Green Catalysis: The Role of Medicinal Plants as Food Waste Decomposition Enhancers/Accelerators
Abstract
1. Introduction
2. Food Waste Management Challenges
3. Current Methods of Food Waste Decomposition
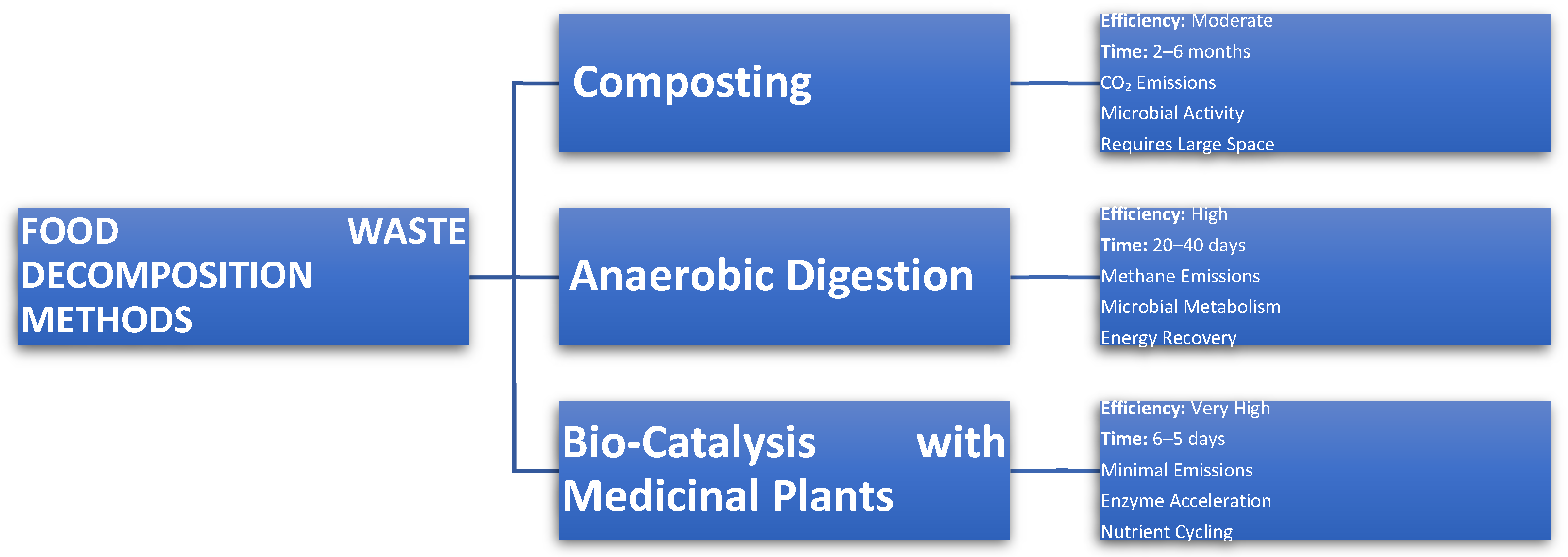
3.1. Anaerobic Digestion
3.2. Composting
- ❖
- Waste reduction: Composting organic waste from landfills, reducing methane emissions associated with anaerobic decomposition [43].
- ❖
- Soil enhancement: The resulting compost improves soil structure, fertility, and moisture retention, promoting sustainable agriculture [44].
- ❖
- Greenhouse gas mitigation: Composting can significantly lower greenhouse gas emissions compared to traditional waste disposal methods [43].
- Challenges and Considerations
- ❖
- Odorous emissions: The release of volatile organic compounds (VOCs) during composting, particularly ammonia and sulfur compounds, poses health risks and environmental concerns [45].
- ❖
- Management techniques: Strategies such as optimal aeration and the addition of bulking agents can mitigate these emissions, enhancing the overall sustainability of composting [45]. While composting is generally seen as eco-friendly, it is essential to address its challenges to maximize its environmental benefits. Balancing effective waste management with odor control remains a critical area for ongoing research and improvement.
- Traditional Composting Duration
- ❖
- Time frame: Generally, composting takes from 2 to 6 months.
- Factors influencing duration:
- ❖
- Ambient temperature.
- ❖
- Moisture content (ideally 30–35%).
- ❖
- Carbon-to-nitrogen (C:N) ratio (should be less than 20) [46].
