Expression of 9-O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells
Abstract
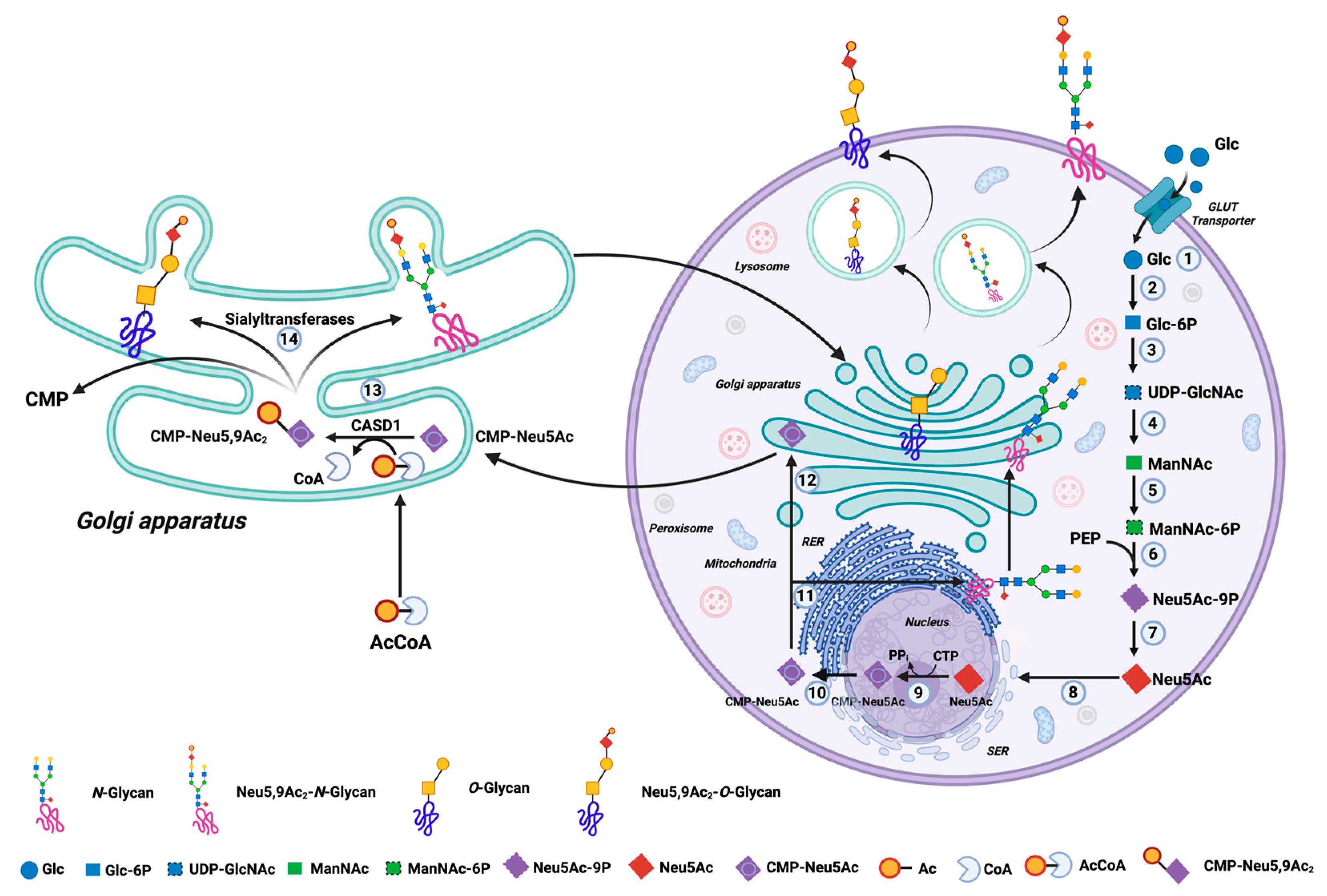
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Macrobrachium Rosenbergii Lectin
2.3. Immunocytochemistry
2.4. Sialidase Treatment of Cells
2.5. Cell Lysate
2.6. Protein Quantification
2.7. One-Dimensional Electrophoresis and Western Blot
2.8. Cellular Proliferation
2.9. Colony Formation Assay
2.10. Statistical Analysis
3. Results
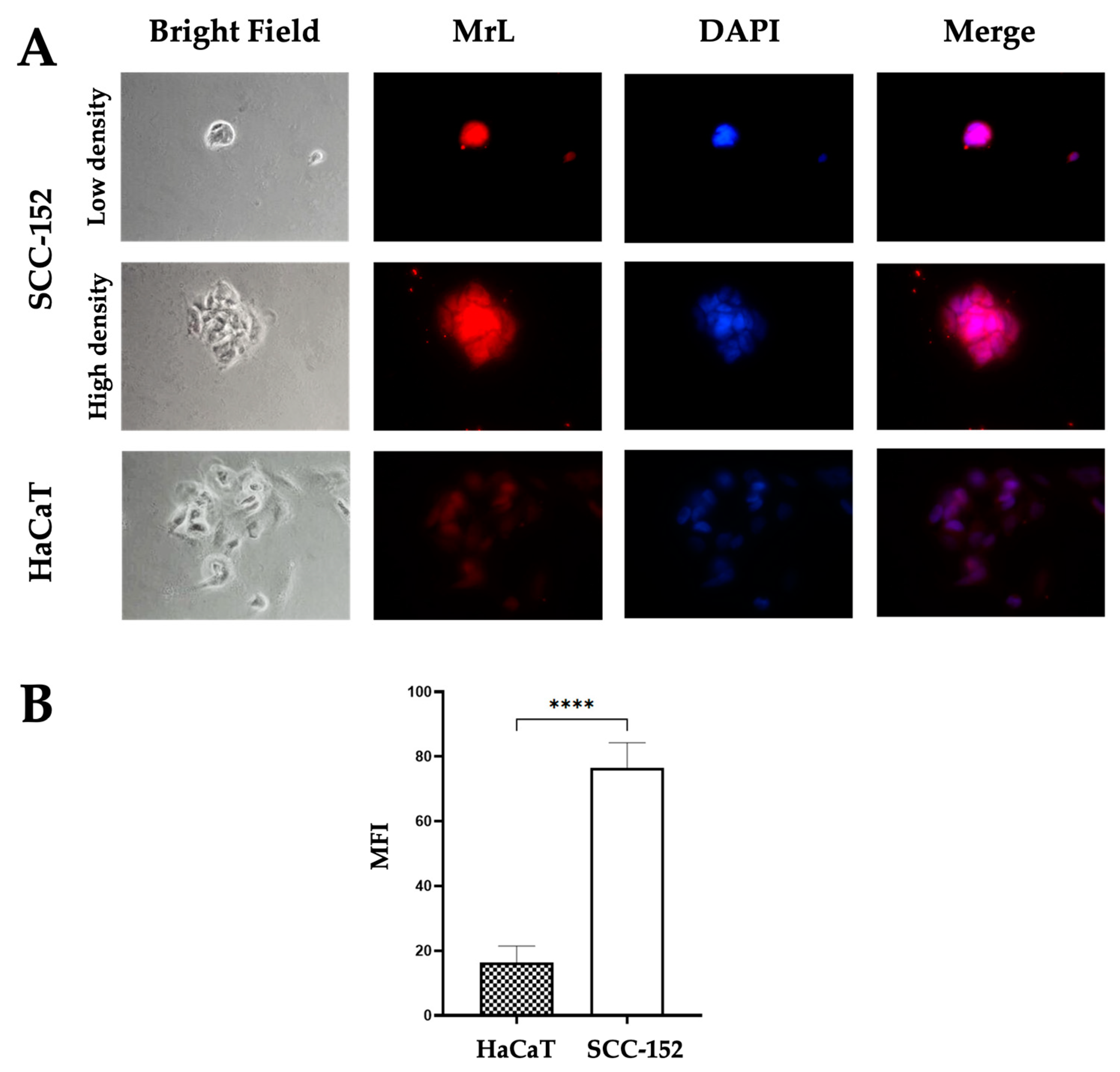
3.1. SCC-152 and HaCaT Cells Express Neu5,9Ac2
3.2. MrL Recognition Is Mediated by Neu5Ac
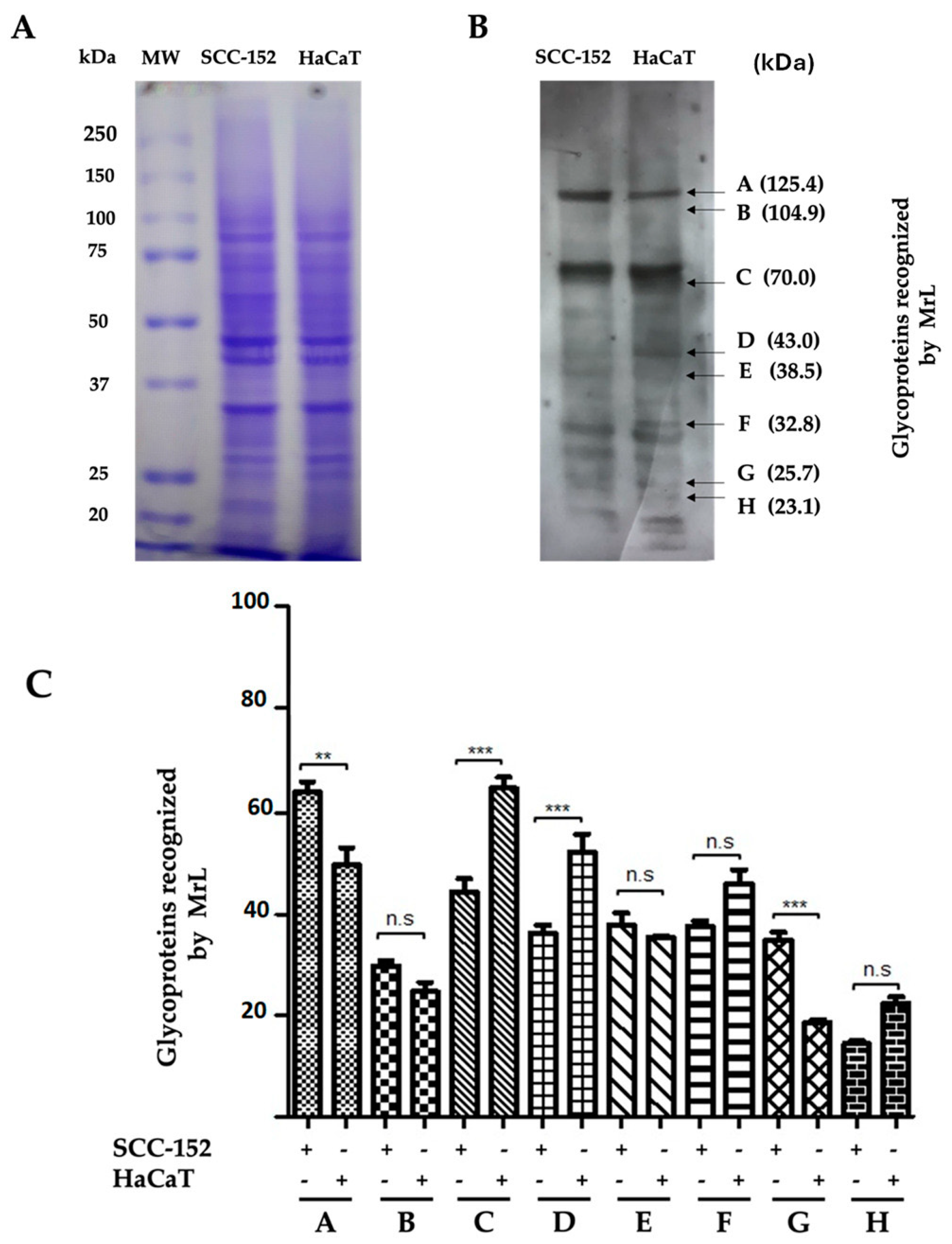
3.3. MrL Lectin Differentially Recognizes Glycoproteins in SCC-152 and HaCaT Cells
3.4. MrL Induces Proliferation of SCC-152 Cells, While It Does Not Affect HaCaT Cells
3.5. MrL Maintains the Structural and Morphological Integrity of SCC-152 Cell Colonies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcCoa | Acetyl-CoA |
| Achatinin-H | Achatina fulica |
| Asn | Asparagine |
| ATCC | American Type Culture Collection |
