Coronary Artery Spasm: From Physiopathology to Diagnosis
Abstract
1. Introduction
2. Imaging Approach of Coronary Artery Spasm
3. Risk Factors for Coronary Artery Spasm
4. Normal Coronary Endothelium
5. Coronary Spasm and Coronary Atherosclerosis—Coronary Spasm and Thrombosis
5.1. Microvascular Dysfunction
5.2. Spasm and Atherosclerosis
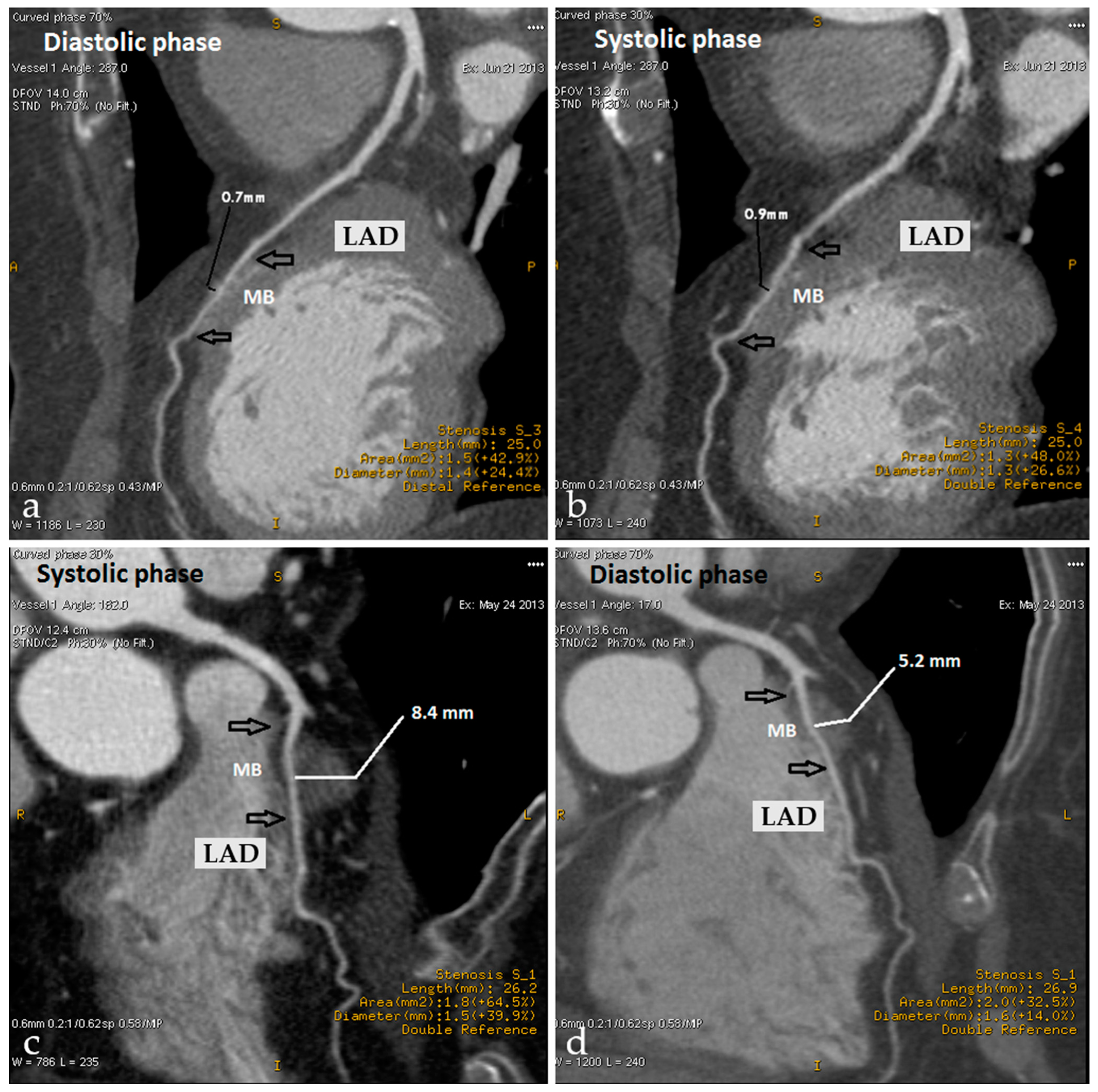
5.3. Myocardial Bridging
5.4. Spasm and Coronary Thrombosis
6. Pathophysiology of CAS
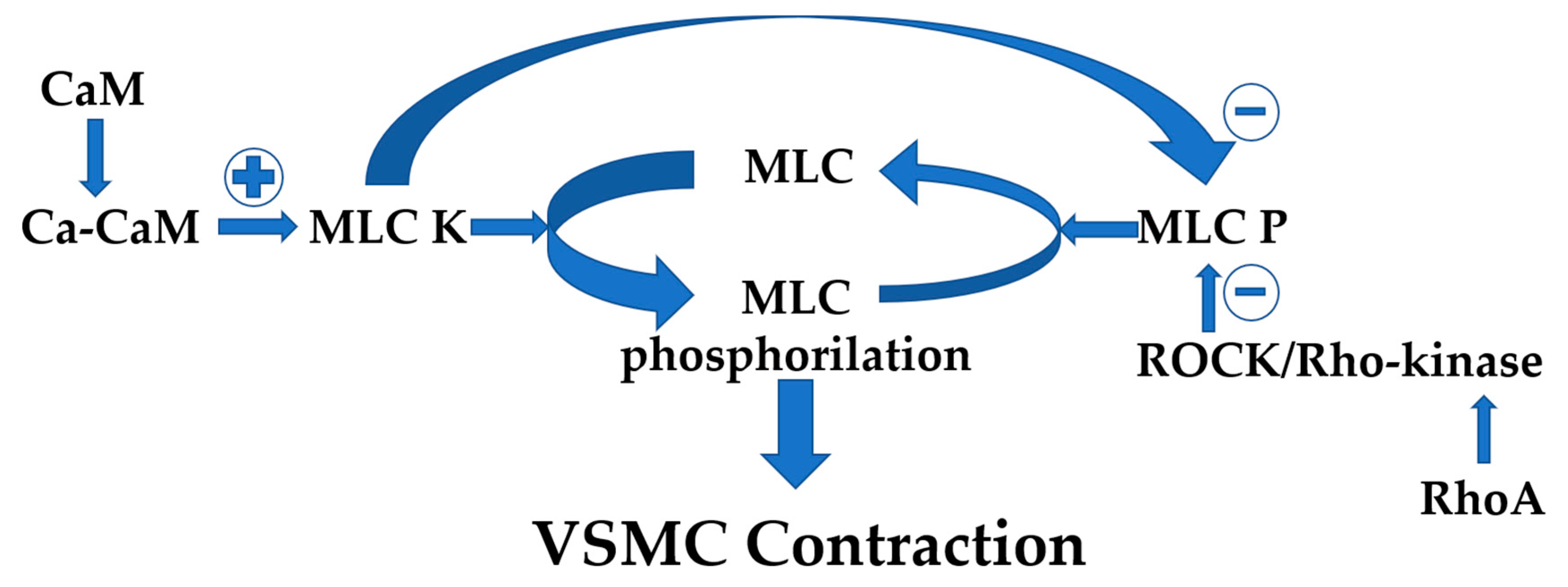
6.1. VSMCs Hypercontractility
6.2. Endothelial Dysfunction
6.3. Inflammation
6.4. Autonomic Nervous System Unbalance
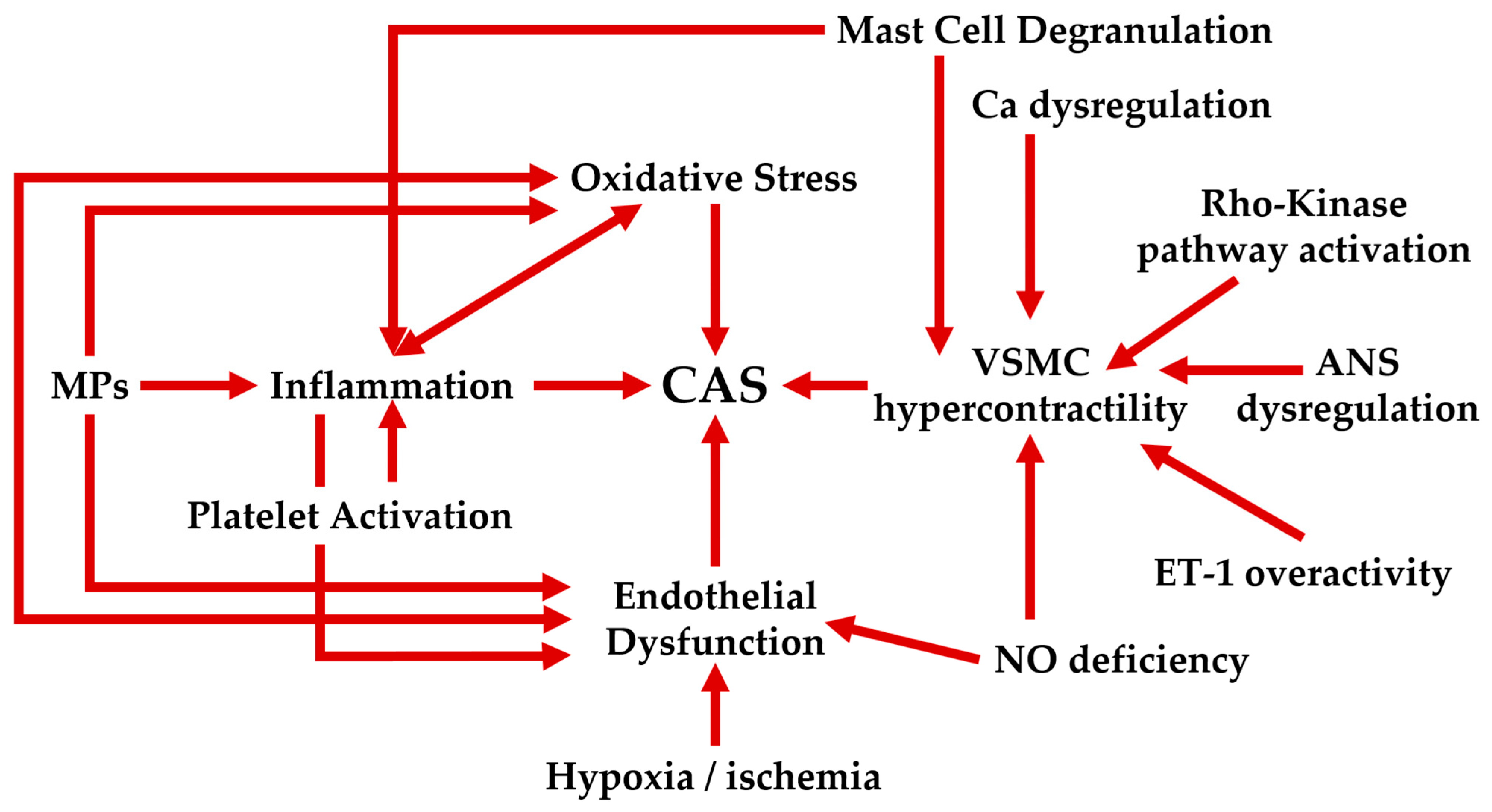
6.5. Interplay of Factors in the Physiopathology of CAS
6.6. Future Directions in the Research of CAS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| ACS | Acute coronary syndrome |
| ALDH2 | Aldehyde dehydrogenase 2 |
| ANS | Autonomic nervous system |
| ATP-CFR | Adenosine triphosphate-induced coronary flow reserve |
| AV | Atrioventricular |
| CAD | Coronary artery disease |
| CaM | Calmodulin |
| CAS | Coronary artery spasm |
| CCT | Cardiac computed tomography |
| cMRI | Cardiac magnetic resonance imaging |
| COX-2 | Cyclooxygenase-2 |
| CPI-17 | C-kinase-activated protein phosphatase-1 inhibitor of 17 kDa |
| CRP | C-reactive protein |
| CSFP | Coronary slow flow phenomenon |
| CTA | Computed tomography angiography |
| EC | Endothelial cells |
| ECG | Electrocardiogram |
| ECM | Extracellular matrix |
| EDHF | Endothelium-derived hyperpolarization factor |
| EMPs | Endothelial microparticles |
| eNOS | Endothelial nitric oxide synthase |
| EPCR | Endothelial cell protein C receptor |
| ET-1 | Endothelin-1 |
| FFR | Fractional flow reserve |
| HRR | Heart rate recovery |
| HRV | Heart rate variability |
| hs-CRP | High-sensitivity C-reactive protein |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| INOCA | Ischemia with non-obstructive coronary arteries |
| iNOS | Inducible nitric oxide synthase |
| IV ER | Intravenous ergonovine |
| IVUS | Intravascular ultrasound |
| LAD | Left anterior descending |
| LDL | Low-density lipoproteins |
| LGE | Late gadolinium enhancement |
| MB | Myocardial bridging |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MI | Myocardial ischemia |
| MINOCA | Myocardial infarction with non-obstructive coronary arteries |
| MLC | Myosin light chain |
| MLCK | Myosin light chain kinase |
| MLCP | Myosin light chain phosphatase |
| MPs | Microparticles |
| NO | Nitric oxide |
| OCT | Optical coherence tomography |
| oxLDL | Oxidized low density lipoprotein |
| PCI | Percutaneous coronary intervention |
| PET/CT | Positron emission tomography/computed tomography |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PVAT | Perivascular adipose tissue |
| ROCK | Rho-associated coiled-coil-containing protein kinase |
| ROS | Reactive oxygen species |
| sCD40L | Soluble CD40 ligand |
| TNF | Tumor necrosis factor |
| TXA2 | Thromboxane A2 |
| VCAM-1 | Vascular adhesion molecule-1 |
| VSA | Vasospastic angina |
| VSMC | Vascular smooth muscle cell |
References
- Beltrame, J.F.; Limaye, S.B.; Wuttke, R.D.; Horowitz, J.D. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am. Heart J. 2003, 146, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev. Esp. Cardiol. 2017, 70, 1082. [Google Scholar] [CrossRef]
- Ioannis, V.; Konstantinos, K.; Antonios, S.; Ioannis, B. Acute Coronary Syndrome with Normal Coronary Arteries: A Case of Spontaneous Spasm Lysis. Med. Arch. 2018, 72, 154–156. [Google Scholar] [CrossRef]
- Cheng, C.W.; Yang, N.I.; Lin, K.J.; Hung, M.J.; Cherng, W.J. Role of coronary spasm for a positive noninvasive stress test result in angina pectoris patients without hemodynamically significant coronary artery disease. Am. J. Med. Sci. 2008, 335, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Morikawa, Y.; Mizuno, Y.; Harada, E.; Ito, T.; Matsui, K.; Saito, Y.; Yasue, H. Coronary spasm preferentially occurs at branch points: An angiographic comparison with atherosclerotic plaque. Circ. Cardiovasc. Interv. 2009, 2, 97–104. [Google Scholar] [CrossRef]
- Raizner, A.E.; Chahine, R.A.; Ishimori, T.; Verani, M.S.; Zacca, N.; Jamal, N.; Miller, R.R.; Luchi, R.J. Provocation of coronary artery spasm by the cold pressor test. Hemodynamic, arteriographic and quantitative angiographic observations. Circulation 1980, 62, 925–932. [Google Scholar] [CrossRef]
- Yeung, A.C.; Vekshtein, V.I.; Krantz, D.S.; Vita, J.A.; Ryan, T.J., Jr.; Ganz, P.; Selwyn, A.P. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N. Engl. J. Med. 1991, 325, 1551–1556. [Google Scholar] [CrossRef]
- Miyagi, H.; Yasue, H.; Okumura, K.; Ogawa, H.; Goto, K.; Oshima, S. Effect of magnesium on anginal attack induced by hyperventilation in patients with variant angina. Circulation 1989, 79, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.; Rosenthal, J.E.; Cohen, L.S.; Hammond, G.; Wolfson, S. Alcohol-induced prinzmetal variant angina. Am. J. Cardiol. 1973, 32, 238–239. [Google Scholar] [CrossRef]
- Beijk, M.A.; Vlastra, W.V.; Delewi, R.; van de Hoef, T.P.; Boekholdt, S.M.; Sjauw, K.D.; Piek, J.J. Myocardial infarction with non-obstructive coronary arteries: A focus on vasospastic angina. Neth. Heart J. 2019, 27, 237–245. [Google Scholar] [CrossRef]
- Matta, A.; Bouisset, F.; Lhermusier, T.; Campelo-Parada, F.; Elbaz, M.; Carrié, D.; Roncalli, J. Coronary Artery Spasm: New Insights. J. Interv. Cardiol. 2020, 2020, 5894586. [Google Scholar] [CrossRef] [PubMed]
- Pitts, W.R.; Lange, R.A.; Cigarroa, J.E.; Hillis, L.D. Cocaine-induced myocardial ischemia and infarction: Pathophysiology, recognition, and management. Prog. Cardiovasc. Dis. 1997, 40, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Yasue, H.; Okumura, K.; Matsuyama, K.; Kugiyama, K.; Miyagi, H.; Higashi, T. Magnesium deficiency detected by intravenous loading test in variant angina pectoris. Am. J. Cardiol. 1990, 65, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Mulcahy, D.; Keegan, J.; Crean, P.; Quyyumi, A.; Shapiro, L.; Wright, C.; Fox, K. Silent myocardial ischaemia in chronic stable angina: A study of its frequency and characteristics in 150 patients. Br. Heart J. 1988, 60, 417–423. [Google Scholar] [CrossRef]
- Ogawa, H.; Yasue, H.; Oshima, S.; Okumura, K.; Matsuyama, K.; Obata, K. Circadian variation of plasma fibrinopeptide A level in patients with variant angina. Circulation 1989, 80, 1617–1626. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Panza, J.A.; Diodati, J.G.; Lakatos, E.; Epstein, S.E. Circadian variation in ischemic threshold. A mechanism underlying the circadian variation in ischemic events. Circulation 1992, 86, 22–28. [Google Scholar] [CrossRef]
- Rocco, M.B.; Nabel, E.G.; Selwyn, A.P. Circadian rhythms and coronary artery disease. Am. J. Cardiol. 1987, 59, 13c–17c. [Google Scholar] [CrossRef]
- Hung, M.J.; Hsu, K.H.; Chang, N.C.; Hung, M.Y. Increased Numbers of Coronary Events in Winter and Spring Due to Coronary Artery Spasm: Effect of Age, Sex, Smoking, and Inflammation. J. Am. Coll. Cardiol. 2015, 65, 2047–2048. [Google Scholar] [CrossRef]
- Enquselassie, F.; Dobson, A.J.; Alexander, H.M.; Steele, P.L. Seasons, temperature and coronary disease. Int. J. Epidemiol. 1993, 22, 632–636. [Google Scholar] [CrossRef]
- Ornato, J.P.; Peberdy, M.A.; Chandra, N.C.; Bush, D.E. Seasonal pattern of acute myocardial infarction in the National Registry of Myocardial Infarction. J. Am. Coll. Cardiol. 1996, 28, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.J.; Hu, P.; Hung, M.Y. Coronary artery spasm: Review and update. Int. J. Med. Sci. 2014, 11, 1161–1171. [Google Scholar] [CrossRef]
- Yasue, H.; Omote, S.; Takizawa, A.; Masao, N.; Hyon, H.; Nishida, S.; Horie, M. Comparison of coronary arteriographic findings during angina pectoris associated with S-T elevation or depression. Am. J. Cardiol. 1981, 47, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Sayah, N.; Spagnoli, V.; Adjedj, J.; Varenne, O. Vasospastic angina: A literature review of current evidence. Arch. Cardiovasc. Dis. 2019, 112, 44–55. [Google Scholar] [CrossRef]
