Exploring a Novel Hypothesis: Could the Eye Function as a Radar or Ultrasound Device in Depth and Distance Perception? Neurophysiological Insights
Abstract
:1. Introduction
2. Materials and Methods
Histopathological Procedures
3. Results
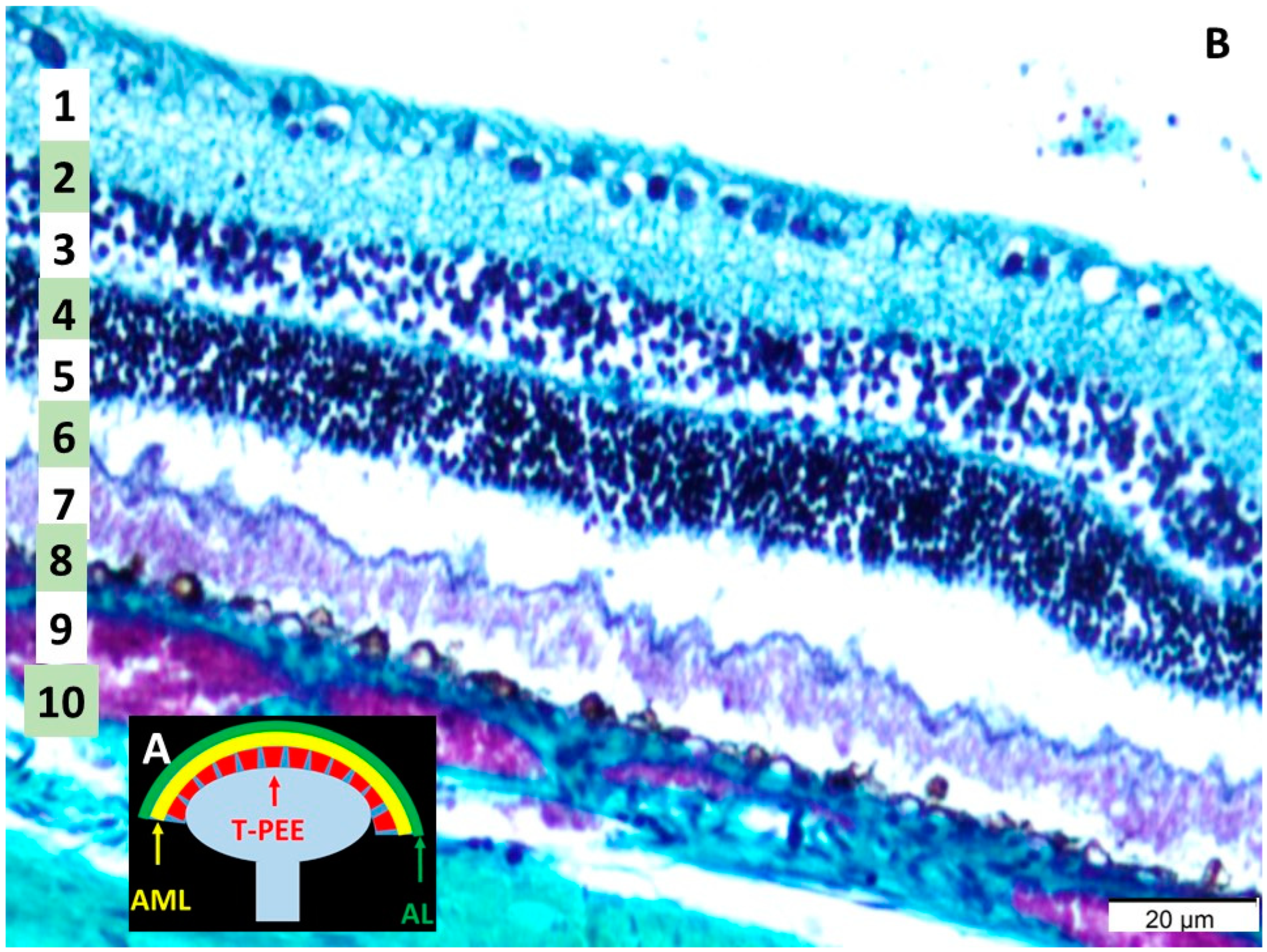
3.1. Histological Analysis of the Retina
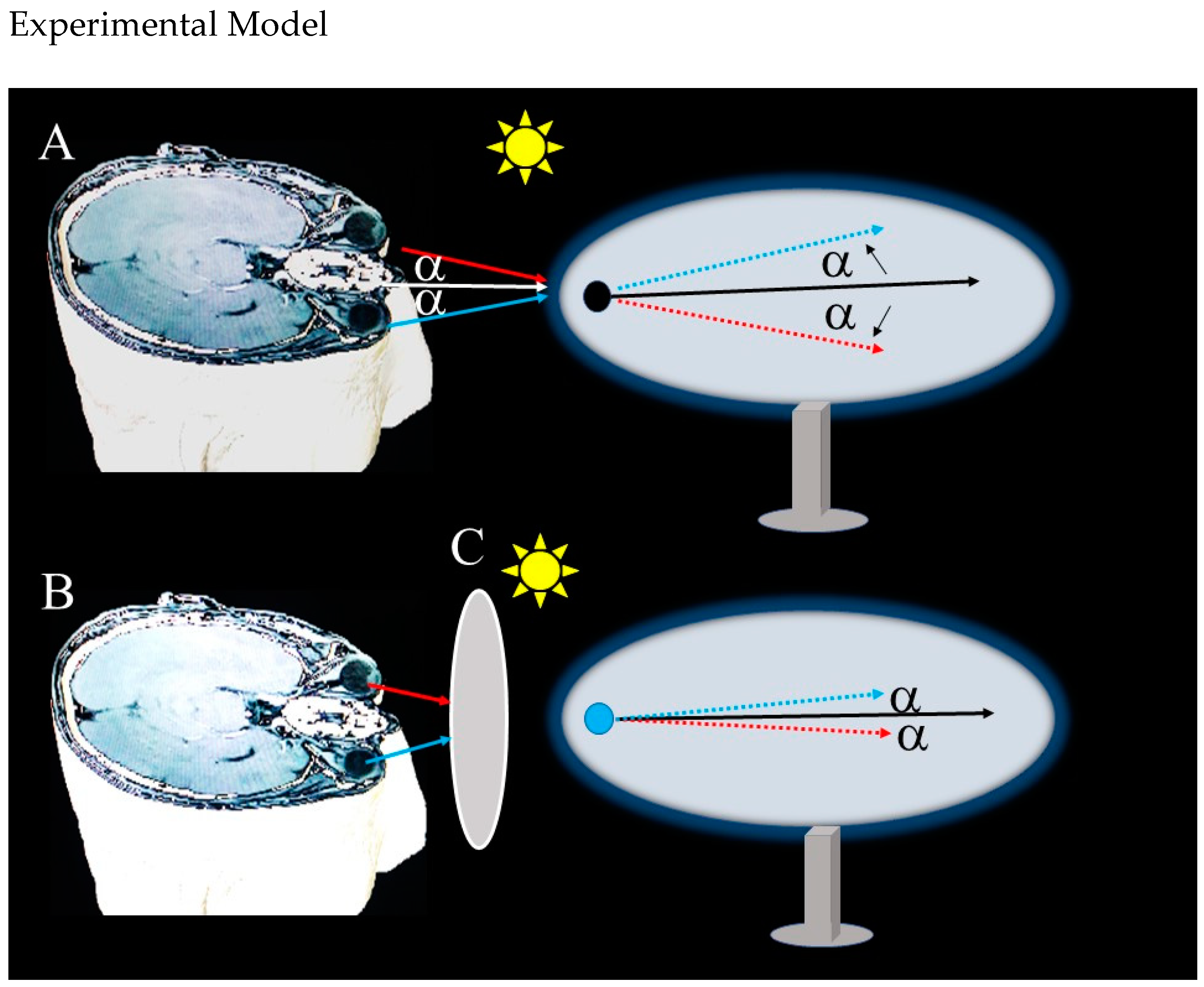
3.2. Retinal Function and Radar Analogy
3.3. Comparative Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakano, Y.; Allison, R.S. Aftereffect of motion-in-depth based on binocular cues: Effects of adaptation duration, interocular correlation, and temporal correlation. J. Vis. 2014, 14, 21. [Google Scholar] [CrossRef]
- Baburaj, V.P.; Gomez, G. Analysis of problems with distance determination using Night Vision Googles. Indian J. Aerosp. Med. 2009, 53, 1–9. [Google Scholar]
- Gordon, B.L. Oculists and occultists: Demonology and the eye. Arch. Ophthalmol. 1939, 22, 25–65. [Google Scholar] [CrossRef]
- Galst, J.M.; Van Alfen, P. Ophthalmologia Optica & Visio in Nummis; Wayenborgh Publishing: Piribebuy, Paraguay, 2018. [Google Scholar]
- Panegyres, K.P.; Panegyres, P.K. The Ancient Greek discovery of the nervous system: Alcmaeon, Praxagoras and Herophilus. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2016, 29, 21–24. [Google Scholar] [CrossRef]
- De Laey, J.J. The eye of Vesalius. Acta Ophthalmol. 2011, 89, 293–300. [Google Scholar] [CrossRef]