3.2.1. Natural Fermentation
3.2.2. Open Windrow Composting
3.2.3. In-Vessel Composting
3.2.4. Vermicomposting
3.3. Bio-Drying
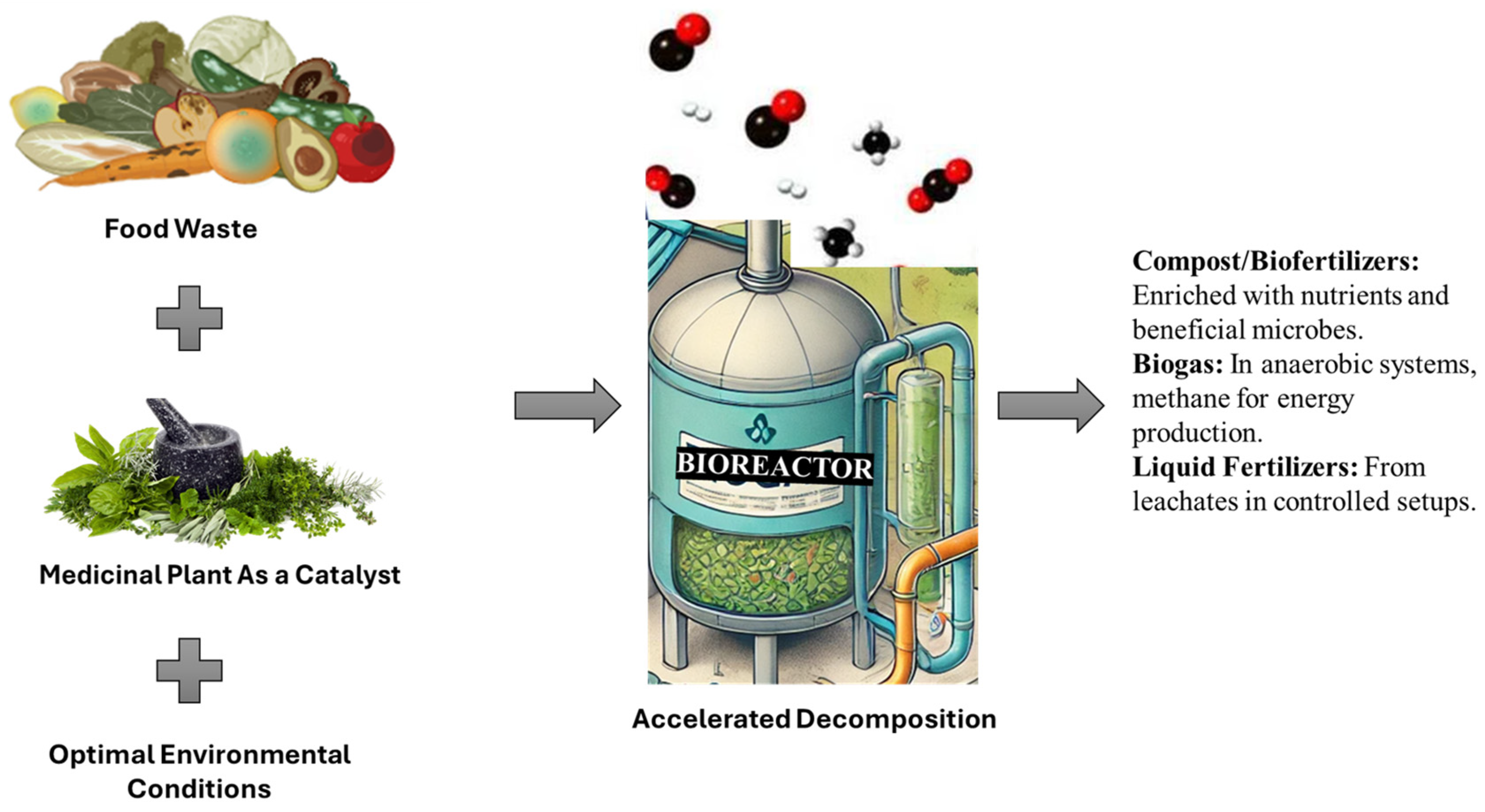
4. Medicinal Plants as Green Catalysts for the Decomposition of Food Waste
5. Mechanisms of Action: How Medicinal Plants Accelerate Decomposition
5.1. Enzymes in Decomposition
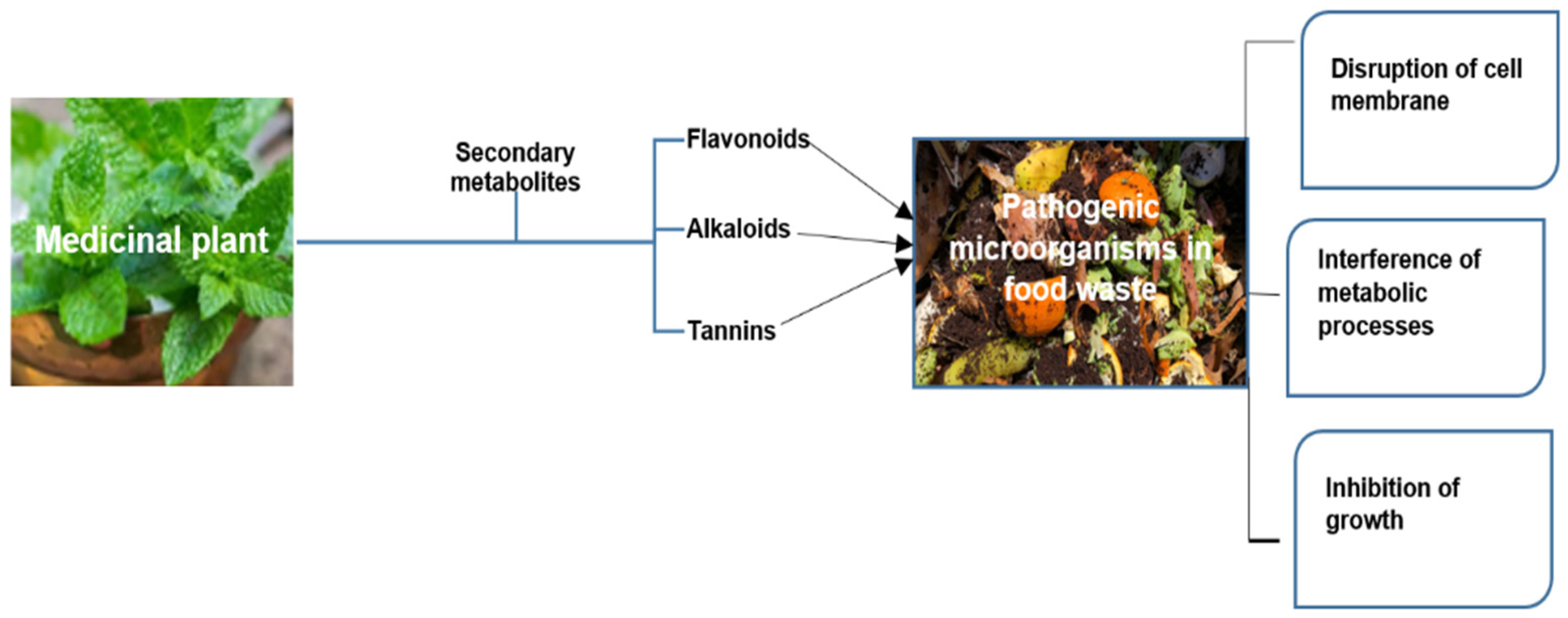
5.2. Secondary Metabolite in Decomposition
5.3. Stimulation of Microbial Activity
5.4. Modification of Soil pH and Structure
5.5. Promotion of Nitrogen Cycling
5.6. Production of Growth Regulators
5.7. Symbiotic Relationships
6. Case Studies: Successful Applications of Medicinal Plants in Food Waste Management
7. Benefits and Challenges of Using Medicinal Plants in Food Waste Decomposition
8. What Is the Way Forward?
9. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Food Loss and Waste 2024. Available online: https://www.fao.org/nutrition/capacity-development/food-loss-and-waste/en/ (accessed on 4 June 2024).
