Applicability of the Instrumented Pendulum Test for Assessing Limb Viscoelastic Properties in Neurological and Internal Diseases: A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Search Criteria
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Analysis
2.5. Instrumentation
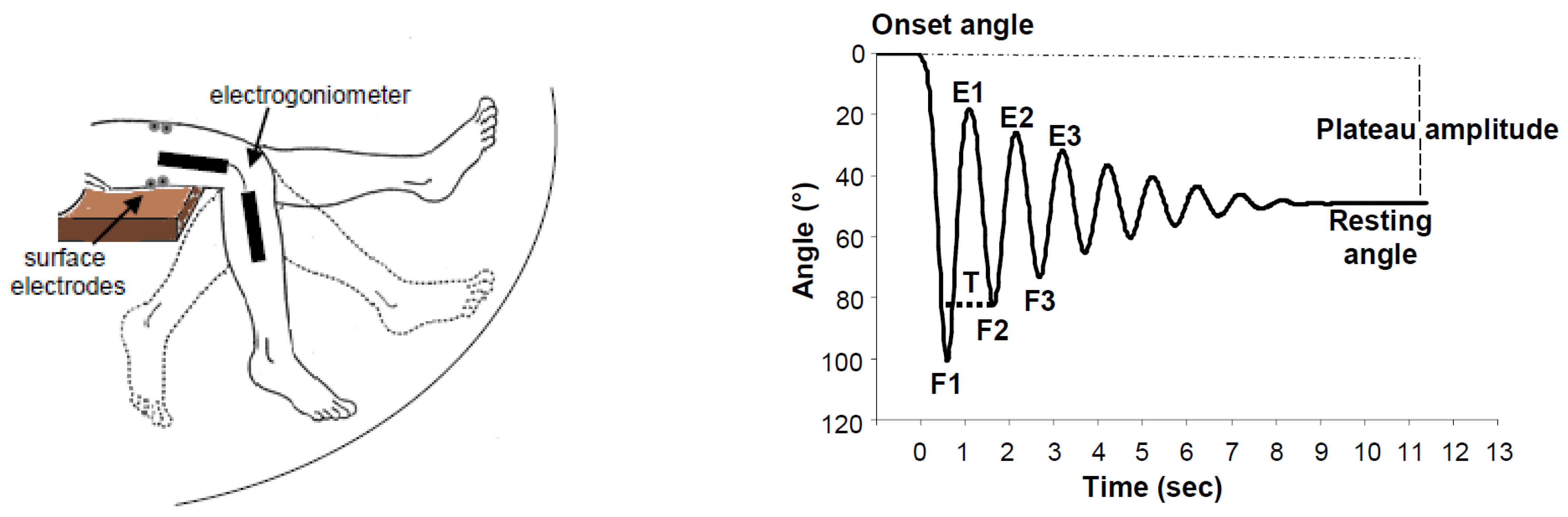
2.6. Methodology of the Instrumented Pendulum Test
2.6.1. Lower Limb Evaluation
2.6.2. Upper Limb Evaluation
2.7. Estimation of Stiffness, Viscosity, and Damping
3. Results
3.1. Internal Diseases
3.1.1. Rheumatic Diseases
3.1.2. Chronic Obstructive Pulmonary Disease (COPD)
3.2. Neurological Diseases
3.2.1. Hypertonia
3.2.2. Hypotonia
3.3. Neural Adaptive Mechanisms and Their Influence on Lower Limb Stiffness
3.4. Methodological Limitations and Proposed Guidelines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, A.V. The Heat of Shortening and the Dynamic Constants of Muscle. Proc. R. Soc. Lond. B Biol. Sci. 1938, 126, 136–195. [Google Scholar] [CrossRef]
- Kodama, Y.; Masuda, S.; Ohmori, T.; Kanamaru, A.; Tanaka, M.; Sakaguchi, T.; Nakagawa, M. Response to mechanical properties and physiological challenges of fascia: Diagnostic and rehabilitative therapeutic intervention for myofascial system disorders. Bioengineering 2023, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Gareis, H.; Solomonow, M.; Baratta, R.; Best, R.; D’Ambrosia, R. The isometric length-force models of nine different skeletal muscles. J. Biomech. 1992, 25, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Quintern, J.; Berger, W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 1981, 104, 431–449. [Google Scholar] [CrossRef]
- Hufschmidt, A.; Mauritz, K.H. Chronic transformation of muscle in spasticity: A peripheral contribution to increased tone. J. Neurol. Neurosurg. Psychiatry 1985, 48, 676–685. [Google Scholar] [CrossRef]
- Meyer, G.A.; McCulloch, A.D.; Lieber, R.L. A nonlinear model of passive muscle viscosity. J. Biomech. Eng. 2011, 133, 091007. [Google Scholar] [CrossRef]
- Mauro, A.; Adams, W.R. The structure of the sarcolemma of the frog skeletal muscle fiber. J. Biophys. Biochem. Cytol. 1961, 10, 177–185. [Google Scholar] [CrossRef]
- Moss, R.L.; Halpern, W. Elastic and viscous properties of resting frog skeletal muscle. Biophys. J. 1977, 17, 213–228. [Google Scholar] [CrossRef]
- Stackhouse, S.K.; Reisman, D.S.; Binder-Macleod, S.A. Challenging the role of pH in skeletal muscle fatigue. Phys. Ther. 2001, 81, 1897–1903. [Google Scholar] [CrossRef]
- Amir, A.; Kim, S.; Stecco, A.; Jankowski, M.P.; Raghavan, P. Hyaluronan homeostasis and its role in pain and muscle stiffness. PM & R 2022, 14, 1490–1496. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Rymer, W.Z. Reflex and intrinsic changes induced by fatigue of human elbow extensor muscles. J. Neurophysiol. 2001, 86, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, P.S. Joint stiffness. In Mechanics of Joint: Physiology, Pathophysiology and Treatment; Wright, V., Radin, E.L., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 203–218. [Google Scholar]
- Timiras, P.S.; Navazio, F.M. The skeleton, joints, and skeletal and cardiac muscles. In The Physiological Basis for Aging and Geriatrics, 4th ed.; Timiras, P.S., Ed.; Informa Healthcare USA: New York, NY, USA, 2007; pp. 329–343. [Google Scholar]
- Bohinc, K.; Vantur, N.; Torkar, D.; Lampe, T.; Hribernik, M.; Jakovljević, M. Knee stiffness and viscosity: New implementation and perspectives in prosthesis development. Bosn. J. Basic Med. Sci. 2017, 17, 164–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.C.; Ju, M.S.; Huang, H.W. Gender and age effects on elbow joint stiffness in healthy subjects. Arch. Phys. Med. Rehabil. 2005, 86, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Creze, M.; Soubeyrand, M.; Yue, J.L.; Gagey, O.; Maître, X.; Bellin, M.F. Magnetic resonance elastography of the lumbar back muscles: A preliminary study. Clin. Anat. 2018, 31, 514–520. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Raghavan, P. Emerging Therapies for Spastic Movement Disorders. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 633–644. [Google Scholar] [CrossRef]