- Hung, M.Y.; Hsu, K.H.; Hung, M.J.; Cheng, C.W.; Cherng, W.J. Interactions among gender, age, hypertension and C-reactive protein in coronary vasospasm. Eur. J. Clin. Investig. 2010, 40, 1094–1103. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Hill, S.; Vogelsberg, H.; Voehringer, M.; Sechtem, U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) Study. J. Am. Coll. Cardiol. 2008, 52, 523–527. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): Digest version. Circ. J. 2010, 74, 1745–1762. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, X.; Zhao, X.; Xu, C.; Yu, B.; Shen, Y.; Li, L. Coronary Artery Spasm: Risk Factors, Pathophysiological Mechanisms and Novel Diagnostic Approaches. Rev. Cardiovasc. Med. 2022, 23, 175. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Takahashi, J.; Shimokawa, H. Diagnosis of Coronary Artery Spasm. In Coronary Vasomotion Abnormalities; Shimokawa, H., Ed.; Springer: Singapore, 2021; pp. 39–57. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Takahashi, J.; Uzuka, H.; Wang, H.; Tsuburaya, R.; Hao, K.; Ohyama, K.; Odaka, Y.; Miyata, S.; et al. Enhanced Adventitial Vasa Vasorum Formation in Patients with Vasospastic Angina: Assessment with OFDI. J. Am. Coll. Cardiol. 2016, 67, 598–600. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Vignali, L.; Montone, R.A.; Rinaldi, R.; Benatti, G.; Solinas, E.; Leone, A.M.; Galante, D.; Campo, G.; Biscaglia, S.; et al. Coronary Spasm Testing with Acetylcholine: A Powerful Tool for a Personalized Therapy of Coronary Vasomotor Disorders. Life 2024, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Colucci, M.; Torre, I.; Ausiello, D.; Bonanni, A.; Basile, M.; Salzillo, C.; Sanna, T.; Liuzzo, G.; Leone, A.M.; et al. Predicting the response to acetylcholine in ischemia or infarction with non-obstructive coronary arteries: The ABCD score. Atherosclerosis 2024, 391, 117503. [Google Scholar] [CrossRef]
- Hirano, Y.; Ozasa, Y.; Yamamoto, T.; Uehara, H.; Yamada, S.; Nakagawa, K.; Ikawa, H.; Ishikawa, K. Hyperventilation and cold-pressor stress echocardiography for noninvasive diagnosis of coronary artery spasm. J. Am. Soc. Echocardiogr. 2001, 14, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H. Ergonovine Provocation Echocardiography for Detection and Prognostication in Patients with Vasospastic Angina. Korean Circ. J. 2018, 48, 917–919. [Google Scholar] [CrossRef]
- Hung, M.J.; Cherng, W.J.; Yang, N.I.; Cheng, C.W.; Li, L.F. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. Am. J. Cardiol. 2005, 96, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Carro, A.; Athanasiadis, A.; Borgulya, G.; Schäufele, T.; Ratge, D.; Gaze, D.; Sechtem, U.; Kaski, J.C. Acetylcholine-induced coronary spasm in patients with unobstructed coronary arteries is associated with elevated concentrations of soluble CD40 ligand and high-sensitivity C-reactive protein. Coron. Artery Dis. 2015, 26, 126–132. [Google Scholar] [CrossRef]
- Watanabe, K.; Shishido, T.; Otaki, Y.; Watanabe, T.; Sugai, T.; Toshima, T.; Takahashi, T.; Yokoyama, M.; Kinoshita, D.; Murase, T.; et al. Increased plasma xanthine oxidoreductase activity deteriorates coronary artery spasm. Heart Vessel. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Lee, S.N.; Shin, D.I.; Jung, M.H.; Choi, I.J.; Seo, S.M.; Her, S.H.; Kim, P.J.; Moon, K.W.; Yoo, K.D.; Baek, S.H.; et al. Impact of cystatin-C level on the prevalence and angiographic characteristics of vasospastic angina in Korean patients. Int. Heart J. 2015, 56, 49–55. [Google Scholar] [CrossRef][Green Version]
- Kikuchi, Y.; Yasuda, S.; Aizawa, K.; Tsuburaya, R.; Ito, Y.; Takeda, M.; Nakayama, M.; Ito, K.; Takahashi, J.; Shimokawa, H. Enhanced Rho-kinase activity in circulating neutrophils of patients with vasospastic angina: A possible biomarker for diagnosis and disease activity assessment. J. Am. Coll. Cardiol. 2011, 58, 1231–1237. [Google Scholar] [CrossRef]
- Ong, P.; Aziz, A.; Hansen, H.S.; Prescott, E.; Athanasiadis, A.; Sechtem, U. Structural and Functional Coronary Artery Abnormalities in Patients with Vasospastic Angina Pectoris. Circ. J. 2015, 79, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Taruya, A.; Shibata, K.; Fuse, K.; Katayama, Y.; Yokoyama, M.; Kashiwagi, M.; Shingo, O.; Akasaka, T.; Kato, N. Coronary artery lumen complexity as a new marker for refractory symptoms in patients with vasospastic angina. Sci. Rep. 2021, 11, 13. [Google Scholar] [CrossRef]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Kang, E.J.; Jin, C.D.; Lee, K.M.; Lim, K.H.; Rha, S.W.; Choi, C.U.; Yong, H.S.; Yun, S.C.; Budoff, M.J.; et al. Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina. J. Clin. Med. 2023, 12, 3753. [Google Scholar] [CrossRef]
- Khattab, M.; Baig, M.; El Zarif, T.; Barac, A.; Ferencik, M.; Henry, M.L.; Lopez-Mattei, J.; Redheuil, A.; Salem, J.E.; Scherrer-Crosbie, M.; et al. How to Use Imaging: Complex Cases of Atherosclerosis, Myocardial Inflammation, and Cardiomyopathy in Cardio-Oncology. Circ. Cardiovasc. Imaging 2025, 18, e015981. [Google Scholar] [CrossRef]
- Angelini, P.; Uribe, C.; Raghuram, A. Coronary Myocardial Bridge Updates: Anatomy, Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment Options. Tex. Heart Inst. J. 2025, 52, e238300. [Google Scholar] [CrossRef]
- Hung, M.J.; Hsu, K.H.; Hu, W.S.; Chang, N.C.; Hung, M.Y. C-reactive protein for predicting prognosis and its gender-specific associations with diabetes mellitus and hypertension in the development of coronary artery spasm. PLoS ONE 2013, 8, e77655. [Google Scholar] [CrossRef]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef]
- Takaoka, K.; Yoshimura, M.; Ogawa, H.; Kugiyama, K.; Nakayama, M.; Shimasaki, Y.; Mizuno, Y.; Sakamoto, T.; Yasue, H. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: Role of cigarette smoking. Int. J. Cardiol. 2000, 72, 121–126. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Surcel, M.; Munteanu, A.; Scheau, C.; Savulescu-Fiedler, I.; Caruntu, C. Diabetic neuropathy: A NRF2 disease? J. Diabetes 2023, 16, e13524. [Google Scholar] [CrossRef]
- Yasue, H.; Mizuno, Y.; Harada, E. Coronary artery spasm—Clinical features, pathogenesis and treatment. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Hizume, T.; Morikawa, K.; Takaki, A.; Abe, K.; Sunagawa, K.; Amano, M.; Kaibuchi, K.; Kubo, C.; Shimokawa, H. Sustained Elevation of Serum Cortisol Level Causes Sensitization of Coronary Vasoconstricting Responses in Pigs In Vivo. Circ. Res. 2006, 99, 767–775. [Google Scholar] [CrossRef]
- Galle, J.; Mameghani, A.; Bolz, S.S.; Gambaryan, S.; Görg, M.; Quaschning, T.; Raff, U.; Barth, H.; Seibold, S.; Wanner, C.; et al. Oxidized LDL and its compound lysophosphatidylcholine potentiate AngII-induced vasoconstriction by stimulation of RhoA. J. Am. Soc. Nephrol. 2003, 14, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Maruko, K.; Stiffel, V.M.; Gilbert, R.D. The Effect of Long-term Hypoxia on Tension and Intracellular Calcium Responses Following Stimulation of the Thromboxane A2 Receptor in the Left Anterior Descending Coronary Artery of Fetal Sheep. Reprod. Sci. 2009, 16, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Ito, A.; Fukumoto, Y.; Kadokami, T.; Nakaike, R.; Sakata, M.; Takayanagi, T.; Egashira, K.; Takeshita, A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J. Clin. Investig. 1996, 97, 769–776. [Google Scholar] [CrossRef]
- Nishino, M.; Mori, N.; Yoshimura, T.; Nakamura, D.; Lee, Y.; Taniike, M.; Makino, N.; Kato, H.; Egami, Y.; Shutta, R.; et al. Higher serum uric acid and lipoprotein(a) are correlated with coronary spasm. Heart Vessel. 2014, 29, 186–190. [Google Scholar] [CrossRef]
- Tsuchida, K.; Hori, T.; Tanabe, N.; Makiyama, Y.; Ozawa, T.; Saigawa, T.; Watanabe, R.; Tanaka, T.; Nasuno, A.; Fukunaga, H.; et al. Relationship between serum lipoprotein(a) concentrations and coronary vasomotion in coronary spastic angina. Circ. J. 2005, 69, 521–525. [Google Scholar] [CrossRef][Green Version]
- Schächinger, V.; Halle, M.; Minners, J.; Berg, A.; Zeiher, A.M. Lipoprotein(a) Selectively Impairs Receptor-Mediated Endothelial Vasodilator Function of the Human Coronary Circulation. J. Am. Coll. Cardiol. 1997, 30, 927–934. [Google Scholar] [CrossRef]
- Miwa, K.; Nakagawa, K.; Yoshida, N.; Taguchi, Y.; Inoue, H. Lipoprotein(a) is a risk factor for occurrence of acute myocardial infarction in patients with coronary vasospasm. J. Am. Coll. Cardiol. 2000, 35, 1200–1205. [Google Scholar] [CrossRef]