- United Nations Environment Programme (UNEP). Food Waste Index Report 2021. United Nations Environment Programme 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 4 June 2024).
- Lins, M.; Zandonadi, R.P.; Raposo, A.; Ginani, V.C. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J.; Dore, M.; Chow, C.C. The progressive increase of food waste in America and its environmental impact. PLoS ONE 2009, 4, e7940. [Google Scholar]
- Poonia, A.; Sindhu, S.; Arya, V.; Panghal, A. Analysis of drivers for anti-food waste behaviour-TISM and MICMAC approach. J. Indian Bus. Res. 2022, 14, 186–212. [Google Scholar]
- Wahbeh, S.; Anastasiadis, F.; Sundarakani, B.; Manikas, I. Exploration of food security challenges towards more sustainable food production: A systematic literature review of the major drivers and policies. Foods 2022, 11, 3804. [Google Scholar] [CrossRef]
- Ardra, S.; Barua, M.K. Halving food waste generation by 2030: The challenges and strategies of monitoring UN sustainable development goal target 12.3. J. Clean. Prod. 2022, 380, 135042. [Google Scholar]
- Han, L.; Li, L.; Xu, X.; Ye, W.; Zhang, F.; Xu, Y.; Peng, X.; Zhen, F. Utilization of short-term high temperature pretreatment for food waste composting: Effects of end-products on soil properties and plant growth. J. Clean. Prod. 2024, 438, 140790. [Google Scholar]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar]
- Greff, B.; Lakatos, E.; Szigeti, J.; Varga, L. Co-composting with herbal wastes: Potential effects of essential oil residues on microbial pathogens during composting. Crit. Rev. Environ. Sci. Technol. 2021, 51, 457–511. [Google Scholar]
- Jara, Y.S.; Mekiso, T.T.; Washe, A.P. Highly efficient catalytic degradation of organic dyes using iron nanoparticles synthesized with Vernonia Amygdalina leaf extract. Sci. Rep. 2024, 14, 6997. [Google Scholar]
- Zhang, L.; Ren, Y.; Liu, W.; Wang, A.; Zhang, T. Single-atom catalyst: A rising star for green synthesis of fine chemicals. Nat. Sci. Rev. 2018, 5, 653–672. [Google Scholar]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food waste utilization for reducing carbon footprints towards sustainable and cleaner environment: A review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Ayati, A.; Krivoshapkin, P.; Tanhaei, B.; Farghali, M.; Yap, P.S.; Abdelhaleem, A. Coordination-driven innovations in low-energy catalytic processes: Advancing sustainability in chemical production. Coord. Chem. Rev. 2024, 514, 215900. [Google Scholar] [CrossRef]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.R.; Rather, R.A.; Farooq, A.; Padder, S.A.; Baba, T.R.; Sharma, S.; Mubarak, N.M.; Khan, A.H.; Singh, P.; Ara, S. New insights in food security and environmental sustainability through waste food management. Environ. Sci. Pollut. Res. 2024, 12, 17835–17857. [Google Scholar]
- Fourie, J.; Engelbrecht, K.; Govender, P.; Pillay, P.; Engel, W. Food Loss and Waste in Farming: Insights from South African Farmers; WWF: Washington, DC, USA, 2023; Available online: https://wwfafrica.awsassets.panda.org/downloads/food_loss_and_waste_report.pdf (accessed on 17 June 2024).