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| CA-125 | Cancer antigen 125 |
| CASD1 | N-acetylneuraminate (7)-9-O-acetyltransferase |
| CFUs | Colony Forming Units |
| CMP | Cytidine monophosphate |
| CTP | Cytidine triphosphate |
| DAPI | 4′,6-diamidino-2-fenilindol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DU145 | Prostate cancer cell line |
| E6 | E6 oncoprotein |
| E6p | Ubiquitin ligase E6p |
| EDTA | Ethylenediaminetetraacetic acid |
| EMEM | Earle’s Minimum Essential Medium |
| FBS | Fetal Bovine Serum |
| GalNAc | N-Acetylgalactosamine |
| GD2 | Ganglioside GD2 |
| GD3-9-O-AcSA | Disialoganglioside GD3-O-Acetylate |
| Glc | Glucose |
| Glc-6P | Glucose-6-phosphate |
| GlcNAc | N-Acetylglucosamine |
| HaCaT | Non-tumorigenic human cutaneous keratinocyte |
| HPV | Human papillomavirus |
| MAA | Maackia amurensis |
| ManNAc | N-Acetylmonosamine |
| ManNAc-6-Phosphate | N-Acetylmonosamine-6-phosphate |
| MrL | Macrobrachium rosenbergii lectin |
| MTT | (4,5-dimethyl-2-thiazolyl)-2,5-diphenium-2H-tetrazolium bromide |
| NaCl | Sodium chloride |