- Funayama, A.; Watanabe, T.; Tamabuchi, T.; Otaki, Y.; Netsu, S.; Hasegawa, H.; Honda, S.; Ishino, M.; Arimoto, T.; Takahashi, H.; et al. Elevated cystatin C levels predict the incidence of vasospastic angina. Circ. J. 2011, 75, 2439–2444. [Google Scholar] [CrossRef]
- Yasue, H.; Nakagawa, H.; Itoh, T.; Harada, E.; Mizuno, Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 2008, 51, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Sugiishi, M.; Takatsu, F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 1993, 87, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Russo, M.; Rinaldi, R.; Caffè, A.; La Vecchia, G.; Bonanni, A.; Iannaccone, G.; Basile, M.; Vergallo, R.; Aurigemma, C.; et al. Air Pollution and Coronary Vasomotor Disorders in Patients with Myocardial Ischemia and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2022, 80, 1818–1828. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.D.; Shin, T.H.; Bae, C.S.; Ahn, T.; Shin, S.S.; Kim, H.J.; Lee, C.M.; Suh, G.H. Respiratory and Systemic Toxicity of Inhaled Artificial Asian Sand Dust in Pigs. Life 2021, 11, 25. [Google Scholar] [CrossRef]
- Rinaldi, R.; Princi, G.; La Vecchia, G.; Bonanni, A.; Chiariello, G.A.; Candreva, A.; Gragnano, F.; Calabrò, P.; Crea, F.; Montone, R.A. MINOCA Associated with a Myocardial Bridge: Pathogenesis, Diagnosis and Treatment. J. Clin. Med. 2023, 12, 3799. [Google Scholar] [CrossRef]
- Boltwood, M.D.; Taylor, C.B.; Burke, M.B.; Grogin, H.; Giacomini, J. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am. J. Cardiol. 1993, 72, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Dakak, N.; Quyyumi, A.A.; Eisenhofer, G.; Goldstein, D.S.; Cannon, R.O., 3rd. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am. J. Cardiol. 1995, 76, 125–130. [Google Scholar] [CrossRef]
- Hung, M.Y.; Mao, C.T.; Hung, M.J.; Wang, J.K.; Lee, H.C.; Yeh, C.T.; Hu, P.; Chen, T.H.; Chang, N.C. Coronary Artery Spasm as Related to Anxiety and Depression: A Nationwide Population-Based Study. Psychosom. Med. 2019, 81, 237–245. [Google Scholar] [CrossRef]
- Mehta, P.K.; Thobani, A.; Vaccarino, V. Coronary Artery Spasm, Coronary Reactivity, and Their Psychological Context. Psychosom. Med. 2019, 81, 233–236. [Google Scholar] [CrossRef]
- Oike, Y.; Hata, A.; Ogata, Y.; Numata, Y.; Shido, K.; Kondo, K. Angiotensin converting enzyme as a genetic risk factor for coronary artery spasm. Implication in the pathogenesis of myocardial infarction. J. Clin. Investig. 1995, 96, 2975–2979. [Google Scholar] [CrossRef]
- Ito, T.; Yasue, H.; Yoshimura, M.; Nakamura, S.; Nakayama, M.; Shimasaki, Y.; Harada, E.; Mizuno, Y.; Kawano, H.; Ogawa, H. Paraoxonase gene Gln192Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum. Genet. 2002, 110, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Zhang, S.Y.; Jo, S.H.; Seo, J.B.; Li, L.; Park, K.W.; Oh, B.H.; Park, Y.B.; Kim, H.S. Common adrenergic receptor polymorphisms as novel risk factors for vasospastic angina. Am. Heart J. 2006, 151, 864–869. [Google Scholar] [CrossRef]
- Kaneda, H.; Taguchi, J.; Kuwada, Y.; Hangaishi, M.; Aizawa, T.; Yamakado, M.; Ogasawara, K.; Aizawa, T.; Ohno, M. Coronary artery spasm and the polymorphisms of the endothelial nitric oxide synthase gene. Circ. J. 2006, 70, 409–413. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yasue, H.; Nakayama, M.; Shimasaki, Y.; Sumida, H.; Sugiyama, S.; Kugiyama, K.; Ogawa, H.; Ogawa, Y.; Saito, Y.; et al. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum. Genet. 1998, 103, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kaumann, A.J.; Levy, F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Kawashima, S.; Kanazawa, K.; Yamada, S.; Akita, H.; Yokoyama, M. Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation 1998, 97, 135–137. [Google Scholar] [CrossRef]
- Mizuno, Y.; Hokimoto, S.; Harada, E.; Kinoshita, K.; Yoshimura, M.; Yasue, H. Variant Aldehyde Dehydrogenase 2 (ALDH2*2) in East Asians Interactively Exacerbates Tobacco Smoking Risk for Coronary Spasm—Possible Role of Reactive Aldehydes. Circ. J. 2016, 81, 96–102. [Google Scholar] [CrossRef]
- Han, H.; Wang, H.; Yin, Z.; Jiang, H.; Fang, M.; Han, J. Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: A meta-analysis. Gene 2013, 525, 134–141. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.Q.; Fu, B.; Zhao, S.L.; Kui, Y. Aldehyde dehydrogenase 2 (ALDH2) polymorphism gene and coronary artery disease risk: A meta-analysis. Genet. Mol. Res. 2015, 14, 18503–18514. [Google Scholar] [CrossRef]
- Fujioka, K.; Gordon, S. Effects of “Essential AD2” Supplement on Blood Acetaldehyde Levels in Individuals Who Have Aldehyde Dehydrogenase (ALDH2) Deficiency. Am. J. Ther. 2019, 26, 583–588. [Google Scholar] [CrossRef]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef]
- Hubert, A.; Seitz, A.; Pereyra, V.M.; Bekeredjian, R.; Sechtem, U.; Ong, P. Coronary Artery Spasm: The Interplay Between Endothelial Dysfunction and Vascular Smooth Muscle Cell Hyperreactivity. Eur. Cardiol. 2020, 15, e12. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Suda, A.; Takahashi, J.; Berry, C.; Camici, P.G.; Crea, F.; Escaned, J.; Ford, T.; Yii, E.; Kaski, J.C.; et al. Clinical characteristics and prognosis of patients with microvascular angina: An international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur. Heart J. 2021, 42, 4592–4600. [Google Scholar] [CrossRef]
- Slavich, M.; Patel, R.S. Coronary artery spasm: Current knowledge and residual uncertainties. Int. J. Cardiol. Heart Vasc. 2016, 10, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Russo, M.; Occhipinti, G.; Laudani, C.; Torre, I.; Colucci, M.; Gurgoglione, F.L.; Animati, F.M.; Lenkowicz, J.; Tudor, A.M.; et al. Sex-Related Differences in the Prognostic Role of Acetylcholine Provocation Testing. J. Am. Heart Assoc. 2025, 14, e037942. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.L.; Thurston, G. Mechanics of endothelial cell architecture and vascular permeability. Crit. Rev. Biomed. Eng. 2001, 29, 247–278. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Vane, J.R. The Croonian Lecture, 1993. The endothelium: Maestro of the blood circulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 343, 225–246. [Google Scholar] [CrossRef]
- Nakashima, Y.; Wight, T.N.; Sueishi, K. Early atherosclerosis in humans: Role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc. Res. 2008, 79, 14–23. [Google Scholar] [CrossRef]
- Chen, Y.X.; Nakashima, Y.; Tanaka, K.; Shiraishi, S.; Nakagawa, K.; Sueishi, K. Immunohistochemical expression of vascular endothelial growth factor/vascular permeability factor in atherosclerotic intimas of human coronary arteries. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 131–139. [Google Scholar] [CrossRef]
- Imanishi, T.; McBride, J.; Ho, Q.; O’Brien, K.D.; Schwartz, S.M.; Han, D.K. Expression of cellular FLICE-inhibitory protein in human coronary arteries and in a rat vascular injury model. Am. J. Pathol. 2000, 156, 125–137. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fujii, H.; Sumiyoshi, S.; Wight, T.N.; Sueishi, K. Early human atherosclerosis: Accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1159–1165. [Google Scholar] [CrossRef]
- Tellides, G.; Pober, J.S. Inflammatory and immune responses in the arterial media. Circ. Res. 2015, 116, 312–322. [Google Scholar] [CrossRef]
- Zorc-Pleskovič, R.; Pleskovič, A.; Vraspir-Porenta, O.; Zorc, M.; Milutinović, A. Immune cells and vasa vasorum in the tunica media of atherosclerotic coronary arteries. Bosn. J. Basic. Med. Sci. 2018, 18, 240–245. [Google Scholar] [CrossRef]
- Milutinović, A.; Šuput, D.; Zorc-Pleskovič, R. Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: An updated review. Bosn. J. Basic. Med. Sci. Udruzenje Basicnih Med. Znan. 2020, 20, 21–30. [Google Scholar] [CrossRef]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell. Mol. Life Sci. 2014, 71, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Waller, B.F.; Orr, C.M.; Slack, J.D.; Pinkerton, C.A.; Van Tassel, J.; Peters, T. Anatomy, histology, and pathology of coronary arteries: A review relevant to new interventional and imaging techniques—Part I. Clin. Cardiol. 1992, 15, 451–457. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Wang, Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediat. Inflamm. 2017, 2017, 8135934. [Google Scholar] [CrossRef]