- Pandey, A. Food wastage: Causes; impacts and solutions. Sci. Herit. J. 2021, 5, 17–20. [Google Scholar] [CrossRef]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A critical review on food waste management for the production of materials and biofuel. J. Haz. Mat. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Arumugam, V.; Ismail, M.H.; Puspadaran, T.A.; Routray, W.; Ngadisih, N.; Karyadi, J.N.; Suwignyo, B.; Suryatmojo, H. Food Waste Treatment Methods and its Effects on the Growth Quality of Plants: A Review. Pertanika J. Trop. Agri. Sci. 2022, 45, 75–101. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Environmental impact assessment of food waste management using two composting techniques. Sustainability 2020, 12, 1595. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste-Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, H.; Badulescu, D.; Bac, D.P. Achieving zero hunger goal through minimizing waste in food supply chain: Evidence from Asian emerging region. Sustainability 2022, 14, 5930. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive compounds in food waste: A review on the transformation of food waste to animal feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Boliko, M.C. FAO and the situation of food security and nutrition in the world. J. Nutr. Sci. Vitaminol. 2019, 65, S4–S8. [Google Scholar] [PubMed]
- Ayare, A.; Phadtare, O.; Tiwari, T.; Sohoni, V. Experimental analysis of organic food waste for degradation by Ayurvedic herbal plants and medicines. J. Mech. Civil Eng. 2016, 13, 9–11. [Google Scholar]
- Hong, G.T.; Samah, M.A.; Nowroji, K.; Chet, S.S. Food waste composting: Natural fermentation method. Int. J. Recent Technol. Eng. 2019, 8. [Google Scholar]
- Raimi, A.; Roopnarain, A.; Chirima, G.J.; Adeleke, R. Insights into the microbial composition and potential efficiency of selected commercial biofertilisers. Heliyon 2020, 6, e04342. [Google Scholar]
- Singh, P.K.; Mohanty, P.; Mishra, S.; Adhya, T.K. Food waste valorisation for biogas-based bioenergy production in circular bioeconomy: Opportunities; challenges, and future developments. Front. Energy Res. 2022, 10, 903775. [Google Scholar]
- Aitken, J.A.; Alaybek, B.; Hartman, R.; Mika, G.; Leib, E.M.; Plekenpol, R.; Branting, K.; Rao, D.; Leets, L.; Sprenger, A. Initial assessment of the efficacy of food recovery policies in US States for increasing food donations and reducing waste. Waste Manag. 2024, 176, 149–158. [Google Scholar]
- Oelofse, S.H.; Nahman, A. Estimating the magnitude of food waste generated in South Africa. Waste Manag. Res. 2013, 31, 80–86. [Google Scholar]
- Wiharyanto, O.; Mochtar, H.; Ika, B.P.; Purwono, P. Decomposition of food waste using bulking agent and bio-drying technology. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 73, p. 05013. [Google Scholar]
- Pham, T.P.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar]
- Lim, W.J.; Chin, N.L.; Yusof, A.Y.; Yahya, A.; Tee, T.P. Food waste handling in Malaysia and comparison with other Asian countries. Int. Food Res. J. 2016, 23, S1. [Google Scholar]
- Lam, C.M.; Iris, K.M.; Medel, F.; Tsang, D.C.; Hsu, S.C.; Poon, C.S. Life-cycle cost-benefit analysis on sustainable food waste management: The case of Hong Kong International Airport. J. Clean. Prod. 2018, 187, 751–762. [Google Scholar]
- Piadeh, F.; Offie, I.; Behzadian, K.; Rizzuto, J.P.; Bywater, A.; Córdoba-Pachón, J.R.; Walker, M. A critical review for the impact of anaerobic digestion on the sustainable development goals. J. Environ. Manag. 2024, 349, 119458. [Google Scholar]
- Enitan-Folami, A.M.; Swalaha, F. Anaerobic Treatment System: A Sustainable Clean Environment and Future Hope of Renewable Energy Production; Springer: Berlin/Heidelberg, Germany, 2021; pp. 169–198. [Google Scholar]
- Owhondah, R.O. Optimization of Small-Scale Low-Cost Anaerobic Digestion System: Model-Based Analysis. Ph.D. Thesis, University of Leeds, Leeds, UK, 2019. [Google Scholar]
- Nagy, G.; Takács, A.; Kallay, A.A.; Mentes, D. The anaerobic digestion of sheep manure in self-designed low-cost biogas reactor. Analecta Tech. Szeged. 2018, 12, 13–23. [Google Scholar]
- Subbarao PM, V.; D’ Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic digestion as a sustainable technology for efficiently utilizing biomass in the context of carbon neutrality and circular economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Y.; Liu, W.B.; Jiang, G.Z.; Zhu, J. Effects of dietary carbohydrate/lipid ratios on growth performance, body composition and glucose metabolism of fingerling blunt snout bream Megalobrama amblycephala. Aquac. Nutr. 2013, 19, 701–708. [Google Scholar]
- Calderon, F.J.; Vigil, M.F.; Benjamin, J. Compost input effect on dryland wheat and forage yields and soil quality. Pedosphere 2018, 28, 451–462. [Google Scholar]
- Hassan, N.Y.I.; El Wahed, N.H.A.; Abdelhamid, A.N.; Ashraf, M.; Abdelfattah, E.A. Composting: An eco-friendly solution for organic waste management to mitigate the effects of climate change. Innovare J. Soc. Sci. 2023, 11, 1–7. [Google Scholar]
- Yadav, K.; Singh, N.; Nayak, S.; Kumar, S. Sustainable vermicomposting: An eco-friendly approach to boost crop productivity. In Futuristic Trends in Agriculture Engineering & Food Sciences; Iterative International Publishers: Chikmagalur, India, 2024; pp. 85–94. [Google Scholar]
- Tran, H.-T.; Binh, Q.A.; Tung, T.V.; Pham, D.T.; Hoang, H.-G.; Nguyen, N.S.H.; Zhang, T.; Mukherjee, S.; Bolan, N. A critical review on characterization, human health risk assessment and mitigation of malodorous gaseous emission during the composting process. Environ. Pollut. 2024, 6, 124115. [Google Scholar]