- Siegel, R.E. Theories of vision and color perception of Empedocles and Democritus; some similarities to the modern approach. Bull. Hist. Med. 1959, 33, 145–159. [Google Scholar]
- Werner, J.S.; Pinna, B.; Spillmann, L. Illusory color & the brain. Novel illusions suggest that the brain does not separate perception of color from perception of form and depth. Sci. Am. 2007, 296, 90–95. [Google Scholar]
- Finger, S. Origins of Neuroscience: A History of Explorations Into Brain Function; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Pizzi, R.; Wang, R.; Rossetti, D. Human Visual System as a Double-Slit Single Photon Interference Sensor: A Comparison between Modellistic and Biophysical Tests. PLoS ONE 2016, 11, e0147464. [Google Scholar] [CrossRef]
- Zheng, W.L.; Lu, B.L. A multimodal approach to estimating vigilance using EEG and forehead EOG. J. Neural Eng. 2017, 14, 026017. [Google Scholar] [CrossRef]
- Arden, G.B.; Constable, P.A. The electro-oculogram. Prog. Retin. Eye Res. 2006, 25, 207–248. [Google Scholar] [CrossRef]
- Kulyabin, M.; Zhdanov, A.; Dolganov, A.; Maier, A. Optimal Combination of Mother Wavelet and AI Model for Precise Classification of Pediatric Electroretinogram Signals. Sensors 2023, 23, 5813. [Google Scholar] [CrossRef]
- Kim, H.R.; Angelaki, D.E.; DeAngelis, G.C. The neural basis of depth perception from motion parallax. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2016, 371, 20150256. [Google Scholar] [CrossRef]
- Rogers, B.; Graham, M. Motion parallax as an independent cue for depth perception. Perception 1979, 8, 125–134. [Google Scholar] [CrossRef]