- Xu, F.; Ji, J.; Li, L.; Chen, R.; Hu, W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: A novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis”. Med. Hypotheses 2007, 69, 908–912. [Google Scholar] [CrossRef]
- Fernández-Alfonso, M.S.; Somoza, B.; Tsvetkov, D.; Kuczmanski, A.; Dashwood, M.; Gil-Ortega, M. Role of Perivascular Adipose Tissue in Health and Disease. Compr. Physiol. 2017, 8, 23–59. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W. Endothelium—Role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef]
- Kirsch, J.; Schneider, H.; Pagel, J.I.; Rehberg, M.; Singer, M.; Hellfritsch, J.; Chillo, O.; Schubert, K.M.; Qiu, J.; Pogoda, K.; et al. Endothelial Dysfunction, and A Prothrombotic, Proinflammatory Phenotype Is Caused by Loss of Mitochondrial Thioredoxin Reductase in Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj Mallat, R.; Mathew John, C.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Garland, C.J.; Dora, K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017, 219, 152–161. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Yasue, H.; Matsuyama, K.; Matsuyama, K.; Okumura, K.; Morikami, Y.; Ogawa, H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation 1990, 81, 482–490. [Google Scholar] [CrossRef]
- Friedberg, C.K. Some comments and reflections on changing interests and new developments in angina pectoris. Circulation 1972, 46, 1037–1047. [Google Scholar] [CrossRef]
- Parker, J.O. Nitrate therapy in stable angina pectoris. N. Engl. J. Med. 1987, 316, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Takeshita, A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Tomoike, H.; Nabeyama, S.; Yamamoto, H.; Araki, H.; Nakamura, M.; Ishii, Y.; Tanaka, K. Coronary artery spasm induced in atherosclerotic miniature swine. Science 1983, 221, 560–562. [Google Scholar] [CrossRef]
- Shimokawa, H.; Tomoike, H.; Nabeyama, S.; Yamamoto, H.; Ishii, Y.; Tanaka, K.; Nakamura, M. Coronary artery spasm induced in miniature swine: Angiographic evidence and relation to coronary atherosclerosis. Am. Heart J. 1985, 110, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Golino, P.; Piscione, F.; Willerson, J.T.; Cappelli-Bigazzi, M.; Focaccio, A.; Villari, B.; Indolfi, C.; Russolillo, E.; Condorelli, M.; Chiariello, M. Divergent effects of serotonin on coronary-artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N. Engl. J. Med. 1991, 324, 641–648. [Google Scholar] [CrossRef]
- McFadden, E.P.; Clarke, J.G.; Davies, G.J.; Kaski, J.C.; Haider, A.W.; Maseri, A. Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. N. Engl. J. Med. 1991, 324, 648–654. [Google Scholar] [CrossRef]
- Odaka, Y.; Takahashi, J.; Tsuburaya, R.; Nishimiya, K.; Hao, K.; Matsumoto, Y.; Ito, K.; Sakata, Y.; Miyata, S.; Manita, D.; et al. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur. Heart J. 2017, 38, 489–496. [Google Scholar] [CrossRef]
- Clarke, J.G.; Davies, G.J.; Kerwin, R.; Hackett, D.; Larkin, S.; Dawbarn, D.; Lee, Y.; Bloom, S.R.; Yacoub, M.; Maseri, A. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet 1987, 1, 1057–1059. [Google Scholar] [CrossRef]
- Herring, N.; Tapoulal, N.; Kalla, M.; Ye, X.; Borysova, L.; Lee, R.; Dall’Armellina, E.; Stanley, C.; Ascione, R.; Lu, C.J.; et al. Neuropeptide-Y causes coronary microvascular constriction and is associated with reduced ejection fraction following ST-elevation myocardial infarction. Eur. Heart J. 2019, 40, 1920–1929. [Google Scholar] [CrossRef]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef]
- Morita, S.; Mizuno, Y.; Harada, E.; Nakagawa, H.; Morikawa, Y.; Saito, Y.; Katoh, D.; Kashiwagi, Y.; Yoshimura, M.; Murohara, T.; et al. Differences and interactions between risk factors for coronary spasm and atherosclerosis--smoking, aging, inflammation, and blood pressure. Intern. Med. 2014, 53, 2663–2670. [Google Scholar] [CrossRef]
- Ishida, M.; Sakai, C.; Kobayashi, Y.; Ishida, T. Cigarette Smoking and Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2024, 31, 189–200. [Google Scholar] [CrossRef]
- Dragosloveanu, C.D.M.; Celea, C.G.; Dragosloveanu, S. Comparison of 360 degrees circumferential trabeculotomy and conventional trabeculotomy in primary pediatric glaucoma surgery: Complications, reinterventions and preoperative predictive risk factors. Int. Ophthalmol. 2020, 40, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Reeh, J.; Therming, C.B.; Heitmann, M.; Højberg, S.; Sørum, C.; Bech, J.; Husum, D.; Dominguez, H.; Sehestedt, T.; Hermann, T.; et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur. Heart J. 2019, 40, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef]
- Lerman, A.; Kwon, T.G.; Lerman, L.O. Morphological Characteristics of Coronary Arteries in Patients with Vasospastic Angina: Another Form of Atherosclerosis? JACC Cardiovasc. Imaging 2015, 8, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Matsuo, Y.; Aoki, T.; Kwon, T.G.; Prasad, A.; Gulati, R.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2473–2477. [Google Scholar] [CrossRef]
- Yasue, H.; Takizawa, A.; Nagao, M.; Nishida, S.; Horie, M.; Kubota, J.; Omote, S.; Takaoka, K.; Okumura, K. Long-term prognosis for patients with variant angina and influential factors. Circulation 1988, 78, 1–9. [Google Scholar] [CrossRef]
- MacAlpin, R.N. Correlation of the location of coronary arterial spasm with the lead distribution of ST segment elevation during variant angina. Am. Heart J. 1980, 99, 555–564. [Google Scholar] [CrossRef]
- Miyao, Y.; Kugiyama, K.; Kawano, H.; Motoyama, T.; Ogawa, H.; Yoshimura, M.; Sakamoto, T.; Yasue, H. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J. Am. Coll. Cardiol. 2000, 36, 432–437. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Kei, C.Y.; Singh, K.; Dautov, R.F.; Nguyen, T.H.; Chirkov, Y.Y.; Horowitz, J.D. Coronary “Microvascular Dysfunction”: Evolving Understanding of Pathophysiology, Clinical Implications, and Potential Therapeutics. Int. J. Mol. Sci. 2023, 24, 11287. [Google Scholar] [CrossRef]
- Hansen, B.; Holtzman, J.N.; Juszczynski, C.; Khan, N.; Kaur, G.; Varma, B.; Gulati, M. Ischemia with No Obstructive Arteries (INOCA): A Review of the Prevalence, Diagnosis and Management. Curr. Probl. Cardiol. 2023, 48, 101420. [Google Scholar] [CrossRef]
- AlShaikh, S.; Rohm, C.L.; Sutton, N.R.; Burgess, S.N.; Alasnag, M. INOCA: Ischemia in non-obstructive coronary arteries. Am. Heart J. Plus 2024, 42, 100391. [Google Scholar] [CrossRef]
- Rinaldi, R.; Russo, M.; Torre, I.; Colucci, M.; Caffè, A.; Scarica, V.; Animati, F.M.; Manzato, M.; Bonanni, A.; Lenkowicz, J.; et al. Prognostic significance of individual COVADIS criteria in patients undergoing acetylcholine provocation testing. EuroIntervention 2025, 21, e296–e306. [Google Scholar] [CrossRef]
- Jo, S.H.; Sim, J.H.; Baek, S.H. Coronary Plaque Characteristics and Cut-Off Stenosis for Developing Spasm in Patients with Vasospastic Angina. Sci. Rep. 2020, 10, 5707. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Woelders, E.; Jansen, T.; Konst, R.; Crooijmans, C.; van de Hoef, T.; Mensink, F.; Los, J.; Pellegrini, D.; Cornel, J.H.; et al. Association Between Coronary Artery Spasm and Atherosclerotic Disease. JACC Cardiovasc. Imaging 2024, 18, 226–239. [Google Scholar] [CrossRef]
- Alegria, J.R.; Herrmann, J.; Holmes, D.R., Jr.; Lerman, A.; Rihal, C.S. Myocardial bridging. Eur. Heart J. 2005, 26, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M. Myocardial Bridging. Braz. J. Cardiovasc. Surg. 2016, 31, 60–62. [Google Scholar] [CrossRef]
- Santucci, A.; Jacoangeli, F.; Cavallini, S.; d’Ammando, M.; de Angelis, F.; Cavallini, C. The myocardial bridge: Incidence, diagnosis, and prognosis of a pathology of uncertain clinical significance. Eur. Heart J. Suppl. 2022, 24, I61–I67. [Google Scholar] [CrossRef]
- Baz, R.O.; Refi, D.; Scheau, C.; Savulescu-Fiedler, I.; Baz, R.A.; Niscoveanu, C. Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review. J. Clin. Med. 2024, 13, 3920. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ishikawa, K. Myocardial bridge: Harmless or harmful. Intern. Med. 2004, 43, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.G.; Butnaru, A.; Lespérance, J.; Tardif, J.C. Symptomatic myocardial bridges: Overview of ischemic mechanisms and current diagnostic and treatment strategies. J. Am. Coll. Cardiol. 2003, 41, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Johnson, N.P. Myocardial Bridges: Lessons in Clinical Coronary Pathophysiology. JACC Cardiovasc. Imaging 2015, 8, 705–709. [Google Scholar] [CrossRef]