- Badj, T.; Bowman, B. Testing and modeling of spasticity. J. Biomed. Eng. 1982, 4, 90–96. [Google Scholar]
- Lin, D.C.; Rymer, W.Z. A quantitative analysis of pendular motion of the lower leg in spastic human subjects. IEEE Trans. Biomed. Eng. 1991, 38, 906–918. [Google Scholar]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Le Cavorzin, P.; Hernot, X.; Bartier, O.; Carrault, G.; Chagneau, F.; Gallien, P.; Allain, H.; Rochcongar, P. Evaluation de la mesure de la spasticité par le pendulum test [Evaluation of pendulum testing of spasticity]. Ann. Readapt. Med. Phys. 2002, 45, 510–516. [Google Scholar] [CrossRef]
- Valle, M.S.; Casabona, A.; Sgarlata, R.; Garozzo, R.; Vinci, M.; Cioni, M. The pendulum test as a tool to evaluate the passive knee stiffness and viscosity of patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2006, 7, 89. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Liang, H.; Wu, J. Knee joint kinematics of the pendulum test in children with and without Down syndrome. Gait Posture 2020, 76, 311–317. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.S.N.; Dos Santos Couto Paz, C.C.; Ribeiro, T.G.; Fachin-Martins, E. Assessment of Passive Upper Limb Stiffness and Its Function in Post-Stroke Individuals Wearing an Inertial Sensor during the Pendulum Test. Sensors 2023, 23, 3487. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Eyvazpour, R.; Salahshour, N.; Azghani, M.R. Objective assessment of spasticity by pendulum test: A systematic review on methods of implementation and outcome measures. Biomed. Eng. Online 2020, 19, 82. [Google Scholar] [CrossRef]
- Wartenberg, R. Pendulousness of the legs as a diagnostic test. Neurology 1951, 1, 8–24. [Google Scholar]
- Oatis, C.A. The use of a mechanical model to describe the stiffness and damping characteristics of the knee joint in healthy adults. Phys. Ther. 1993, 73, 740–749, Erratum in Phys. Ther. 2005, 85, 1390–1391. [Google Scholar] [CrossRef]
- Fowler, E.G.; Nwigwe, A.I.; Ho, T.W. Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Dev. Med. Child Neurol. 2000, 42, 182–189. [Google Scholar]
- Szopa, A.; Domagalska-Szopa, M.; Kidoñ, Z.; Syczewska, M. Quadriceps femoris spasticity in children with cerebral palsy: Measurement with the pendulum test and relationship with gait abnormalities. J. Neuroeng. Rehabil. 2014, 11, 166. [Google Scholar] [CrossRef]
- Lin, C.C.; Ju, M.S.; Lin, C.W. The pendulum test for evaluating spasticity of the elbow joint. Arch. Phys. Med. Rehabil. 2003, 84, 69–74. [Google Scholar] [CrossRef]
- Winter, D.A. Anthropometry. In Biomechanics and Motor Control of Human Movement; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Casabona, A.; Valle, M.S.; Pisasale, M.; Pantò, M.R.; Cioni, M. Functional assessments of the knee joint biomechanics by using pendulum test in adults with Down syndrome. J. Appl. Physiol. 2012, 113, 1747–1755. [Google Scholar] [CrossRef]
- Burks, K.; Keegan, K. Objective measurement of stiffness in knee osteoarthritis. Orthop. Nurs. 2006, 25, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wachter, K.C.; Kaeser, H.E.; Gühring, H.; Ettlin, T.M.; Mennet, P.; Müller, W. Muscle damping measured with a modified pendulum test in patients with fibromyalgia, lumbago, and cervical syndrome. Spine 1996, 21, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.S.; Casabona, A.; Di Fazio, E.; Crimi, C.; Russo, C.; Malaguarnera, L.; Crimi, N.; Cioni, M. Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system. Sci. Rep. 2021, 11, 18077. [Google Scholar] [CrossRef] [PubMed]