- Wortmann, C.S.; Shapiro, C.A. Composting Manure and Other Organic Materials. In NebGuide; University of Nebraska-Lincoln: Lincoln, NE, USA, 2012; p. G1315. [Google Scholar]
- Mohamad, M.; Firra, R. Percepatan Waktu Pengomposan menggunakan Kombinasi Aktivator EM4 dan Starbio dengan Metode Bersusun. J. Ilm. Tek. Lingkung. 2016, 5, 70–76. [Google Scholar]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar]
- Waqas, M.; Nizami, A.S.; Aburiazaiza, A.S.; Barakat, M.A.; Rashid, M.I.; Ismail, I.M. Optimizing the process of food waste compost and valorizing its applications: A case study of Saudi Arabia. J. Clean. Prod. 2018, 176, 426–438. [Google Scholar]
- Shilev, S.; Naydenov, M.; Vancheva, V.; Aladjadjiyan, A. Composting of Food. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: Berlin/Heidelberg, Germany, 2006; Volume 23, p. 283. [Google Scholar]
- Daniel-Gromke, J.; Liebetrau, J.; Denysenko, V.; Krebs, C. Digestion of bio-waste-GHG emissions and mitigation potential. Energy Sustain. Soc. 2015, 5, 3. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, C.; Geng, B.; Liu, X.; Ye, J.; Tian, Y.; Peng, X. Improved fermentation performance in an expanded ectopic fermentation system inoculated with thermophilic bacteria. Bioresour. Technol. 2015, 198, 867–875. [Google Scholar]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Conv. Bioref. 2021, 13, 5445–5468. [Google Scholar]
- Liang, C.; Liu, L.X.; Liu, J.; Aihaiti, A.; Tang, X.J.; Liu, Y.G. New insights on low-temperature fermentation for food. Fermentation 2023, 9, 477. [Google Scholar] [CrossRef]
- Pajura, R. Composting municipal solid waste and animal manure in response to the current fertilizer crisis-a recent review. Sci. Total Environ. 2024, 912, 169221. [Google Scholar]
- Nur, F.M.S.; Nursyahida, B.N.B.; Shahrom, M.d.Z.S.M. Windrow composting of yard wastes and food waste. Aust. J. Basic Appl. Sci. 2014, 8, 64–68. [Google Scholar]
- Mason, I.G.; Milke, M.W. Physical modelling of the composting environment: A review. Part 1: Reactor systems. Waste Manag. 2005, 25, 481–500. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Susilawati, K.; Majid, N.M. Compost maturity and nitrogen availability by co-composting of paddy husk and chicken manure amended with clinoptilolite zeolite. Waste Manag. Res. 2015, 33, 322–331. [Google Scholar]
- Makan, A.B.; Assobhei, O.M.; Mountadar, M.O. Initial air pressure influence on in-vessel composting for the biodegradable fraction of municipal solid waste in Morocco. Int. J. Environ. Sci. Technol. 2014, 11, 53–58. [Google Scholar]
- Ajmal, M.; Aiping, S.; Uddin, S.; Awais, M.; Faheem, M.; Ye, L.; Rehman, K.U.; Ullah, M.S.; Shi, Y. A review on mathematical modeling of in-vessel composting process and energy balance. Biomass Conv. Bioref. 2020, 12, 4201–4213. [Google Scholar]
- Cao, Y.; Tian, Y.; Wu, Q.; Li, J.; Zhu, H. Vermicomposting of livestock manure as affected by carbon-rich additives (straw, biochar and nanocarbon): A comprehensive evaluation of earthworm performance, microbial activities, metabolic functions and vermicompost quality. Bioresour. Technol. 2021, 320, 124404. [Google Scholar]
- Jayaprakash, S.; Lohit, H.S.; Abhilash, B.S. Design and development of compost bin for Indian kitchen. Int. J. Waste Resour. 2018, 8, 2. [Google Scholar]
- Rada, E.C.; Ragazzi, M.; Fiori, L.; Antolini, D. Bio-drying of grape marc and other biomass: A comparison. Water Sci. Technol. 2009, 60, 1065–1070. [Google Scholar]
- Zhang, J.; Cai, X.; Qi, L.; Shao, C.; Lin, Y.; Zhang, J.; Zhang, Y.; Shen, P.; Wei, Y. Effects of aeration strategy on the evolution of dissolved organic matter (DOM) and microbial community structure during sludge bio-drying. Appl. Microbiol. Biotechnol. 2015, 99, 7321–7331. [Google Scholar]
- Salem, O.; Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Świerk, D.; Stuper-Szablewska, K. New Insights into Bioactive Compounds of Wild-Growing Medicinal Plants. Appl. Sci. 2023, 13, 13196. [Google Scholar] [CrossRef]
- Topi, D.; Risto, J. Medicinal plant effluents, a source of bioactive compounds and their applications in processed foods. Int. J. Chem. Mater. Sci. 2024, 9, 13–21. [Google Scholar] [CrossRef]
- Oladipo, A.T.; Enwemiwe, V.N.; Ejeromedoghene, O.; Adebayo, A.O.; Ogunyemi, O.; Fu, F. Production and Functionalities of Specialized Metabolites from Different Organic Sources. Metabolites 2022, 12, 534. [Google Scholar] [CrossRef]
- Ata, A. Bioactive Natural Products from Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2023; pp. 417–434. [Google Scholar] [CrossRef]
- Mironov, V.; Moldon, I.; Shchelushkina, A.; Zhukov, V.; Zagustina, N. Bio-Drying of Municipal Wastewater Sludge: Effects of High Temperature, Low Moisture Content and Volatile Compounds on the Microbial Community. Fermentation 2023, 9, 570. [Google Scholar] [CrossRef]
- Czekała, W.; Nowak, M.; Bojarski, W. Anaerobic Digestion and Composting as Methods of Bio-Waste Management. Žemės Ūkio Inžinerija 2023, 27, 173–186. [Google Scholar]
- Náthia-Neves, G.; Berni, M.D.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar]
- Kate, G.U.; Krishnani, K.K.; Kumar, N.; Sukhdhane, K.; Verma, A.K.; Brahmane, M.P.; Chadha, N.K.; Kumar, J. Abiotic and biotic stress alleviating effects of the medicinal and aromatic plant-derived product on striped catfish Pangasianodon hypophthalmus. Fish Shellfish Immun. 2023, 135, 108625. [Google Scholar]
- Shul’ts, E.E.; Tolstikov, G.A. Modification of biologically active plant metabolites via the metal complex catalysis reactions as a promising direction in medicinal chemistry. Russ. Chem. Bull. 2013, 62, 605–621. [Google Scholar]
