the quality of ECG acquisition is not always the best, could be a problem. Moreover, there is a concern that these algorithms may not be generalizable to diverse patient populations (e.g., different ethnicities) and thus will require more rigorous validation across healthcare systems.
- Ethical and legal challenges: Data ownership remains another unresolved issue: the use of each patient’s data within the ML could be seen as a potential privacy violation; the exchange of patient data between various research centers around the world is a complex process that raises concerns about the security and protection of sensitive patient information that could be susceptible to cyber-attacks. It should also be considered that the use of AI in medical decision-making has not yet been legally defined and correctly regularized, leaving numerous debates open in multiple scenarios. For instance, in the event of misclassification by the AI model, in the absence of a precise regulation, the question of to whom the liability belongs must be addressed—to the physician using the model, to the programmer, or to AI itself—with the latter, nowadays, still not being recognized as a legal entity, thus necessitating more clarity by regulatory and health agencies (the AI Act from the European Union AI Office still pending).
- Clinical implementation challenges: It is crucial to cautiously integrate these advanced tools with physician decision-making. While AI has shown promise in enhancing diagnostic accuracy, streamlining workflows, and offering prognostic forecasting, it cannot be seamlessly incorporated into our daily clinical practice. One of the primary hurdles is ensuring that AI tools are user-friendly and compatible with existing clinical infrastructure, such as Electronic Medical Records (EMRs) systems, to minimize disruptions and optimize efficiency. Moreover, AI’s possible role in decision-making raises concerns about the potential erosion of physicians’ clinical autonomy. To address these challenges, there is an urgent need for robust training programs that equip healthcare professionals with the skills to effectively use AI systems. These should include not only training on how to deal with AI tools, but also on how to critically assess and integrate AI recommendations into clinical practice. Physicians and other healthcare providers must understand the underlying algorithms, recognize their limitations, and develop the capability to make decisions that incorporate both human expertise and machine learning-based insights.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2021, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Kazakiewicz, D.; Townsend, N.; Huculeci, R.; Aboyans, V.; Vardas, P. Global epidemiology of acute coronary syndromes. Nat. Rev. Cardiol. 2023, 20, 778–788. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Beiser, D.G.; Cifu, A.S.; Paul, J. Evaluation and Diagnosis of Chest Pain 2022. JAMA 2022, 328, 292–293. [Google Scholar] [CrossRef]
- Agzew, Y. Elevated serum cardiac troponin in non-acute coronary syndrome. Clin. Cardiol. 2009, 32, 15–20. [Google Scholar] [CrossRef]
- Kilic, A. Artificial Intelligence and Machine Learning in Cardiovascular Health Care. Ann. Thorac. Surg. 2020, 109, 1323–1329. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer. Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC) Ibanez * †, (Chairperson) (Spain), and ESC Scientific Document Group 2023. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Diercks, D.B.; Peacock, W.F.; Hiestand, B.C.; Chen, A.Y.; Pollack, C.V., Jr.; Kirk, J.D.; Smith, S.C., Jr.; Gibler, W.B.; Ohman, E.M.; Blomkalns, A.L.; et al. Frequency and consequences of recording an electrocardiogram >10 minutes after arrival in an emergency room in non-ST-segment elevation acute coronary syndromes (from the CRUSADE initiative). Am. J. Cardiol. 