- Joghtaei, M.; Arab, A.M.; Hashemi-Nasl, H.; Joghataei, M.T.; Tokhi, M.O. Assessment of passive knee stiffness and viscosity in individuals with spinal cord injury using pendulum test. J. Spinal Cord Med. 2015, 38, 170–177. [Google Scholar] [CrossRef]
- Bianchi, L.; Monaldi, F.; Paolucci, S.; Iani, C.; Lacquaniti, F. Quantitative analysis of the pendulum test: Application to multiple sclerosis patients treated with botulinum toxin. Funct. Neurol. 1999, 14, 79–92. [Google Scholar]
- Huang, H.W.; Ju, M.S.; Lin, C.C. Flexor and extensor muscle tone evaluated using the quantitative pendulum test in stroke and parkinsonian patients. J. Clin. Neurosci. 2016, 27, 48–52. [Google Scholar] [CrossRef]
- Huang, H.W.; Ju, M.S.; Wang, W.C.; Lin, C.C. Muscle tone of upper limbs evaluated by quantitative pendulum test in patients with acute cerebellar stroke. Acta Neurol. Taiwan 2009, 18, 250–254. [Google Scholar]
- Ferreira, D.M.; Liang, H.; Wu, J. Effect of body position and external ankle load on the pendulum test in adults. Knee 2023, 42, 99–106. [Google Scholar] [CrossRef]
- Lin, C.C.; Ju, M.S.; Huang, H.W. Muscle tone in diabetic polyneuropathy evaluated by the quantitative pendulum test. Arch. Phys. Med. Rehabil. 2007, 88, 368–373. [Google Scholar] [CrossRef]
- Valle, M.S.; Cioni, M.; Pisasale, M.; Pantò, M.R.; Casabona, A. Timing of Muscle Response to a Sudden Leg Perturbation: Comparison between Adolescents and Adults with Down Syndrome. PLoS ONE 2013, 8, e81053. [Google Scholar] [CrossRef]
- Casabona, A.; Valle, M.S.; Dominante, C.; Laudani, L.; Onesta, M.P.; Cioni, M. Effects of Functional Electrical Stimulation Cycling of Different Duration on Viscoelastic and Electromyographic Properties of the Knee in Patients with Spinal Cord Injury. Brain Sci. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.B.; Nossent, J.C.; Preen, D.B.; Keen, H.I.; Inderjeeth, C.A. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-based Studies. J. Rheumatol. 2021, 48, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ropes, M.W.; Bennett, G.A.; Cobb, S.; Jacox, R.; Jessar, R.A. Revision of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1959, 2, 16–20. [Google Scholar] [CrossRef]
- Wright, V.; Johns, R.J. Physical factors concerned with the stiffness of normal or diseased joints. Bull. Johns Hopkins Hosp. 1960, 160, 215–231. [Google Scholar]
- Such, C.H.; Unsworth, A.; Wright, V.; Dowson, D. Quantitative study of stiffness in the knee joint. Ann. Rheum. Dis. 1975, 34, 286–291. [Google Scholar]
- Helliwell, P.S.; Howe, A.; Wright, V. The measurement of stiffness in the rheumatoid hand. Eng. Med. 1987, 16, 203–207. [Google Scholar]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef]
- Yentes, J.M.; Wai-Yan Liu, W.Y.; Zhang, K.; Markvicka, E.; Rennard, S.D.I. Updated Perspectives on the Role of Biomechanics in COPD: Considerations for the Clinicians. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2653–2675. [Google Scholar] [CrossRef]
- Deng, M.; Zhou, X.; Li, Y.; Yin, Y.; Liang, C.; Zhang, Q.; Lu, J.; Wang, M.; Wang, Y.; Sun, Y.; et al. Ultrasonic Elastography of the Rectus Femoris, a Potential Tool to Predict Sarcopenia in Patients With Chronic Obstructive Pulmonary Disease. Front. Physiol. 2022, 12, 783421. [Google Scholar] [CrossRef]
- Brown, R.A.; Lawson, D.A.; Leslie, G.C.; MacArthur, A.; MacLennan, W.J.; McMurdo, M.E.; Mutch, W.J.; Part, N.J. Does the Wartenberg pendulum test differentiate quantitatively between spasticity and rigidity? A study in elderly stroke and Parkinsonian patients. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1178–1186. [Google Scholar] [CrossRef]