- Kim, P.J.; Hur, G.; Kim, S.Y.; Namgung, J.; Hong, S.W.; Kim, Y.H.; Lee, W.R. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: A comparison between computed tomography and invasive coronary angiography. Circulation 2009, 119, 1408–1416. [Google Scholar] [CrossRef]
- Ciampricotti, R.; el Gamal, M. Vasospastic coronary occlusion associated with a myocardial bridge. Catheter. Cardiovasc. Diagn. 1988, 14, 118–120. [Google Scholar] [CrossRef]
- Saito, Y.; Kitahara, H.; Shoji, T.; Tokimasa, S.; Nakayama, T.; Sugimoto, K.; Fujimoto, Y.; Kobayashi, Y. Relation between severity of myocardial bridge and vasospasm. Int. J. Cardiol. 2017, 248, 34–38. [Google Scholar] [CrossRef]
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’Amario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients with Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535. [Google Scholar] [CrossRef]
- Scalone, G.; Niccoli, G.; Crea, F. Editor’s Choice- Pathophysiology, diagnosis and management of MINOCA: An update. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 54–62. [Google Scholar] [CrossRef]
- Montone, R.A.; Meucci, M.C.; De Vita, A.; Lanza, G.A.; Niccoli, G. Coronary provocative tests in the catheterization laboratory: Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis 2021, 318, 14–21. [Google Scholar] [CrossRef]
- Heo, Y.; Oh, S.; Cho, K.H.; Kim, M.C.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Coronary vasospasm and cardiovascular outcomes in patients with isolated myocardial bridging: A retrospective study. Cardiol. J. 2024, 31, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef]
- Bil, J.; Pietraszek, N.; Pawlowski, T.; Gil, R.J. Advances in Mechanisms and Treatment Options of MINOCA Caused by Vasospasm or Microcirculation Dysfunction. Curr. Pharm. Des. 2018, 24, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Oshima, S.; Yasue, H.; Ogawa, H.; Okumura, K.; Matsuyama, K. Fibrinopeptide A is released into the coronary circulation after coronary spasm. Circulation 1990, 82, 2222–2225. [Google Scholar] [CrossRef] [PubMed]
- Panduranga, P.; Riyami, A.A. Acute intracoronary thrombosis in a normal coronary artery following coronary angiography: Thromboaspiration using a guide catheter. Heart Views 2010, 11, 68–70. [Google Scholar] [CrossRef]
- Nihei, T.; Takahashi, J.; Tsuburaya, R.; Ito, Y.; Shiroto, T.; Hao, K.; Takagi, Y.; Matsumoto, Y.; Nakayama, M.; Miyata, S.; et al. Circadian variation of Rho-kinase activity in circulating leukocytes of patients with vasospastic angina. Circ. J. 2014, 78, 1183–1190. [Google Scholar] [CrossRef][Green Version]
- Saito, T.; Hirano, M.; Ide, T.; Ichiki, T.; Koibuchi, N.; Sunagawa, K.; Hirano, K. Pivotal role of Rho-associated kinase 2 in generating the intrinsic circadian rhythm of vascular contractility. Circulation 2013, 127, 104–114. [Google Scholar] [CrossRef]
- Rivero, F.; Antuña, P.; Cuesta, J.; Alfonso, F. Severe coronary spasm in a COVID-19 patient. Catheter. Cardiovasc. Interv. 2021, 97, E670–E672. [Google Scholar] [CrossRef]
- Daia, C.; Scheau, C.; Neagu, G.; Andone, I.; Spanu, A.; Popescu, C.; Stoica, S.I.; Verenca, M.C.; Onose, G. Nerve conduction study and electromyography findings in patients recovering from COVID-19—Case report. Int. J. Infect. Dis. 2021, 103, 420–422. [Google Scholar] [CrossRef]
- Forman, M.B.; Oates, J.A.; Robertson, D.; Robertson, R.M.; Roberts, L.J., 2nd; Virmani, R. Increased adventitial mast cells in a patient with coronary spasm. N. Engl. J. Med. 1985, 313, 1138–1141. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Shimokawa, H.; Ito, A.; Kadokami, T.; Yonemitsu, Y.; Aikawa, M.; Owada, M.K.; Egashira, K.; Sueishi, K.; Nagai, R.; et al. Inflammatory cytokines cause coronary arteriosclerosis-like changes and alterations in the smooth-muscle phenotypes in pigs. J. Cardiovasc. Pharmacol. 1997, 29, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shimokawa, H.; Fukumoto, Y.; Kadokami, T.; Nakaike, R.; Takayanagi, T.; Egashira, K.; Sueishi, K.; Takeshita, A. The role of fibroblast growth factor-2 in the vascular effects of interleukin-1 beta in porcine coronary arteries in vivo. Cardiovasc. Res. 1996, 32, 570–579. [Google Scholar]
- Ito, A.; Shimokawa, H.; Kadokami, T.; Fukumoto, Y.; Owada, M.K.; Shiraishi, T.; Nakaike, R.; Takayanagi, T.; Egashira, K.; Takeshita, A. Tyrosine kinase inhibitor suppresses coronary arteriosclerotic changes and vasospastic responses induced by chronic treatment with interleukin-1 beta in pigs in vivo. J. Clin. Investig. 1995, 96, 1288–1294. [Google Scholar] [CrossRef]
- Miyata, K.; Shimokawa, H.; Yamawaki, T.; Kunihiro, I.; Zhou, X.; Higo, T.; Tanaka, E.; Katsumata, N.; Egashira, K.; Takeshita, A. Endothelial vasodilator function is preserved at the spastic/inflammatory coronary lesions in pigs. Circulation 1999, 100, 1432–1437. [Google Scholar] [CrossRef]
- Katsumata, N.; Shimokawa, H.; Seto, M.; Kozai, T.; Yamawaki, T.; Kuwata, K.; Egashira, K.; Ikegaki, I.; Asano, T.; Sasaki, Y.; et al. Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1beta. Circulation 1997, 96, 4357–4363. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, J.; Shimokawa, H.; Higashi, M.; Morikawa, K.; Kandabashi, T.; Kawamura, N.; Kubota, T.; Ichiki, T.; Amano, M.; Kaibuchi, K.; et al. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J. Mol. Cell Cardiol. 2004, 37, 537–546. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef]
- Fukata, Y.; Amano, M.; Kaibuchi, K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001, 22, 32–39. [Google Scholar] [CrossRef]
- Takai, Y.; Sasaki, T.; Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 2001, 81, 153–208. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2002, 39, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Liao, J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Kandabashi, T.; Shimokawa, H.; Mukai, Y.; Matoba, T.; Kunihiro, I.; Morikawa, K.; Ito, M.; Takahashi, S.; Kaibuchi, K.; Takeshita, A. Involvement of rho-kinase in agonists-induced contractions of arteriosclerotic human arteries. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 243–248. [Google Scholar] [CrossRef]
- Kamiunten, H.; Koike, J.; Mashiba, J.; Shimokawa, H.; Takeshita, A. A comprehensive analysis of a novel missense mutation in Rho-kinase that causes coronary vasospasm in the Japanese. Circ. J. 2004, 68, 211. [Google Scholar]
- Takemoto, M.; Sun, J.; Hiroki, J.; Shimokawa, H.; Liao, J.K. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 57–62. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Uwatoku, T.; Oi, K.; Abe, K.; Hattori, T.; Morishige, K.; Eto, Y.; Fukumoto, Y.; Nakamura, K.; Shibata, Y.; et al. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: Involvement of multiple mechanisms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 181–186. [Google Scholar] [CrossRef]
- Shibata, R.; Kai, H.; Seki, Y.; Kusaba, K.; Takemiya, K.; Koga, M.; Jalalidin, A.; Tokuda, K.; Tahara, N.; Niiyama, H.; et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J. Cardiovasc. Pharmacol. 2003, 42 (Suppl. S1), S43–S47. [Google Scholar] [CrossRef]
- Shimokawa, H. Reactive oxygen species in cardiovascular health and disease: Special references to nitric oxide, hydrogen peroxide, and Rho-kinase. J. Clin. Biochem. Nutr. 2020, 66, 83–91. [Google Scholar] [CrossRef]
- Oi, K.; Shimokawa, H.; Hiroki, J.; Uwatoku, T.; Abe, K.; Matsumoto, Y.; Nakajima, Y.; Nakajima, K.; Takeichi, S.; Takeshita, A. Remnant lipoproteins from patients with sudden cardiac death enhance coronary vasospastic activity through upregulation of Rho-kinase. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 918–922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, K.; Shimokawa, H.; Kandabashi, T.; Higo, T.; Morishige, K.; Eto, Y.; Egashira, K.; Kaibuchi, K.; Takeshita, A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2351–2358. [Google Scholar] [CrossRef]
- Kandabashi, T.; Shimokawa, H.; Miyata, K.; Kunihiro, I.; Kawano, Y.; Fukata, Y.; Higo, T.; Egashira, K.; Takahashi, S.; Kaibuchi, K.; et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation 2000, 101, 1319–1323. [Google Scholar] [CrossRef]
- Rikitake, Y.; Liao, J.K. Rho GTPases, statins, and nitric oxide. Circ. Res. 2005, 97, 1232–1235. [Google Scholar] [CrossRef]