- Kardam, V.; Kalita, S.; Dubey, K.D. Computations reveal a crucial role of an aromatic dyad in the catalytic function of plant cytochrome P450 mint superfamily. J. Inorg. Biochem. 2022, 237, 111990. [Google Scholar]
- Akhtar, M.F.; Parveen, A.; Hussain, A.; Mumtaz, M.Z.; Kamran, M.; Farooqi, M.A.; Ahmad, M. Exploring the potential of four medicinal plants for antioxidant enzymes activity, proximate and nutritional composition. Acta Bot. Hung. 2019, 61, 219–231. [Google Scholar]
- Hosseini Mohtasham, N.; Gholizadeh, M. Nano silica extracted from horsetail plant as a natural silica support for the synthesis of H3PW12O40 immobilized on aminated magnetic nanoparticles (Fe3O4@ SiO2-EP-NH-HPA): A novel and efficient heterogeneous nanocatalyst for the green one-pot synthesis of pyrano [2; 3-c] pyrazole derivatives. Res. Chem. Int. 2020, 46, 3037–3066. [Google Scholar]
- Qian, X.; Lei, J.; Wickramasinghe, S.R. Novel polymeric solid acid catalysts for cellulose hydrolysis. RSC Adv. 2013, 3, 24280–24287. [Google Scholar]
- Kumar, M.P.; Suresh, D.; Sneharani, A.H. Senna mediated facile green synthesis of nano ceria and its photo-catalytic and biological application. Mater. Today Proc. 2022, 49, 882–890. [Google Scholar]
- Zhang, Y.; Chen, Y.; Liu, Q. Green synthesis of MCM-41 derived from renewable biomass and construction of VOx-Modified nickel phyllosilicate catalyst for CO2 methanation. Int. J. Hydrogen Energy 2021, 46, 32003–32016. [Google Scholar]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar]
- Khadija, A.E.; Mansour, S.; Ali, B.; Abdelaziz, Y. Chemically degraded soil rehabilitation process using medicinal and aromatic plants. Environ. Sci. Pollut. Res. Int. 2021, 28, 73–93. [Google Scholar]
- Da Silva, A.C. Valorisation of Organic Wastes: Assessment of Compost Quality. Doctoral Dissertation, Universidade do Minho, Minho, Portugal, 2022. [Google Scholar]
- Luo, J.; Yang, R.; Ma, F.; Jiang, W.; Han, C. Recycling utilization of Chinese medicine herbal residues resources: Systematic evaluation on industrializable treatment modes. Environ. Sci. Pollut. Res. Int. 2023, 30, 32153–32167. [Google Scholar]
- Forti, J.C.; Loretti, G.H.; Tadayozzi, Y.S.; de Andrade, A.R. A phytotoxicity assessment of the efficiency 2; 4-D degradation by different oxidative processes. J. Environ. manag. 2020, 266, 110588. [Google Scholar] [CrossRef]
- Zarevúcka, M.; Wimmer, Z. Plant products for pharmacology: Application of enzymes in their transformations. Int. J. Mol. Sci. 2008, 9, 2447–2473. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar]
- Wei, S.; Ding, S.; Li, Y.; Zhang, E.; Duan, X. Differential responses of soil cellulase enzymes and oxidative enzymes to soil erosion. Catena 2024, 241, 108015. [Google Scholar]
- Khan, A.L.; Shahzad, R.; Al-Harrasi, A.; Lee, I.J. Endophytic microbes: A resource for producing extracellular enzymes. In Endophytes: Crop Productivity and Protection; Springer: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 95–110. [Google Scholar]
- Sunitha, V.H.; Devi, D.N.; Srinivas, C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J. Agri. Sci. 2013, 9, 1–9. [Google Scholar]
- Hawar, S.N. Extracellular enzyme of endophytic fungi isolated from Ziziphus spina leaves as medicinal plant. Inter. J. Biomater. 2022, 2022, 2135927. [Google Scholar]
- Zerva, A.; Pentari, C.; Ferousi, C.; Nikolaivits, E.; Karnaouri, A.; Topakas, E. Recent Advances on Key Enzymatic Activities for the Utilisation of Lignocellulosic. Biomass. Bioresour. Technol. 2021, 342, 126058. [Google Scholar]
- Wang, F.W.; Jiao, R.H.; Cheng, A.B.; Tan, S.H.; Song, Y.C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J. Microbiol. Biotechnol. 2007, 23, 79–83. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Siika-Aho, M.; Viikari, L. Carbohydrate-binding modules (CBMs) revisited: Reduced amount of water counterbalances the need for CBMs. Biotechnol. Biofuels 2013, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Aubert, J.P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Biely, P. Microbial xylanolytic systems. Trends Biotechnol. 1985, 3, 286–290. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Ladd, J.N. Origin and range of enzyme in soil. In Soil Enzyme; Academic Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Rao, K.K.; Gravelle, M.; Valente, J.S.; Figueras, F. Activation of Mg–Al hydrotalcite catalysts for aldol condensation reactions. J. Catal. 1998, 173, 115–121. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M.A. Glycogen; hyaluronate; and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [PubMed]
- Jaeger, K.E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [PubMed]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production; purification; characterization; and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar]
- Bollag, J.M. Decontaminating soil with enzymes. Environ. Sci. Technol. 1992, 26, 1876–1881. [Google Scholar]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase; peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar]
- Theuerl, S.; Buscot, F. Laccases: Toward disentangling their diversity and functions in relation to soil organic matter cycling. Biol. Fertil. Soils 2010, 46, 215–225. [Google Scholar]
- Stevenson, F.G. Humus Chemistry: Genesis; Composition; Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types; synthesis; and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Baldy, V.; Guittonny, M.; Greff, S.; DesRochers, A. Litter leachates have stronger impact than leaf litter on Folsomia candida fitness. Soil Biol. Biochem. 2020, 147, 107850. [Google Scholar]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar]