- Jamshidi, M.; Smith, A.W. Clinical measurement of spasticity usiong the pendulum test: Comparison of electrogoniometric and videotape analyses. Arch. Phys. Med. Rehabil. 1996, 77, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Nordmark, E.; Andersson, G. Wartenberg pendulum test: Objective quantification of muscle tone in children with spastic diplegia undergoing selective dorsal rhizotomy. Dev. Med. Child Neurol. 2002, 44, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.H. Dynamics of postural control in the child with Down syndrome. Phys. Ther. 1985, 65, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Korenberg, J.R.; Chen, X.N.; Schipper, R.; Sun, Z.; Gonsky, R.; Gerwehr, S.; Carpenter, N.; Daumer, C.; Dignan, P.; Disteche, C.; et al. Down syndrome phenotypes: The consequences of chromosomal imbalance. Proc. Natl. Acad. Sci. USA 1994, 91, 4997–5001. [Google Scholar] [CrossRef]
- Aimola, E.; Valle, M.S.; Casabona, A. Effects of predictability of load magnitude on the response of the Flexor Digitorum Superficialis to a sudden fingers extension. PLoS ONE 2014, 9, e109067. [Google Scholar] [CrossRef]
- de Azevedo, E.R.; Maria, R.M.; Alonso, K.C.; Cliquet, A., Jr. Posture Influence on the Pendulum Test of Spasticity in Patients with Spinal Cord Injury. Artif. Organs 2015, 39, 1033–1037. [Google Scholar] [CrossRef]
- Willaert, J.; Desloovere, K.; Van Campenhout, A.; Ting, L.H.; De Groote, F. Movement History Influences Pendulum Test Kinematics in Children With Spastic Cerebral Palsy. Front. Bioeng. Biotechnol. 2020, 8, 920. [Google Scholar] [CrossRef]

| Study | Design | Objective | Population | Methods | Results |
|---|---|---|---|---|---|
| Knee stiffness and viscosity: New implementation and perspectives in prosthesis development [14] | Experimental study | To identify the contribution of periarticular and intraarticular soft tissues to stiffness and viscosity of knee. | One female cadaver | The instrumented pendulum test at knee was executed by a machine vision system equipped with 6 passive infrareds marked. Progressive removal of soft tissues around the knee with contemporary and multiple trial of evaluation. | Knee damping was reduced. The contribution to the knee damping was of 10% for skin, 20% for ligaments and 40% for muscles and tendons. |
| Gender and age effects on elbow joint stiffness in healthy [15] | Experimental study | To study the relationships among biomechanical parameters and demographic factors at elbow. | 192 healthy men (n.111) and women (n. 81) aged 20~70 y. | The instrumented pendulum test at elbow was done by an electrogoniometer to measure number of swings, relaxation index, stiffness coefficient and damping coefficient. | Stiffness and damping coefficients of the elbow joint were similar in men and women when the data were normalized for body weight. |
| A quantitative analysis of pendular motion of the lower leg in spastic human subjects [20] | Experimental study | To investigate gravity induced oscillations of the lower leg in subjects with and without spasticity. | 3 patients with spastic hemiparesis (1 woman and two men aged respectively 36, 79 and 45 y). 1 healthy woman (25 y) | The instrumented pendulum test at knee was done by wearing a plastic splint with a metal rod connected to a potentiometer to record pendular oscillation. Surface EMG recordings from muscles extensors and flexors of knee. | Motion of a spastic limb during the pendulum test follows a nonlinear model with a variance usually exceeding 90%. |
| Evaluation of pendulum testing of spasticity [22] | Experimental study | To identify which parameters of the pendulum test are sensitive to spasticity. | 15 subjects with spasticity (mean age 61.5 ± 9.0 y, 4 females) and 10 control subjects (mean age 32 ± 9.4 y, 3 females), matched for age, sex and morphometric criteria. | The instrumented pendulum test at knee was performed by an electrogoniometer. Surface EMG recordings of m. rectus femoris and m. semimebranosus during pendulum test. Mathematic model of lower limb swinging. | Viscosity coefficient was found to be significantly different (p = 0.014) in the group of patients with spasticity in respect to the control group. Elasticity was not significantly different between the two groups (p = 0.064). |