- Sauzeau, V.; Rolli-Derkinderen, M.; Marionneau, C.; Loirand, G.; Pacaud, P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J. Biol. Chem. 2003, 278, 9472–9480. [Google Scholar] [CrossRef]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef]
- Nihei, T.; Takahashi, J.; Kikuchi, Y.; Takagi, Y.; Hao, K.; Tsuburaya, R.; Shiroto, T.; Ito, Y.; Matsumoto, Y.; Nakayama, M.; et al. Enhanced Rho-kinase activity in patients with vasospastic angina after the Great East Japan Earthquake. Circ. J. 2012, 76, 2892–2894. [Google Scholar] [CrossRef]
- Seasholtz, T.M.; Majumdar, M.; Kaplan, D.D.; Brown, J.H. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ. Res. 1999, 84, 1186–1193. [Google Scholar] [CrossRef]
- Masumoto, A.; Mohri, M.; Shimokawa, H.; Urakami, L.; Usui, M.; Takeshita, A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002, 105, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Seto, M.; Katsumata, N.; Amano, M.; Kozai, T.; Yamawaki, T.; Kuwata, K.; Kandabashi, T.; Egashira, K.; Ikegaki, I.; et al. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 1999, 43, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Chutkow, W.A.; Pu, J.; Wheeler, M.T.; Wada, T.; Makielski, J.C.; Burant, C.F.; McNally, E.M. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J. Clin. Investig. 2002, 110, 203–208. [Google Scholar] [CrossRef]
- Kakkar, R.; Ye, B.; Stoller, D.A.; Smelley, M.; Shi, N.Q.; Galles, K.; Hadhazy, M.; Makielski, J.C.; McNally, E.M. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ. Res. 2006, 98, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tao, T.; Zhao, W.; Wei, L.; She, F.; Wang, P.; Li, Y.; Zheng, Y.; Chen, X.; Wang, W.; et al. CPI-17-mediated contraction of vascular smooth muscle is essential for the development of hypertension in obese mice. J. Genet. Genom. 2019, 46, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Yasue, H.; Matsuyama, K.; Ogawa, H.; Kugiyama, K.; Ishizaka, H.; Sumida, H.; Fujii, H.; Matsunaga, T.; Tsunoda, R. Diffuse disorder of coronary artery vasomotility in patients with coronary spastic angina. Hyperreactivity to the constrictor effects of acetylcholine and the dilator effects of nitroglycerin. J. Am. Coll. Cardiol. 1996, 27, 45–52. [Google Scholar] [CrossRef]
- Kugiyama, K.; Yasue, H.; Okumura, K.; Ogawa, H.; Fujimoto, K.; Nakao, K.; Yoshimura, M.; Motoyama, T.; Inobe, Y.; Kawano, H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation 1996, 94, 266–271. [Google Scholar] [CrossRef]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Kugiyama, K.; Ogawa, H.; Motoyama, T.; Saito, Y.; Ogawa, Y.; Miyamoto, Y.; et al. T-786-->C mutation in the 5’-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation 1999, 99, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of coronary artery spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef]
- Urakami-Harasawa, L.; Shimokawa, H.; Nakashima, M.; Egashira, K.; Takeshita, A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J. Clin. Investig. 1997, 100, 2793–2799. [Google Scholar] [CrossRef]
- Nakayama, M.; Yoshimura, M.; Sakamoto, T.; Abe, K.; Yamamuro, M.; Shono, M.; Suzuki, S.; Nishijima, T.; Miyamoto, Y.; Saito, Y.; et al. A -786T>C polymorphism in the endothelial nitric oxide synthase gene reduces serum nitrite/nitrate levels from the heart due to an intracoronary injection of acetylcholine. Pharmacogenet Genom. 2006, 16, 339–345. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, Z.; Dai, L.; Zhang, X.; Yan, C.; Han, Y. Association between the—786T>C 1polymorphism in the promoter region of endothelial nitric oxide synthase (eNOS) and risk of coronary artery disease: A systematic review and meta-analysis. Gene 2014, 545, 175–183. [Google Scholar] [CrossRef]
- Kawano, H.; Ogawa, H. Endothelial dysfunction and coronary artery spasm. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Kawano, H.; Kugiyama, K.; Hirashima, O.; Ohgushi, M.; Tsunoda, R.; Moriyama, Y.; Miyao, Y.; Yoshimura, M.; Ogawa, H.; et al. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J. Am. Coll. Cardiol. 1998, 32, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Kawano, H.; Kugiyama, K.; Hirashima, O.; Ohgushi, M.; Yoshimura, M.; Ogawa, H.; Yasue, H. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: Effect of vitamin C. Am. J. Physiol. 1997, 273, H1644–H1650. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, K.; Yodoi, J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997, 15, 351–369. [Google Scholar] [CrossRef]
- Miwa, K.; Miyagi, Y.; Igawa, A.; Nakagawa, K.; Inoue, H. Vitamin E deficiency in variant angina. Circulation 1996, 94, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kawano, H.; Sakamoto, T.; Soejima, H.; Kajiwara, I.; Hokamaki, J.; Hirai, N.; Sugiyama, S.; Yoshimura, M.; Yasue, H.; et al. Increased plasma levels of thioredoxin in patients with coronary spastic angina. Antioxid. Redox Signal 2004, 6, 75–80. [Google Scholar] [CrossRef]
- Takahashi, J.; Nihei, T.; Takagi, Y.; Miyata, S.; Odaka, Y.; Tsunoda, R.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: Multicentre registry study of the Japanese coronary spasm association. Eur. Heart J. 2015, 36, 228–237. [Google Scholar] [CrossRef]
- Kaski, J.C.; Elliott, P.M.; Salomone, O.; Dickinson, K.; Gordon, D.; Hann, C.; Holt, D.W. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br. Heart J. 1995, 74, 620–624. [Google Scholar] [CrossRef]
- Kyriakides, Z.S.; Kremastinos, D.T.; Bofilis, E.; Tousoulis, D.; Antoniadis, A.; Webb, D.J. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart 2000, 84, 176–182. [Google Scholar] [CrossRef][Green Version]
- Nakayama, K.; Ishigai, Y.; Uchida, H.; Tanaka, Y. Potentiation by endothelin-1 of 5-hydroxytryptamine-induced contraction in coronary artery of the pig. Br. J. Pharmacol. 1991, 104, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, D.; Rao, V.; Tumiati, L.C.; Xu, N.; Sheshgiri, R.; Miriuka, S.; Delgado, D.H.; Ross, H.J. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation 2006, 114, I319–I326. [Google Scholar] [CrossRef] [PubMed]
- Kaku, B.; Mizuno, S.; Ohsato, K.; Murakami, T.; Moriuchi, I.; Arai, Y.; Nio, Y.; Ohe, K.; Mabuchi, T.; Takahashi, Y.; et al. Plasma endothelin-1 elevation associated with alcohol-induced variant angina. Jpn. Circ. J. 1999, 63, 554–558. [Google Scholar] [CrossRef][Green Version]
- Wilbert-Lampen, U.; Seliger, C.; Zilker, T.; Arendt, R.M. Cocaine increases the endothelial release of immunoreactive endothelin and its concentrations in human plasma and urine: Reversal by coincubation with sigma-receptor antagonists. Circulation 1998, 98, 385–390. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Shindo, T.; Hanawa, K.; Hasebe, Y.; Tsuburaya, R.; Shiroto, T.; Takahashi, J.; Ito, K.; Ishibashi-Ueda, H.; et al. Association of Adventitial Vasa Vasorum and Inflammation with Coronary Hyperconstriction After Drug-Eluting Stent Implantation in Pigs In Vivo. Circ. J. 2015, 79, 1787–1798. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamoto, M.; Wada, H.; Ito, M.; Nakano, T.; Sasaki, Y.; Narumiya, S.; Shiku, H.; Nishikawa, M. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood 1999, 93, 3408–3417. [Google Scholar] [CrossRef]
- Yasue, H.; Hirai, N.; Mizuno, Y.; Harada, E.; Itoh, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ. J. 2006, 70, 8–13. [Google Scholar] [CrossRef]
- Kaikita, K.; Ogawa, H.; Yasue, H.; Sakamoto, T.; Suefuji, H.; Sumida, H.; Okumura, K. Soluble P-selectin is released into the coronary circulation after coronary spasm. Circulation 1995, 92, 1726–1730. [Google Scholar] [CrossRef]
- Hung, M.Y.; Hung, M.J. Relationship between Inflammation and Vasospastic Angina. Medicina 2023, 59, 318. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Sukhija, R.; Romeo, F.; Sepulveda, J.L. Value of CRP in coronary risk determination. Indian. Heart J. 2007, 59, 173–177. [Google Scholar]
- Verma, S.; Wang, C.H.; Li, S.H.; Dumont, A.S.; Fedak, P.W.; Badiwala, M.V.; Dhillon, B.; Weisel, R.D.; Li, R.K.; Mickle, D.A.; et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Prasad, M.; Zhang, M.; Lennon, R.J.; Herrmann, J.; Lerman, L.O.; Lerman, A. High-sensitivity C-reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non-obstructive coronary artery disease. Am. Heart J. 2017, 190, 1–11. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur. Heart J. 2020, 41, 2952–2961. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients with Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Hunter, I.; Cobban, H.J.; Vandenabeele, P.; MacEwan, D.J.; Nixon, G.F. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: Role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol. Pharmacol. 2003, 63, 714–721. [Google Scholar] [CrossRef]