| Neu5,9Ac | 9-O-acetylated sialic acid |
| Neu5Ac | Sialic acid |
| Neu5Ac-9-phosphate | N-Acetylneuraminic acid-9-phosphate |
| OAcGD2 | Ganglioside GD2-9-O-acetylated |
| OPMDs | Oral potentially malignant disorders |
| OSCC | Oral squamous cell carcinoma |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate-Buffered Saline |
| PBS-Ca++ | PBS-Calcio |
| PC-3 | Prostate cancer cell line |
| PEP | Phosphoenolpyruvate |
| PFA | Paraformaldehyde |
| PHA | Phytohemagglutinin |
| pRB | Retinoblastoma protein |
| pre-B ALL | B-cell acute lymphoblastic leukemia |
| PSA | Prostate-specific antigen |
| RER | Rough endoplasmic reticulum |
| SCC | Squamous cell carcinoma |
| SCC-152 | Squamous cell carcinoma cell line |
| SDS-PAGE | Sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| SER | Smooth endoplasmic reticulum |
| SIAE | Sialiac acid acetylesterase |
| SNA | Sambucus nigra |
| ST6Gal-I | α2,6-sialyltransferase I |
| Thr | Threonine |
| TP | Total protein |
| TSA | Total Neu5Ac |
| UDP-GlcNAc | UDP-N-Acetyl-D-glucosamine |
References