| The pendulum test as a tool to evaluate the passive knee stiffness and viscosity of patients with rheumatoid arthritis [23] | Experimental study | To study the biomechanical changes of knee caused by rheumatoid arthritis | 8 women with rheumatoid arthritis (mean age 52 ± 10 y) and 8 healthy women (mean age 49 ± 10.5 y). | The instrumented pendulum test at knee was done with a motion analysis system (Zebris CMS H) to evaluate kinematic parameters and to calculate the viscoelastic properties of knee joint. | Knee stiffness increased significantly in the group of patients in respect to the control group and it was the main factor causing a reduction of swing oscillations. Knee stiffness was significantly correlated to the severity of disease. |
| Knee joint kinematics of the pendulum test in children with and without Down syndrome [24] | Experimental analytical study | To identify the difference of knee kinematics between children with and without Down syndrome | 15 children with Down syndrome (DS) (4 males, mean age 8.46 ± 1.75) and 15 children without DS (7 males, 8.33 ± 1.87) | The instrumented pendulum test at knee was performed by an eight cameras motion capture system (VICON). Measurements of kinematic parameters of pendulum test. EMG recording from m. m. rectus femoris, vastus lateralis and vastus medialis. Calculation of stiffness and damping coefficients. | Authors suggested a greater stiffness of knee joint, in the group of children with DS. Inclusion of an ankle load improved the joint knee stiffness by significantly increasing the number of cycles and the stiffness coefficient in both groups of children with and without DS. |
| Assessment of Passive Upper Limb Stiffness and Its Function in Post-Stroke Individuals Wearing an Inertial Sensor during the Pendulum Test [25] | Observational analytical study | To identify the correlations between pendulum test parameters and some clinical scales. | 7 subjects chronic post stroke (2 men, mean age 51.9 ± 11.2). | The instrumented pendulum test at elbow was executed by a single wearable sensor attached to the forearm. Measurements of kinematic parameters and natural frequency. MAS, MAL and FM clinical scales. Calculation of damping and stiffness coefficients and damping ratio. | Stiffness coefficient was correlated to the natural frequency of pendulum oscillations (r = 0.96, p = 0.003). No correlations of stiffness or damping coefficients with clinical scales. |
| The pendulum test for evaluating spasticity of the elbow joint [31] | Experimental study | To test a modified pendulum test to quantify spasticity at elbow. | 11 men with chronic post -stroke condition (mean age 57.7 ± 16.1 y) and 11 able body men (mean age 59.5 ± 11.8 y). | A clock pendulum test with an electrogoniometer was set up to allow a more comfortable posture and an increased inertia. The number of swings, relaxation index, stiffness and damping coefficients were measured. | Damping coefficient and damping ratio increased in the affected side and worsened with the severity of spasticity. |
| Functional assessments of the knee joint biomechanics by using pendulum test in adults with Down syndrome [33] | Experimental study | To study the biophysical characteristics of hypotonic muscles | 10 adults (4 women, mean age 26 ± 5 y) with Down syndrome (DS) and 10 adults without DS (5 women, mean age 24 ± 5 y). | The instrumented pendulum test at knee was done by using an electrogoniometer to assess kinematic and viscoelastic properties of lower limbs in adults with DS. | Damping coefficient was significantly reduced in persons with DS than control group. Stiffness was similar between subjects with or without DS. |