- Shimokawa, H. Cellular and molecular mechanisms of coronary artery spasm: Lessons from animal models. Jpn. Circ. J. 2000, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wang, B.; Shirvaikar, A.; Khan, S.; Kamat, S.; Schelling, J.R.; Konieczkowski, M.; Sedor, J.R. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J. Clin. Investig. 1999, 103, 1561–1570. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Nishimiya, K.; Hao, K.; Tsuburaya, R.; Ota, H.; Amamizu, H.; Uzuka, H.; Takahashi, J.; Ito, K.; et al. Increased Coronary Perivascular Adipose Tissue Volume in Patients with Vasospastic Angina. Circ. J. 2016, 80, 1653–1656. [Google Scholar] [CrossRef]
- Aalbaek, F.; Bonde, L.; Kim, S.; Boedtkjer, E. Perivascular tissue inhibits rho-kinase-dependent smooth muscle Ca2+ sensitivity and endothelium-dependent H2S signalling in rat coronary arteries. J. Physiol. 2015, 593, 4747–4764. [Google Scholar] [CrossRef]
- França, C.N.; Izar, M.C.; Amaral, J.B.; Tegani, D.M.; Fonseca, F.A. Microparticles as potential biomarkers of cardiovascular disease. Arq. Bras. Cardiol. 2015, 104, 169–174. [Google Scholar] [CrossRef]
- Przybycien-Szymanska, M.M.; Ashley, W.W., Jr. Biomarker Discovery in Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2015, 24, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Scoazec, A.; Ebrahimian, T.; Henry, P.; Mathieu, E.; Tedgui, A.; Mallat, Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001, 104, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Hugel, B.; Ohan, J.; Lesèche, G.; Freyssinet, J.M.; Tedgui, A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation 1999, 99, 348–353. [Google Scholar] [CrossRef]
- Gao, Y.; Li, K.; Li, X.; Li, Q.; Wang, J.; Zhang, S.; Zhang, J. Exploration of cerebral vasospasm from the perspective of microparticles. Front. Neurosci. 2022, 16, 1013437. [Google Scholar] [CrossRef]
- Mezentsev, A.; Merks, R.M.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1106–H1114. [Google Scholar] [CrossRef]
- Angelillo-Scherrer, A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 2012, 110, 356–369. [Google Scholar] [CrossRef]
- Barry, O.P.; Pratico, D.; Lawson, J.A.; FitzGerald, G.A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Investig. 1997, 99, 2118–2127. [Google Scholar] [CrossRef]
- Barry, O.P.; Praticò, D.; Savani, R.C.; FitzGerald, G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998, 102, 136–144. [Google Scholar] [CrossRef]
- Mesri, M.; Altieri, D.C. Endothelial Cell Activation by Leukocyte Microparticles1. J. Immunol. 1998, 161, 4382–4387. [Google Scholar] [CrossRef]
- Mesri, M.; Altieri, D.C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 1999, 274, 23111–23118. [Google Scholar] [CrossRef]
- Caruntu, A.; Moraru, L.; Surcel, M.; Munteanu, A.; Costache, D.O.; Tanase, C.; Constantin, C.; Scheau, C.; Neagu, M.; Caruntu, C. Persistent Changes of Peripheral Blood Lymphocyte Subsets in Patients with Oral Squamous Cell Carcinoma. Healthcare 2022, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Nhek, S.; Clancy, R.; Lee, K.A.; Allen, N.M.; Barrett, T.J.; Marcantoni, E.; Nwaukoni, J.; Rasmussen, S.; Rubin, M.; Newman, J.D.; et al. Activated Platelets Induce Endothelial Cell Activation via an Interleukin-1β Pathway in Systemic Lupus Erythematosus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 707–716. [Google Scholar] [CrossRef]
- Goto, A.; Ito, S.; Kondo, H.; Nomura, Y.; Yasue, N.; Suzumura, H.; Takeda, Y.; Tomimoto, S.; Yamada, Y.; Horio, T.; et al. Evaluation of adjunctive intracoronary administration of acetylcholine following intravenous infusion of ergonovine to provoke coronary artery spasm. J. Cardiol. 1999, 34, 309–316. [Google Scholar]
- Imam, H.; Nguyen, T.H.; Stafford, I.; Liu, S.; Heresztyn, T.; Chirkov, Y.Y.; Horowitz, J.D. Impairment of platelet NO signalling in coronary artery spasm: Role of hydrogen sulphide. Br. J. Pharmacol. 2021, 178, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Waterbury, T.M.; Tarantini, G.; Vogel, B.; Mehran, R.; Gersh, B.J.; Gulati, R. Non-atherosclerotic causes of acute coronary syndromes. Nat. Rev. Cardiol. 2020, 17, 229–241. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Qian, J.; Lin, H.; Zhu, G.; Chen, F.; Liu, X. New Concepts on the Pathophysiology of Acute Coronary Syndrome. Rev. Cardiovasc. Med. 2023, 24, 112. [Google Scholar] [CrossRef]
- Miwa, K.; Igawa, A.; Miyagi, Y.; Nakagawa, K.; Inoue, H. Alterations of autonomic nervous activity preceding nocturnal variant angina: Sympathetic augmentation with parasympathetic impairment. Am. Heart J. 1998, 135, 762–771. [Google Scholar] [CrossRef]
- Agorastos, A.; Boel, J.A.; Heppner, P.S.; Hager, T.; Moeller-Bertram, T.; Haji, U.; Motazedi, A.; Yanagi, M.A.; Baker, D.G.; Stiedl, O. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress 2013, 16, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.B.; Deegan, T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin. Chim. Acta 1974, 55, 389–397. [Google Scholar] [CrossRef]
- Floras, J.S.; Jones, J.V.; Johnston, J.A.; Brooks, D.E.; Hassan, M.O.; Sleight, P. Arousal and the circadian rhythm of blood pressure. Clin. Sci. Mol. Med. Suppl. 1978, 4, 395s–397s. [Google Scholar] [CrossRef]
- Millar-Craig, M.W.; Bishop, C.N.; Raftery, E.B. Circadian variation of blood-pressure. Lancet 1978, 1, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Yasue, H.; Horio, Y.; Nakamura, N.; Fujii, H.; Imoto, N.; Sonoda, R.; Kugiyama, K.; Obata, K.; Morikami, Y.; Kimura, T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: Possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation 1986, 74, 955–963. [Google Scholar] [CrossRef]
- Maseri, A.; L’Abbate, A.; Baroldi, G.; Chierchia, S.; Marzilli, M.; Ballestra, A.M.; Severi, S.; Parodi, O.; Biagini, A.; Distante, A.; et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. N. Engl. J. Med. 1978, 299, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G. Coronary hypersensitivity disorder: The Kounis syndrome. Clin. Ther. 2013, 35, 563–571. [Google Scholar] [CrossRef]
- Portaluppi, F.; Tiseo, R.; Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Fabbian, F. Circadian rhythms and cardiovascular health. Sleep. Med. Rev. 2012, 16, 151–166. [Google Scholar] [CrossRef]
- Kusama, Y.; Kodani, E.; Nakagomi, A.; Otsuka, T.; Atarashi, H.; Kishida, H.; Mizuno, K. Variant angina and coronary artery spasm: The clinical spectrum, pathophysiology, and management. J. Nippon. Med. Sch. 2011, 78, 4–12. [Google Scholar] [CrossRef]
- Watanabe, T.; Kim, S.; Akishita, M.; Kario, K.; Sekiguchi, H.; Fujikawa, H.; Mitsuhashi, T.; Ouchi, Y.; Shimada, K. Circadian variation of autonomic nervous activity in patients with multivessel coronary spasm. Jpn. Circ. J. 2001, 65, 593–598. [Google Scholar] [CrossRef]
- Kim, H.; Cho, S.H.; Cho, K.I.; Kim, B.J.; Im, S.I.; Heo, J.H. Blunted heart rate recovery is associated with coronary artery spasm in patients with suspected vasospastic angina. Clin. Hypertens. 2017, 23, 24. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Kono, S.; Stiffel, V.M.; Gilbert, R.D. Effects of long-term, high-altitude hypoxia on tension and intracellular calcium responses in coronary arteries of fetal and adult sheep. J. Soc. Gynecol. Investig. 2006, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Toyo-oka, T.; Aizawa, T.; Suzuki, N.; Hirata, Y.; Miyauchi, T.; Shin, W.S.; Yanagisawa, M.; Masaki, T.; Sugimoto, T. Increased plasma level of endothelin-1 and coronary spasm induction in patients with vasospastic angina pectoris. Circulation 1991, 83, 476–483. [Google Scholar] [CrossRef]
- Li, L.; Jin, Y.P.; Xia, S.D.; Feng, C. The Biochemical Markers Associated with the Occurrence of Coronary Spasm. BioMed Res. Int. 2019, 2019, 4834202. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; Paini, A.; De Ciuceis, C.; Withers, S.; Greenstein, A.; Heagerty, A.M.; Rizzoni, D. Modulation of Vascular Reactivity by Perivascular Adipose Tissue (PVAT). Curr. Hypertens. Rep. 2018, 20, 44. [Google Scholar] [CrossRef]