- Grimes, W.N.; Schwartz, G.W.; Rieke, F. The synaptic and circuit mechanisms underlying a change in spatial encoding in the retina. Neuron 2014, 82, 460–473. [Google Scholar] [CrossRef]
- Purgert, R.J.; Lukasiewicz, P.D. Differential encoding of spatial information among retinal on cone bipolar cells. J. Neurophysiol. 2015, 114, 1757–1772. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, A.; Sirota, A.; Lopes-Dos-Santos, V.; Dupret, D. Over and above frequency: Gamma oscillations as units of neural circuit operations. Neuron 2023, 111, 936–953. [Google Scholar] [CrossRef]
- Arab, F.; Rostami, S.; Dehghani-Habibabadi, M.; Mateos, D.M.; Braddell, R.; Scholkmann, F.; Ismail Zibaii, M.; Rodrigues, S.; Salari, V.; Safari, M.S. Effects of optogenetic and visual stimulation on gamma activity in the visual cortex. Neurosci. Lett. 2023, 816, 137474. [Google Scholar] [CrossRef]
- Fetsch, C.R.; Deangelis, G.C.; Angelaki, D.E. Visual-vestibular cue integration for heading perception: Applications of optimal cue integration theory. Eur. J. Neurosci. 2010, 31, 1721–1729. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Klier, E.M.; Snyder, L.H. A vestibular sensation: Probabilistic approaches to spatial perception. Neuron 2009, 64, 448–461. [Google Scholar] [CrossRef]
- Joyce, C.; Le, P.H.; Sadiq, N.M. Histology, Retina. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; [Updated 8 August 2023]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546692/ (accessed on 4 January 2025).