| Objective measurements of stiffness in knee osteoarthritis [34] | Observational study | To test reliability of pendulum test for evaluation of knee stiffness in older adults with or without knee osteo-arthritis. | 41 participants (8 men) (29 with a diagnosis of knee osteoarthritis and 12 without), mean age 67.6 ± 7.6. | The instrumented pendulum test at knee was done by a three cameras motion capture system (VICON). Stiffness and damping coefficients were calculated within and between each group. | Within-participant variability for stiffness and damping was low (respectively 0.55% and 8.92%) whereas between-participant variability was high (respectively 99.45% and 91.08%). |
| Muscle damping measured with a modified pendulum test in patients with fibromyalgia, lumbago, and cervical syndrome [35] | Observational study | To quantify the muscle tension and in particular muscle damping. | 33 able bodied subjects (aged 20–62 yrs), 15 patients with fibromyalgia (aged 41~82 y), 22 with lumbago (aged 27~68 y), 21 with cervicalgia (aged 27~68 y). | The instrumented pendulum test at knee was executed by means of an electrogoniometer. Muscle damping was calculated for each group. | In respect to the control group, in patients with fibromyalgia and in most of patients with chronic lumbago and cervical syndrome, damping values are elevated (overdamping) |
| Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system [36] | Experimental study | To quantify the viscoelastic properties of lower limbs of patients with chronic obstructive pulmonary disease (COPD). | 11 patients with COPD (5 men, mean age 63.8 ± 11.4) | The instrumented pendulum test at knee was done by an electrogoniometer to evaluate kinematic and viscoelastic parameters of lower limbs and electromyographic reflex responses of m. rectus femoris and biceps femoris caput longus. | Significant reduction of knee stiffness and viscosity coefficients, in comparison with the control group. |
| Assessment of passive knee stiffness and viscosity in individuals with spinal cord injury using pendulum test [37] | Observational study | Measurements of stiffness and viscosity in patients with spinal cord injury. | 15 subjects with paraplegia due to incomplete spinal cord injury (grade C, AI-C) (men 8, mean age 34.60 ± 9.18) and 15 able-body subjects (men 8, 30.66 ± 11.13 yrs). | The instrumented pendulum test at knee was done by using an electrogoniometer to measure viscoelastic parameters of the knee. | Stiffness of subjects with paraplegia was significantly increased in respect to the control group (p = 0.01). Viscosity was not significantly different. |
| Quantitative analysis of the pendulum test: application to multiple sclerosis patients treated with botulinum toxin [38] | Experimental study | To set up a quantitative analytical method to quantify the effects of botulinum toxin type A (BoNT-A) on patients with spasticity. | 6 patients affected by progressive multiple sclerosis (aged 29–55 y, 1 male). | The instrumented pendulum test at knee was done with a 3-D motion analysis system (ELITE system). Recordings were done before and after the treatment with botulinum toxin type A. | Before treatment with BoNT-A of knee flexor muscles, both viscosity and stiffness coefficients were significantly higher than the control group. After BoNT-A both knee stiffness and viscosity coefficients were decreased. |
| Flexor and extensor muscle tone evaluated using the quantitative pendulum test in stroke and parkinsonian patients [39] | Experimental study | To investigate the difference in hypertonia between spasticity and rigidity. | 21 subjects with Parkinson’s disease (men 9, mean age 68.3 ± 9.9) and 14 subjects with post-stroke (men 9, mean age 65.1 ± 8.8). Control group was composed by 22 subjects (men 11, mean age 63.2 ± 7.5). | The instrumented pendulum test at elbow was performed by using an electrogoniometer to quantify kinematic parameters, stiffness, viscosity and damping. | Stiffness coefficient was similar between patients with pyramidal or extrapyramidal hypertonia. The instrumented pendulum test does not allow to differentiate hypertonia of different origin. The clinical characteristics of these patients are not reported. |