2006, 97, 437–442. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time Delay to Treatment and Mortality in Primary Angioplasty for Acute Myocardial Infarction. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Lee, K.K.; Chapman, A.R.; Sandeman, D.; Stables, C.L.; Adamson, P.D.; Andrews, J.P.M.; et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: A stepped-wedge, cluster-randomised controlled trial. Lancet 2018, 392, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.T.; Sörensen, N.A.; Schwemer, T.; Ojeda, F.; Bourry, R.; Sciacca, V.; Schaefer, S.; Waldeyer, C.; Sinning, C.; Renné, T.; et al. Diagnosis of Myocardial Infarction Using a High-Sensitivity Troponin I 1-Hour Algorithm. JAMA Cardiol. 2016, 1, 397–404. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Bayés de Luna, A.; Fiol, M.; Nikus, K.; Macfarlane, P.; Gorgels, A.; Sionis, A.; Cinca, J.; Barrabes, J.A.; Pahlm, O.; et al. Common pitfalls in the interpretation of electrocardiograms from patients with acute coronary syndromes with narrow QRS: A consensus report. J. Electrocardiol. 2012, 45, 463–475. [Google Scholar] [CrossRef]
- Cook, D.A.; Oh, S.Y.; Pusic, M.V. Accuracy of Physicians’ Electrocardiogram Interpretations: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 1461–1471. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chen, Y.H.; Wu, K.H.; Chen, Y.C. Validation of the diagnosis and triage algorithm for acute myocardial infarction in the setting of left bundle branch block. Am. J. Emerg. Med. 2020, 38, 2614–2619. [Google Scholar] [CrossRef]
- Nestelberger, T.; Cullen, L.; Lindahl, B.; Reichlin, T.; Greenslade, J.H.; Giannitsis, E.; Christ, M.; Morawiec, B.; Miro, O.; Martín-Sánchez, F.J.; et al. Diagnosis of acute myocardial infarction in the presence of left bundle branch block. Heart 2019, 105, 1559–1567. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zhang, M.; Fu, Y.; Armstrong, P.W.; Newby, L.K.; Gibson, C.M.; Moliterno, D.J.; Van de Werf, F.; White, H.D.; Harrington, R.A.; et al. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am. Heart J. 2009, 157, 716–723. [Google Scholar] [CrossRef]
- Khan, A.R.; Golwala, H.; Tripathi, A.; Bin Abdulhak, A.A.; Bavishi, C.; Riaz, H.; Mallipedi, V.; Pandey, A.; Bhatt, D.L. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2017, 38, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Fu, P.; Jia, C.; Dong, Y.; Liang, C.; Cao, Q.; Yang, Z.; Fu, R.; Zhang, X.; Sun, Z. The delayed activation wave in non-ST-elevation myocardial infarction. Int. J. Cardiol. 2013, 162, 107–111. [Google Scholar] [CrossRef] [PubMed]
- De Winter, R.J.; Verouden, N.J.W.; Wellens, H.J.J.; Wilde, A.A.M. A New ECG Sign of Proximal LAD Occlusion. N. Engl. J. Med. 2008, 359, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Neumann, J.T.; Lindahl, B.; Giannitsis, E.; Sörensen, N.A.; Badertscher, P.; Jann, J.E.; Wussler, D.; et al. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur. Heart J. 2018, 39, 3780–3794. [Google Scholar] [CrossRef]
- Hillinger, P.; Twerenbold, R.; Wildi, K.; Rubini Gimenez, M.; Jaeger, C.; Boeddinghaus, J.; Nestelberger, T.; Grimm, K.; Reichlin, T.; Stallone, F.; et al. Gender-specific uncertainties in the diagnosis of acute coronary syndrome. Clin. Res. Cardiol. 2017, 106, 28–37. [Google Scholar] [CrossRef]
- Twerenbold, R.; Badertscher, P.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Puelacher, C.; Sabti, Z.; Rubini Gimenez, M.; Tschirky, S.; du Fay de Lavallaz, J.; et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients with Renal Dysfunction. Circulation 2018, 137, 436–451. [Google Scholar] [CrossRef]
- Anand, A.; Lee, K.K.; Chapman, A.R.; Ferry, A.V.; Adamson, P.D.; Strachan, F.E.; Berry, C.; Findlay, I.; Cruikshank, A.; Reid, A.; et al. High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction: A Stepped-Wedge Cluster Randomized Controlled Trial. Circulation 2021, 143, 2214–2224. [Google Scholar] [CrossRef]