- Wang, C.; Bókkon, I.; Dai, J.; Antal, I. Spontaneous and visible light-induced ultraweak photon emission from rat eyes. Brain Res. 2011, 1369, 1–9. [Google Scholar] [CrossRef]
- Cifra, M.; Pospíšil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B Biol. 2014, 139, 2–10. [Google Scholar] [CrossRef]
- Bókkon, I. Visual perception and imagery: A new molecular hypothesis. Biosystems 2009, 96, 178–184. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Biophoton signal transmission and processing in the brain. J. Photochem. Photobiol. B Biol. 2014, 139, 71–75. [Google Scholar] [CrossRef]
- Rahnama, M.; Tuszynski, J.A.; Bókkon, I.; Cifra, M.; Sardar, P.; Salari, V. Emission of mitochondrial biophotons and their effect on electrical activity of membrane via microtubules. J. Integr. Neurosci. 2011, 10, 65–88. [Google Scholar] [CrossRef]
- Sharkova, M.; Aparicio, G.; Mouzaaber, C.; Zolessi, F.R.; Hocking, J.C. Photoreceptor calyceal processes accompany the developing outer segment, adopting a stable length despite a dynamic core. J. Cell Sci. 2024, 137, jcs261721. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bolhasani, H. Photonic Neural Networks: A Compact Review. arXiv 2023, arXiv:2302.08390. [Google Scholar]
- Wang, M.; Chen, N. Three-dimensional cellular imaging in thick biological tissue with confocal detection of one-photon fluorescence in the near-infrared II window. J. Biophotonics 2019, 12, e201800459. [Google Scholar] [CrossRef]
- Wu, Y.; Christensen, R.; Colón-Ramos, D.; Shroff, H. Advanced optical imaging techniques for neurodevelopment. Curr. Opin. Neurobiol. 2013, 23, 1090–1097. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B Biol. 2014, 139, 11–23. [Google Scholar] [CrossRef]
- Tsai, C.J.; Marino, J.; Adaixo, R.; Pamula, F.; Muehle, J.; Maeda, S.; Flock, T.; Taylor, N.M.; Mohammed, I.; Matile, H.; et al. Cryo-EM structure of the rhodopsin-Gαi-βγ complex reveals binding of the rhodopsin C-terminal tail to the gβ subunit. eLife 2019, 8, e46041. [Google Scholar] [CrossRef]
- Molday, R.S.; Moritz, O.L. Photoreceptors at a glance. J. Cell Sci. 2015, 128, 4039–4045. [Google Scholar] [CrossRef]
- Baccus, S.A.; Olveczky, B.P.; Manu, M.; Meister, M. A retinal circuit that computes object motion. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 6807–6817. [Google Scholar] [CrossRef]
- Gollisch, T.; Meister, M. Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron 2010, 65, 150–164. [Google Scholar] [CrossRef]
- Werblin, F.S. The retinal hypercircuit: A repeating synaptic interactive motif underlying visual function. J. Physiol. 2011, 589, 3691–3702. [Google Scholar] [CrossRef]
- Molnar, A.; Hsueh, H.A.; Roska, B.; Werblin, F.S. Crossover inhibition in the retina: Circuitry that compensates for nonlinear rectifying synaptic transmission. J. Comput. Neurosci. 2009, 27, 569–590. [Google Scholar] [CrossRef]
- Azeredo da Silveira, R.; Rieke, F. The Geometry of Information Coding in Correlated Neural Populations. Annu. Rev. Neurosci. 2021, 44, 403–424. [Google Scholar] [CrossRef]
- Field, G.D.; Chichilnisky, E.J. Information processing in the primate retina: Circuitry and coding. Annu. Rev. Neurosci. 2007, 30, 1–30. [Google Scholar] [CrossRef]
- Kastner, D.B.; Baccus, S.A. Insights from the retina into the diverse and general computations of adaptation, detection, and prediction. Curr. Opin. Neurobiol. 2014, 25, 63–69. [Google Scholar] [CrossRef]
- Escobar, M.J.; Pezo, D.; Orio, P. Mathematical analysis and modeling of motion direction selectivity in the retina. J. Physiol. 2013, 107, 349–359. [Google Scholar] [CrossRef]
- Noor, N.M.M.; Mustafa, M.A.M. Eye movement activity that affected the eye signals using electrooculography (EOG) technique. In Proceedings of the 2016 6th IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 25–27 November 2016; pp. 91–95. [Google Scholar]