| Muscle tone of upper limbs evaluated by quantitative pendulum test in patients with acute cerebellar stroke [40] | Observational study | To quantify the muscle tone of upper limbs. | 8 subjects (4 females, mean age 70 ± 8.2 y ) with acute cerebellar stroke. | The instrumented pendulum test at elbow was done by an electrogoniometer to measure number of swings, relaxation index, stiffness coefficient and damping coefficient. | Damping coefficient of the affected side was significantly smaller than that on the intact arm. |
| Effect of body position and external ankle load on the pendulum test in adults [41] | Experimental analytical study | To test if the body position affects the lower limb kinematics and the estimation of viscosity and stiffness. To investigate the effects of external ankle loads on kinematics of pendulum test. | 20 young healthy adults (10 men, mean age 22.4 ± 3.1). | The instrumented pendulum test at knee was done by using 8 cameras motion capture system (VICON). EMG recording from m. rectus femoris, vastus lateralis and vastus medialis. Calculation of stiffness and damping coefficients | Damping coefficient was lower in the upright position than both the supine and inclined positions (p < 0.001) Heavier load conditions (3% and 6% of body weight) produced lower stiffness and damping coefficients that 0% load coefficient. |
| Muscle tone in diabetic polyneuropathy evaluated by the quantitative pendulum test [42] | Case control study | To quantify the muscle tone of upper limbs | n. 53 subjects (mean age 58.4 ± 9.5 y) with diabetic neuropathy and 128 healthy subjects (mean age 58.1 ± 11.1 y). | The instrumented pendulum test at elbow was done by using an electrogoniometer to measure number of swings, relaxation index, stiffness constant and damping coefficient. | Damping coefficient decreased significantly in patients with diabetic neuropathy in respect to the control group. Stiffness constant was not significantly different. |
| Timing of Muscle Response to a Sudden Leg Perturbation: Comparison between Adolescents and Adults with Down Syndrome [43] | Experimental study | To identify the adaptive response to a sudden lower limb load perturbation in adolescents and adults with Down syndrome. | 10 adult subjects with Down syndrome (DS) (age 25.5 ± 3.7 yrs) and 10 adolescents with DS (age 13.9 ± 3.1 yrs). | The instrumented pendulum test at knee was done with an electrogoniometer to evaluate kinematic and viscoelastic parameters and electromyographic reflex responses of m. rectus femoris and biceps femoris. | Significant differences of damping and stiffness were observed between adolescents and adults with Down Syndrome. Different latencies of surface EMG responses of rectus femoris in the two groups. |
| Effects of Functional Electrical Stimulation Cycling of Different Duration on Viscoelastic and Electromyographic Properties of the Knee in Patients with Spinal Cord Injury [44] | Experimental study | To identify the best temporal dose of Functional Electrical Stimulation (FES). | 7 young adults (1 woman, mean age 32.3 ± 4.8) with flaccid paraplegia due to a lesion of spinal cord. AIS-A (5 subjects) and B (2 subjects). | The instrumented pendulum test at knee was done by an electrogoniomter to identify changes induced by functional electric stimulation (FES) on knee angle excursion, stiffness and viscosity. | Stiffness of knee increased statistically both after 20- and 40-min exercises of FES cycling. The best significant functional changes of knee mobility were obtained with the FES dose of 20 min. Viscosity was not significantly modified. |
| Study | Trunk/Hip | sEMG | Trials | Stiffness | Viscosity |
|---|---|---|---|---|---|
| Angle | Muscles | n° | K | B | |
| Knee stiffness and viscosity: New implementation and perspectives in prosthesis development [14] | 0° | - | 1 | Kg m2/s2 | Kg m2/s |