- Reichlin, T.; Twerenbold, R.; Reiter, M.; Steuer, S.; Bassetti, S.; Balmelli, C.; Winkler, K.; Kurz, S.; Stelzig, C.; Freese, M.; et al. Introduction of high-sensitivity troponin assays: Impact on myocardial infarction incidence and prognosis. Am. J. Med. 2012, 125, 1205–1213. [Google Scholar] [CrossRef]
- Karatzia, L.; Aung, N.; Aksentijevic, D. Artificial intelligence in cardiology: Hope for the future and power for the present. Front. Cardiovasc. Med. 2022, 9, 945726. [Google Scholar] [CrossRef]
- Makimoto, H.; Kohro, T. Adopting artificial intelligence in cardiovascular medicine: A scoping review. Hypertens. Res. 2023, 47, 685–699. [Google Scholar] [CrossRef]
- Al-Zaiti, S.; Besomi, L.; Bouzid, Z.; Faramand, Z.; Frisch, S.; Martin-Gill, C.; Gregg, R.; Saba, S.; Callaway, C.; Sejdić, E. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat. Commun. 2020, 11, 3966. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Wang, Y.C.; Liu, M.H.; Tsai, B.Y.; Wu, M.Y.; Hsieh, P.H.; Wei, J.T.; Shih, E.S.C.; Shiao, Y.T.; Hwang, M.J.; et al. Artificial intelligence-assisted remote detection of ST-elevation myocardial infarction using a mini-12-lead electrocardiogram device in prehospital ambulance care. Front. Cardiovasc. Med. 2022, 9, 1001982. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, J.; Hou, Y.; Zhu, M.; Lu, Y.; Xu, Y.; Teliewubai, J.; Liu, W.; Xu, X.; Li, X.; et al. Early detection of ST-segment elevated myocardial infarction by artificial intelligence with 12-lead electrocardiogram. Int. J. Cardiol. 2020, 317, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, M.J.; Ko, Y.; Soh, M.S.; Kim, H.M.; Kim, C.H.; Lee, E.; Kim, J. Artificial intelligence versus physicians on interpretation of printed ECG images: Diagnostic performance of ST-elevation myocardial infarction on electrocardiography. Int. J. Cardiol. 2022, 363, 6–10. [Google Scholar] [CrossRef]
- Herman, R.; Meyers, H.P.; Smith, S.W.; Bertolone, D.T.; Leone, A.; Bermpeis, K.; Viscusi, M.M.; Belmonte, M.; Demolder, A.; Boza, V.; et al. International evaluation of an artificial intelligence–powered electrocardiogram model detecting acute coronary occlusion myocardial infarction. Eur. Heart J. Digit. Health 2024, 5, 123. [Google Scholar] [CrossRef]
- Liu, W.C.; Lin, C.S.; Tsai, C.S.; Tsao, T.P.; Cheng, C.C.; Liou, J.T.; Lin, W.S.; Cheng, S.M.; Lou, Y.S.; Lee, C.C.; et al. A deep learning algorithm for detecting acute myocardial infarction. EuroIntervention 2021, 17, 765–773. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, W.D.; Islam, M.M.; Poly, T.N.; Yang, H.C.; Nguyen, P.A.; Wang, Y.C.; Li, Y.J. An artificial intelligence approach to early predict non-ST-elevation myocardial infarction patients with chest pain. Comput. Methods Programs Biomed. 2019, 173, 109–117. [Google Scholar] [CrossRef]
- Qin, L.; Qi, Q.; Aikeliyaer, A.; Hou, W.Q.; Zuo, C.X.; Ma, X. Machine learning algorithm can provide assistance for the diagnosis of non-ST-segment elevation myocardial infarction. Postgrad. Med. J. 2022, 99, 442–454. [Google Scholar] [CrossRef]
- Berikol, G.B.; Yildiz, O.; Özcan, T. Diagnosis of Acute Coronary Syndrome with a Support Vector Machine. J. Med. Syst. 2016, 40, 84. [Google Scholar] [CrossRef]
- Than, M.P.; Pickering, J.W.; Sandoval, Y.; Shah, A.S.V.; Tsanas, A.; Apple, F.S.; Blankenberg, S.; Cullen, L.; Mueller, C.; Neumann, J.T.; et al. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation 2019, 140, 899–909. [Google Scholar] [CrossRef]