- Pratap, G.M.; Manoj, K.M.; Sai, S.A.; Sujatha, B.; Sreedevi, E. Evaluation of three medicinal plants for anti-microbial activity. AYU 2012, 33, 423–428. [Google Scholar]
- Chandler-Ezell, K.; Austin, S.F. Understanding Medicinal Plants. Their Chemistry and Therapeutic Action. Econ. Bot. 2006, 60, 298. [Google Scholar]
- Park, M.; Baek, S.J.; Park, S.M.; Yi, J.M.; Cha, S. Comparative study of the mechanism of natural compounds with similar structures using docking and transcriptome data for improving in silico herbal medicine experimentations. Brief. Bioinform. 2023, 24, bbad344. [Google Scholar]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms; anti-quorum-sensing actions; and clinical trials of medicinal plant bioactive compounds against bacteria: A comprehensive review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef]
- Spiridonov, N.A.; Foigel, A.G.; Fomkina, M.G.; Arkhipov, V.V.; Shipulina, L.D. Mechanism of action of some antimicrobial preparations of plant origin. Pharm. Chem. J. 1996, 30, 400–403. [Google Scholar]
- Tao, J.; Zuo, J.; He, Z.; Wang, Y.; Liu, J.; Liu, W.; Cornelissen, J.H. Traits including leaf dry matter content and leaf pH dominate over forest soil pH as drivers of litter decomposition among 60 species. Funct. Ecol. 2019, 33, 1798–1810. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar]
- Du, L.; Zhao, J.; Abbas, F.; Liu, W. Higher nitrates; P and lower pH in soils under medicinal plants versus crop plants. Environ. Chem. Lett. 2013, 11, 385–390. [Google Scholar]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil microbiome: Diversity; benefits and interactions with plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Yang, W.; Zhang, Y.; Wang, N.; Fan, P.; You, C.; Yu, L.; Gao, Q.; Wang, H.; et al. Response mechanisms of 3 typical plants nitrogen and phosphorus nutrient cycling to nitrogen deposition in temperate meadow grasslands. Front. Plant Sci. 2023, 14, 1140080. [Google Scholar]
- Knops, J.M.; Bradley, K.L.; Wedin, D.A. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar]
- Meister, A.; Bohm, K.; Gutiérrez-Ginés, M.J.; Gaw, S.; Dickinson, N.; Robinson, B. Effects of native plants on nitrogen cycling microorganisms in soil. Appl. Soil Ecol. 2023, 191, 105031. [Google Scholar]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar]
- Pan, S.; Neeraj, A.; Srivastava, K.S.; Kishore, P.; Sarethy, I.P. Effects of growth regulators on in vitro response and multiple shoot induction in some endangered medicinal plants. OA Biotechnol. 2013, 2. [Google Scholar] [CrossRef][Green Version]
- Chauhan, H.K.; Bisht, A.K.; Bhatt, I.D. Role of Plant Growth Regulators for Augmenting Secondary Metabolites Production in Medicinal Plants. In Vitro Propagation and Secondary Metabolite Production from Medicinal Plants: Current Trends (Part 1); Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 120–141. [Google Scholar]
- Tarasov, S.S.; Mikhalev, E.V.; Rechkin, A.I.; Krutova, E.K. Plant Growth and Development Regulators: Classification; Nature and Mechanism of Action. Agrohimiâ 2023, 9, 65–80. [Google Scholar] [CrossRef]
- Bell-Dereske, L.; Takacs-Vesbach, C.; Kivlin, S.N.; Emery, S.M.; Rudgers, J.A. Leaf endophytic fungus interacts with precipitation to alter belowground microbial communities in primary successional dunes. FEMS Microbiol. Ecol. 2017, 93, fix036. [Google Scholar] [CrossRef] [PubMed]
- Schalk, F.; Gostinčar, C.; Kreuzenbeck, N.B.; Conlon, B.H.; Sommerwerk, E.; Rabe, P.; Burkhardt, I.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A.; et al. The termite fungal cultivar Termitomyces combines diverse enzymes and oxidative reactions for plant biomass conversion. mBio 2021, 12, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant growth-promoting rhizobacteria (PGPR): Approaches to alleviate abiotic stresses for enhancement of growth and development of medicinal plants. Sustainability 2022, 14, 15514. [Google Scholar] [CrossRef]
- Wu, D.L.; Liu, P.; Luo, Y.Z.; Tian, G.M.; Mahmood, Q. Nitrogen transformations during co-composting of herbal residues; spent mushrooms; and sludge. J. Zhejiang Univ. Sci. B. 2010, 11, 497–505. [Google Scholar] [CrossRef]
- Chopra, A.; Doiphode, V.V. Ayurvedic medicine: Core concept; therapeutic principles; and current relevance. Med. Clin. 2002, 86, 75–89. [Google Scholar]
- Kulkarni, K.V.; Adavirao, B.V. A review on: Indian traditional shrub Tulsi (Ocimum sanctum): The unique medicinal plant. J. Med. Plants Stud. 2018, 6, 106–110. [Google Scholar]
- Zhou, Y.; Selvam, A.; Wong, J.W. Effect of Chinese medicinal herbal residues on microbial community succession and anti-pathogenic properties during co-composting with food waste. Bioresour. Technol. 2016, 217, 190–199. [Google Scholar] [CrossRef]
- Otunola, B.O.; Aghoghovwia, M.P.; Thwala, M.; Gómez-Arias, A.; Jordaan, R.; Hernandez, J.C.; Ololade, O.O. Influence of clay mineral amendments characteristics on heavy metals uptake in vetiver grass (Chrysopogon zizanioides L. Roberty) and Indian mustard (Brassica juncea L. Czern). Sustainability 2022, 14, 5856. [Google Scholar] [CrossRef]

| Decomposition Method | Advantages | Disadvantages | Environmental Impact | Refs. |
|---|---|---|---|---|
| Anaerobic Digestion | Low capital cost, simple process, produces biogas (methane and CO2) as an energy source. Can be used as soil fertilizers. | Long decomposition period (20–40 days). Inhibition of bacteria by toxins. High ammonia levels harmful to methanogenic bacteria. Initial costs are usually quite high. | Contributes to greenhouse gas emissions (methane). Potential environmental pollution through leachate. | [70,71] |