| Evaluation of pendulum testing of spasticity [22] | 90° | - | 5–10 | N/A | N/A |
| The pendulum test as a tool to evaluate the passive knee stiffness and viscosity of patients with rheumatoid arthritis [23] | 40° | - | 10 | N/rad m4 | N-m-s/rad |
| Knee joint kinematics of the pendulum test in children with and without Down syndrome [24] | 90° | - | 5 | N-m/rad | N-m-s/rad |
| Functional assessments of the knee joint biomechanics by using the pendulum test in adults with Down syndrome [33] | 40° | RF | 10 | N-m/rad | N-m-s/rad |
| Objective measurements of stiffness in knee osteoarthritis [34] | 90° | - | 3 | N-m/rad | N-m/rad s |
| Muscle damping measured with a modified pendulum test in patients with fibromyalgia, lumbago, and cervical syndrome [35] | 40° | VL, BF | 2–3 | N/A | N/A |
| Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system [36] | 40° | RF, BF | 10 | N-m/rad-Normalized for G.T. | N-m-s/rad |
| Assessment of passive knee stiffness and viscosity in individuals with spinal cord injury using the pendulum test [37] | 45° | - | 3 | N-m/rad m4 | N-m-s/rad m4 |
| Quantitative analysis of the pendulum test: application to multiple sclerosis patients treated with botulinum toxin [38] | 45° | - | 56–61 | N-m/rad | N-m-s/rad |
| Effect of body position and external ankle load on the pendulum test in adults [41] | 0°, 45°, 90° | RF, VL, VM | 5 | N-m/rad | N-m-s/rad |
| Timing of muscle response to a sudden leg perturbation: comparison between adolescents and adults with Down syndrome [43] | 40° | RF, BF | 10 | N-m/rad | N-m-s/rad |
| Effects of functional electrical stimulation cycling of different duration on viscoelastic and electromyographic properties of the knee in patients with spinal cord injury [44] | 45° | RF, BF | 10 | N-m/rad Normalized for G.T. | N-m-s/rad Normalized for G.T. |
| Actions | |
|---|---|
| 1 | History and clinical evaluation of patients. |
| 2 | Body position (trunk-hip angle: between 40° and 45°) with backrest. Arms in lap. |
| 3 | The examiner should be in front of the patient. |
| 4 | Check for some pain or limitation of knee flexion/extension movements. |
| 5 | Explain and allow the patient to practice for some trials. |
| 6 | Check for the patient’s compliance and ability to relax. |
| 7 | Pre-trial movements before each trial (for patients with spasticity). |
| 8 | Online recordings of kinematics by an electrogoniometer, IMU or 2D/3D motion analysis systems. |
| 9 | Online recordings of sEMG activity of m. rectus femoris and m. biceps femoris. |
| 10 | Collect between 5 and 10 qualified trials. |
| 11 | Data elaboration. |
| 12 | Report with clinical data, kinematics, sEMG, stiffness, and viscosity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, M.S.; Cioni, M.; Russo, C.; Malaguarnera, L.; Casabona, A. Applicability of the Instrumented Pendulum Test for Assessing Limb Viscoelastic Properties in Neurological and Internal Diseases: A Narrative Review. Life 2025, 15, 535. https://doi.org/10.3390/life15040535
Valle MS, Cioni M, Russo C, Malaguarnera L, Casabona A. Applicability of the Instrumented Pendulum Test for Assessing Limb Viscoelastic Properties in Neurological and Internal Diseases: A Narrative Review. Life. 2025; 15(4):535. https://doi.org/10.3390/life15040535
Chicago/Turabian StyleValle, Maria Stella, Matteo Cioni, Cristina Russo, Lucia Malaguarnera, and Antonino Casabona. 2025. "Applicability of the Instrumented Pendulum Test for Assessing Limb Viscoelastic Properties in Neurological and Internal Diseases: A Narrative Review" Life 15, no. 4: 535. https://doi.org/10.3390/life15040535
APA StyleValle, M. S., Cioni, M., Russo, C., Malaguarnera, L., & Casabona, A. (2025). Applicability of the Instrumented Pendulum Test for Assessing Limb Viscoelastic Properties in Neurological and Internal Diseases: A Narrative Review. Life, 15(4), 535. https://doi.org/10.3390/life15040535