- Doudesis, D.; Lee, K.K.; Boeddinghaus, J.; Bularga, A.; Ferry, A.V.; Tuck, C.; Lowry, M.T.H.; Lopez-Ayala, P.; Nestelberger, T.; Koechlin, L.; et al. Machine learning for diagnosis of myocardial infarction using cardiac troponin concentrations. Nat. Med. 2023, 29, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Gi, W.T.; Sedaghat-Hamedani, F.; Lehmann, D.H.; Frese, K.S.; Haas, J.; Tappu, R.; Samani, O.S.; Nietsch, R.; Kahraman, M.; et al. microRNA neural networks improve diagnosis of acute coronary syndrome (ACS). J. Mol. Cell. Cardiol. 2021, 151, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ulvenstam, A.; Graipe, A.; Irewall, A.L.; Söderström, L.; Mooe, T. Incidence and predictors of cardiovascular outcomes after acute coronary syndrome in a population-based cohort study. Sci. Rep. 2023, 13, 3447. [Google Scholar] [CrossRef]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.L.; et al. Use of Machine Learning Models to Predict Death After Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef]
- Hadanny, A.; Shouval, R.; Wu, J.; Shlomo, N.; Unger, R.; Zahger, D.; Matetzky, S.; Goldenberg, I.; Beigel, R.; Gale, C.; et al. Predicting 30-day mortality after ST elevation myocardial infarction: Machine learning- based random forest and its external validation using two independent nationwide datasets. J. Cardiol. 2021, 78, 439–446. [Google Scholar] [CrossRef]
- Sherazi, S.W.A.; Jeong, Y.J.; Jae, M.H.; Bae, J.W.; Lee, J.Y. A machine learning-based 1-year mortality prediction model after hospital discharge for clinical patients with acute coronary syndrome. Health Inform. J. 2020, 26, 1289–1304. [Google Scholar] [CrossRef]
- Sherazi, S.W.A.; Bae, J.W.; Lee, J.Y. A soft voting ensemble classifier for early prediction and diagnosis of occurrences of major adverse cardiovascular events for STEMI and NSTEMI during 2-year follow-up in patients with acute coronary syndrome. PLoS ONE 2021, 16, e0249338. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Quadri, G.; et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): A modelling study of pooled datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Olesen, K.K.W.; Koul, S.; Gale, C.P.; Rylance, R.; Jernberg, T.; Baron, T.; Spaak, J.; James, S.; Lindahl, B.; et al. Development and validation of an artificial neural network algorithm to predict mortality and admission to hospital for heart failure after myocardial infarction: A nationwide population-based study. Lancet Digit. Health 2022, 4, e37–e45. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Woo, S.I.; Choi, S.H.; Bae, J.W.; Jung, S.; Jeong, M.H.; Lee, W.K. Machine learning enhances the performance of short and long-term mortality prediction model in non-ST-segment elevation myocardial infarction. Sci. Rep. 2021, 11, 12886. [Google Scholar] [CrossRef]
- Shavadia, J.S.; Chen, A.Y.; Fanaroff, A.C.; de Lemos, J.A.; Kontos, M.C.; Wang, T.Y. Intensive Care Utilization in Stable Patients With ST-Segment Elevation Myocardial Infarction Treated With Rapid Reperfusion. JACC Cardiovasc. Interv. 2019, 12, 709–717. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, A.; Spaccarotella, C.A.M.; Rea, F.S.; Franzone, A.; Piccolo, R.; Castiello, D.S.; Indolfi, C.; Esposito, G. Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature. Life 2025, 15, 515. https://doi.org/10.3390/life15040515
Mariani A, Spaccarotella CAM, Rea FS, Franzone A, Piccolo R, Castiello DS, Indolfi C, Esposito G. Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature. Life. 2025; 15(4):515. https://doi.org/10.3390/life15040515
Chicago/Turabian StyleMariani, Andrea, Carmen Anna Maria Spaccarotella, Francesco Saverio Rea, Anna Franzone, Raffaele Piccolo, Domenico Simone Castiello, Ciro Indolfi, and Giovanni Esposito. 2025. "Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature" Life 15, no. 4: 515. https://doi.org/10.3390/life15040515
APA StyleMariani, A., Spaccarotella, C. A. M., Rea, F. S., Franzone, A., Piccolo, R., Castiello, D. S., Indolfi, C., & Esposito, G. (2025). Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature. Life, 15(4), 515. https://doi.org/10.3390/life15040515