- Polishchuk, O.V.; Tykhanova, O.F. Biophysical aspects of electromagnetic theory of human vision perception of light information in the visible range. Ukr. Neurosurg. J. 2022, 28, 17–24. [Google Scholar] [CrossRef]
- Sun, Z. Neurons Can Generate Electromagnetic Waves. Nat. Sci. 2022, 14, 463–471. [Google Scholar] [CrossRef]
- Bachofer, C.S.; Wittry, S.E. Electroretinogram in response to x-ray stimulation. Science 1961, 133, 642–644. [Google Scholar] [CrossRef]
- Doly, M.; Isabelle, D.B.; Vincent, P.; Gaillard, G.; Meyniel, G. Mechanism of the formation of X-ray-induced phosphenes. I. Electrophysiological investigations. Radiat. Res. 1980, 82, 93–105. [Google Scholar] [CrossRef]
- Feller, M.B. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009, 4, 24. [Google Scholar] [CrossRef]
- Howard, I.P.; Rogers, B.J. Binocular Vision and Stereopsis; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Hibbard, P.B.; Goutcher, R.; Hornsey, R.L.; Hunter, D.W.; Scarfe, P. Luminance contrast provides metric depth information. R. Soc. Open Sci. 2023, 10, 220567. [Google Scholar] [CrossRef]
- Linton, P. Minimal theory of 3D vision: New approach to visual scale and visual shape. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20210455. [Google Scholar] [CrossRef]
- Vishwanath, D. Toward a new theory of stereopsis. Psychol. Rev. 2014, 121, 151–178. [Google Scholar] [CrossRef]
- Livingstone, M.; Hubel, D. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 1988, 240, 740–749. [Google Scholar] [CrossRef]
- Marr, D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information; The MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Shim, S.; Eom, K.; Jeong, J.; Kim, S.J. Retinal Prosthetic Approaches to Enhance Visual Perception for Blind Patients. Micromachines 2020, 11, 535. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res 2010, 91, 48–53. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, R.; Zhao, L.M.; Lu, B.L. Multimodal Emotion Recognition from Eye Image, Eye Movement and EEG Using Deep Neural Networks. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 3071–3074. [Google Scholar] [CrossRef]
- Zheng, W.L.; Dong, B.N.; Lu, B.L. Multimodal emotion recognition using EEG and eye tracking data. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5040–5043. [Google Scholar]
- Singh, K.D. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. NeuroImage 2012, 62, 1121–1130. [Google Scholar] [CrossRef]
- Westheimer, G. Path dissociation of visual signals entering the cortex: Disparity, contour orientation and position. Vis. Res. 2011, 51, 1058–1063. [Google Scholar] [CrossRef]
- Westheimer, G. Three-dimensional displays and stereo vision. Proc. R. Soc. B Biol. Sci. 2011, 278, 2241–2248. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovation. Bioinspiration Biomim. 2006, 1, P1. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findik, H.; Kaim, M.; Uzun, F.; Kanat, A.; Keleş, O.N.; Aydin, M.D. Exploring a Novel Hypothesis: Could the Eye Function as a Radar or Ultrasound Device in Depth and Distance Perception? Neurophysiological Insights. Life 2025, 15, 536. https://doi.org/10.3390/life15040536
Findik H, Kaim M, Uzun F, Kanat A, Keleş ON, Aydin MD. Exploring a Novel Hypothesis: Could the Eye Function as a Radar or Ultrasound Device in Depth and Distance Perception? Neurophysiological Insights. Life. 2025; 15(4):536. https://doi.org/10.3390/life15040536
Chicago/Turabian StyleFindik, Hüseyin, Muhammet Kaim, Feyzahan Uzun, Ayhan Kanat, Osman Nuri Keleş, and Mehmet Dumlu Aydin. 2025. "Exploring a Novel Hypothesis: Could the Eye Function as a Radar or Ultrasound Device in Depth and Distance Perception? Neurophysiological Insights" Life 15, no. 4: 536. https://doi.org/10.3390/life15040536
APA StyleFindik, H., Kaim, M., Uzun, F., Kanat, A., Keleş, O. N., & Aydin, M. D. (2025). Exploring a Novel Hypothesis: Could the Eye Function as a Radar or Ultrasound Device in Depth and Distance Perception? Neurophysiological Insights. Life, 15(4), 536. https://doi.org/10.3390/life15040536