- Saxton, S.N.; Withers, S.B.; Nyvad, J.; Mazur, A.; Matchkov, V.; Heagerty, A.M.; Aalkjær, C. Perivascular Adipose Tissue Contributes to the Modulation of Vascular Tone in vivo. J. Vasc. Res. 2019, 56, 320–332. [Google Scholar] [CrossRef]
- Yun, K.H.; Oh, S.K.; Park, E.M.; Kim, H.J.; Shin, S.H.; Lee, E.M.; Rhee, S.J.; Yoo, N.J.; Kim, N.H.; Jeong, J.W.; et al. An increased monocyte count predicts coronary artery spasm in patients with resting chest pain and insignificant coronary artery stenosis. Korean J. Intern. Med. 2006, 21, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Osei, J.; Vaccarino, V.; Wang, M.; Shah, A.S.; Lampert, R.; Li, L.Y.; Ko, Y.A.; Pearce, B.D.; Kutner, M.; Garcia, E.V.; et al. Stress-Induced Autonomic Dysfunction is Associated with Mental Stress-Induced Myocardial Ischemia in Patients with Coronary Artery Disease. Circ. Cardiovasc. Imaging 2024, 17, e016596. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef]
- Lambert, J.; Oc, S.; Worssam, M.D.; Häußler, D.; Solomon, C.U.; Figg, N.L.; Baxter, R.; Imaz, M.; Taylor, J.C.K.; Foote, K.; et al. Network-based prioritization and validation of regulators of vascular smooth muscle cell proliferation in disease. Nat. Cardiovasc. Res. 2024, 3, 714–733. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Carreira, J.; Maia, T.M.; Macedo, A.L.; Matos, A.P.; Neves, J.; Simes, D. Gla Rich Protein (GRP) Mediates Vascular Smooth Muscle Cell (VSMC) Osteogenic Differentiation, Extracellular Vesicle (EV) Calcification Propensity, and Immunomodulatory Properties. Int. J. Mol. Sci. 2024, 25, 12406. [Google Scholar] [CrossRef] [PubMed]
- McChord, J.; Ong, P. Bridging the Gender Gap in Cardiovascular Medicine: Addressing Drug Intolerances and Personalized Care for Women with Angina/Ischemia with Non-Obstructive Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, G.-P.; Tomescu, D.-R.; Pleș, L.; Panaitescu, A.-M.; Dragosloveanu, Ș.; Scheau, C.; Sima, R.-M.; Coman, I.-S.; Grigorean, V.-T.; Cochior, D. Implications of using artificial intelligence in the diagnosis of sepsis/sepsis shock. Germs 2024, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Karpouzas, G.A.; Rezaeian, P.; Ormseth, S.R.; Hollan, I.; Budoff, M.J. Epicardial Adipose Tissue Volume As a Marker of Subclinical Coronary Atherosclerosis in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1412–1420. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Quan, X.Q.; Wang, R.C.; Zhang, Q.; Zhang, C.T.; Sun, L. The predictive value of lymphocyte-to-monocyte ratio in the prognosis of acute coronary syndrome patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 338. [Google Scholar] [CrossRef]
- Albayati, S.; Li, N.; Unsworth, A.J.; Liverani, E. Platelet-lymphocyte co-culture serves as an ex vivo platform of dynamic heterotypic cross-talk. J. Cell Commun. Signal 2022, 16, 661–675. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, N.; Hui, H.; Wang, Y.; Liu, F.; Xu, L.; Liu, M.; Rao, Z.; Yuan, Z.; Shang, Y.; et al. Endothelial cell-derived tetrahydrobiopterin prevents aortic valve calcification. Eur. Heart J. 2022, 43, 1652–1664. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Ravankhah, M.; Ahmadi, M.; Keshavarzian, O.; Azari, I.; Abdollahi, M.; Rezaei, M.; Akbari, H. L-arginine impact on inflammatory and cardiac markers in patients undergoing coronary artery bypass graft: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2024, 24, 641. [Google Scholar] [CrossRef]
- Wei, C.; Gao, Z.; Knabel, M.; Ulbricht, M.; Senekowitsch, S.; Erfurt, P.; Maggi, N.; Zwick, B.; Eickner, T.; Matin-Mann, F.; et al. Development of a drug delivering round window niche implant for cochlear pharmacotherapy. Drug Deliv. 2024, 31, 2392755. [Google Scholar] [CrossRef] [PubMed]
- Brami, P.; Fischer, Q.; Pham, V.; Seret, G.; Varenne, O.; Picard, F. Evolution of Coronary Stent Platforms: A Brief Overview of Currently Used Drug-Eluting Stents. J. Clin. Med. 2023, 12, 6711. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.-A.; Georgatos-Garcia, S.; Scheau, A.-E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Vermeltfoort, I.A.; Raijmakers, P.G.; Kamphuisen, P.W. Improved myocardial perfusion preceding clinical response on bosentan treatment for coronary vasospasm. Acta Cardiol. 2009, 64, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, H.; Dieudé, M.; Hébert, M.J. Endothelial Dysfunction in Kidney Transplantation. Front. Immunol. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Dragosloveanu, C.D.M.; Potop, V.; Coviltir, V.; Dinu, V.; Păsărică, M.; Ducan, I.L.; Maier, C.; Dragosloveanu, Ş. Prematurity-Risk Factor or Coincidence in Congenital Glaucoma? Medicina 2022, 58, 334. [Google Scholar] [CrossRef]
- Li, Q.; Pang, B.; Dang, E.; Wang, G. Endothelial Dysfunction in Psoriasis: An Integrative Review. J. Investig. Dermatol. 2024, 144, 1935–1942. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, N.G.; Im, J.Y.; Lee, J.H.; Kim, J.; Jeon, Y.; Choi, Y.J.; Lee, J.H.; Uemura, A.; Park, D.H.; et al. Targeting the Mitochondrial Chaperone TRAP1 Alleviates Vascular Pathologies in Ischemic Retinopathy. Adv. Sci. 2024, 11, e2302776. [Google Scholar] [CrossRef]
| Diagnostic Modality | Key Features | Advantages | Limitations |
|---|---|---|---|
| Electrocardiography |
|
|
|
| Exercise Stress Testing |
|
|
|
| Holter Monitoring |
|
|
|
| Coronary Angiography with Spasm Provocation Test |
|
|
|
| Intravascular Ultrasound |
|
|
|
| Optical Coherence Tomography |
|
|
|
| Cardiac Magnetic Resonance Imaging |
|
|
|
| Computed Tomography Angiography |
|
|
|
| Dual-Acquisition Cardiac CT |
|
|
|
| Positron Emission Tomography |
|
|
|
| Pathophysiological Factor | Mechanism of Action | Clinical Relevance |
|---|---|---|
| microparticles | ↑ endothelial inflammation ↑ expression of E-selectin, ICAM-1, VCAM-1 | potential biomarker for risk stratification target for novel anti-inflammatory therapies |
| endothelial dysfunction | ↓ availability of NO ↑ expression of adhesion molecule | justifies use of endothelial-protective drugs (e.g., statins, ACE inhibitors, NO donors) |
| platelet activation | IL-1β-dependent endothelial activation impaired NO response hyperaggregability | supports the role of P2Y12 inhibitors, aspirin, and novel platelet-targeted therapies |
| inflammatory mediators | ↑ inflammation ↑ vasoconstriction vascular remodeling | suggests IL-6, COX-2, and other inflammatory pathway inhibitors could improve CAS management |
| vascular smooth muscle hypercontractility | ↑ vascular tone due (hypercontractility of smooth muscle cells) | provides rationale for vasodilators targeting vascular smooth muscle relaxation |
| Rho-kinase pathway activation | ↑ sensitivity to vasoconstrictors impaired vascular relaxation | potential target for Rho-kinase inhibitors in CAS treatment |
| myosin light chain phosphorylation | ↑ vasoconstriction increased spasm severity | correlates with CAS severity may predict recurrent vasospastic events |
| mast cell activation | ↑ vascular hyperreactivity endothelial dysfunction | potential role for mast cell stabilizers as ancillary treatment of CAS |
| mild atherosclerotic lesions | predisposition for vasospasm by altering vascular reactivity | lipid-lowering therapies may reduce susceptibility for CAS in mild atherosclerosis |
| serotonin/histamine sensitivity | ↑ vasospastic responses (in segments with mild atherosclerosis) | potential therapeutic targets in serotonin pathway |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savulescu-Fiedler, I.; Baz, R.O.; Baz, R.A.; Scheau, C.; Gegiu, A. Coronary Artery Spasm: From Physiopathology to Diagnosis. Life 2025, 15, 597. https://doi.org/10.3390/life15040597
Savulescu-Fiedler I, Baz RO, Baz RA, Scheau C, Gegiu A. Coronary Artery Spasm: From Physiopathology to Diagnosis. Life. 2025; 15(4):597. https://doi.org/10.3390/life15040597
Chicago/Turabian StyleSavulescu-Fiedler, Ilinca, Radu Octavian Baz, Radu Andrei Baz, Cristian Scheau, and Andrei Gegiu. 2025. "Coronary Artery Spasm: From Physiopathology to Diagnosis" Life 15, no. 4: 597. https://doi.org/10.3390/life15040597
APA StyleSavulescu-Fiedler, I., Baz, R. O., Baz, R. A., Scheau, C., & Gegiu, A. (2025). Coronary Artery Spasm: From Physiopathology to Diagnosis. Life, 15(4), 597. https://doi.org/10.3390/life15040597