| Composting | Eco-friendly. Inexpensive. Produces valuable organic fertilizers. Enhances soil structure and fertility. | Slow process (2 to 6 months). Requires management of moisture, aeration, pH, etc. Needs space for composting facilities. Odorous emissions (Tran et al., 2024 [45]). | Reduces landfill waste. Helps with soil regeneration. May release greenhouse gases (CO2). | [72,73,74] |
| Natural Fermentation | Accelerates decomposition. Enhances microbial growth. Produces nutrient-rich compost for farming. | Requires control of microbial activity. Requires temperature, moisture, and ventilation management. | Reduces food waste. May release heat and CO2 into the atmosphere. | [53,55] |
| Open Windrow Composting | Simple to implement. Low cost. Allows for aeration and temperature control during decomposition. | Slow process (12–20 weeks). Requires large area. Needs frequent turning and aeration. | Reduces organic waste. Potential odor emissions. Releases CO2 and other gases during decomposition. | [56] |
| In-Vessel Composting | Faster decomposition. Better control over temperature and aeration. Produces mature compost quicker. | Higher initial setup cost. Requires more space. Mechanical systems needed for aeration. | Reduces food waste. Decreases landfill use. Releases CO2 and possible odors. | [60] |
| Vermicomposting | Fast decomposition. Produces nutrient-rich worm castings. Improves soil health. Eco-friendly. | Cannot handle high-temperature food waste. Not suitable for large-scale operations. | Reduces waste and produces high-nutrient organic fertilizer. Minimal environmental pollution. | [58] |
| Bio-Drying | Accelerates composting process. Reduces water content and volume. Low emissions of CO2. Improves handling and transport of waste. | Requires use of bulking agents like leaf waste and manure. May not be suitable for all types of food waste. | Reduces food waste volume. Lower CO2 emissions compared to traditional methods. May still emit some gases. | [63] |
| Medicinal Plant | Key Bioactive Compounds | Decomposition Mechanism | Environmental Benefits | Decomposition Timeframe | Limitations/Challenges | Case Studies | Refs. |
|---|---|---|---|---|---|---|---|
| Neem (Azadirachta indica) | Alkaloids, flavonoids, terpenoids | Enhances microbial activity; antimicrobial effects reduce pathogens | Reduces chemical fertilizer use; lowers pathogen load | 6 days | Overharvesting risk due to widespread usage | Proven effective with 90% degradation in 6 days. | [26,136] |
| Tulsi (Ocimum sanctum) | Essential oils, polyphenols | Promotes microbial enzymatic activity and nutrient cycling | Improves compost quality; enhances microbial diversity | 6 days | Sensitive to water availability | Effective decomposition with enhanced nutrient content. | [26,138] |
| Chinese Herbal Residues | Flavonoids, alkaloids | Accelerates humification and inhibits pathogens | Prolongs thermophilic phase; reduces pathogen activity | 9 days | Requires proper ratio mixing with food waste | Extends thermophilic phase and improves compost quality. | [23,139] |
| Aloe vera | Polysaccharides, anthraquinones | Aids moisture retention during decomposition; fosters fungal growth | Increases soil organic matter; retains moisture | 20+ days | Slower degradation; fungus growth needs control | Slower but contributes to compost moisture retention. | [26,138] |
| Horsetail (Equisetum spp.) | Silica, flavonoids, alkaloids | Natural acid catalysis; promotes enzymatic hydrolysis | High catalytic activity for organic matter breakdown | Varies (depending on method) | Limited data on large-scale application | Effective hydrolysis catalyst in smaller systems. | [60] |
| Asafetida (Ferula assa-foetida) | Resins, essential oils, tannins | Stimulates microbial activity; antimicrobial effects | Reduces pathogen load; improves microbial activity | 20+ days | Ineffective without additional water or bulking | Long degradation time despite microbial stimulation. | [26] |
| Senna (Cassia spp.) | Anthraquinones, tannins, flavonoids | Promotes breakdown of lignocellulosic biomass | Enhances lignin degradation; boosts soil fertility | 15 days | Can inhibit non-target microbial communities | Effective for cellulose and lignin degradation. | [60,63] |
| Tulip Tree (Liriodendron tulipifera) | Terpenoids, polyphenols | Enhances microbial enzyme production | Accelerates organic matter breakdown in acidic soils | 10–14 days | Adapted to specific soil pH ranges | Notable for accelerating decomposition in acidic environments. | [26] |
| Vernonia amygdalina | Saponins, tannins, flavonoids | Produces iron nanoparticles; enhances microbial oxidation | Catalyzes reactions for nutrient release | 7–10 days | Requires controlled conditions for maximum effect | Promising results in small-scale decomposition trials. | [11,137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugivhisa, L.L.; Manganyi, M.C. Green Catalysis: The Role of Medicinal Plants as Food Waste Decomposition Enhancers/Accelerators. Life 2025, 15, 552. https://doi.org/10.3390/life15040552
Mugivhisa LL, Manganyi MC. Green Catalysis: The Role of Medicinal Plants as Food Waste Decomposition Enhancers/Accelerators. Life. 2025; 15(4):552. https://doi.org/10.3390/life15040552
Chicago/Turabian StyleMugivhisa, Liziwe L., and Madira C. Manganyi. 2025. "Green Catalysis: The Role of Medicinal Plants as Food Waste Decomposition Enhancers/Accelerators" Life 15, no. 4: 552. https://doi.org/10.3390/life15040552
APA StyleMugivhisa, L. L., & Manganyi, M. C. (2025). Green Catalysis: The Role of Medicinal Plants as Food Waste Decomposition Enhancers/Accelerators. Life, 15(4), 552. https://doi.org/10.3390/life15040552