- Fatima, J.; Fatima, E.; Mehmood, F.; Ishtiaq, I.; Khan, M.A.; Khurshid, H.M.S.; Kashif, M. Comprehensive Analysis of Oral Squamous Cell Carcinomas: Clinical, Epidemiological, and Histopathological Insights with a Focus on Prognostic Factors and Survival Time. Cureus 2024, 16, e54394. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, D.; George, N.A.; Thomas, S.; Iype, E.M. Factors associated with delay in diagnosis of oral cancers. Cancer Treat. Res. Commun. 2024, 40, 100831. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Dong, L.; Xue, L.; Cheng, W.; Tang, J.; Ran, J.; Li, Y. Comprehensive survival analysis of oral squamous cell carcinoma patients undergoing initial radical surgery. BMC Oral Health 2024, 24, 919. [Google Scholar] [CrossRef]
- Lenoci, D.; Moresco, E.; Cavalieri, S.; Bergamini, C.; Torchia, E.; Botta, L.; Canevari, S.; Trama, A.; Licitra, L.; De Cecco, L. Oral cancer in young adults: Incidence, risk factors, prognosis, and molecular biomarkers. Front. Oncol 2024, 14, 1452909. [Google Scholar] [CrossRef] [PubMed]
- Ajila, V.; Babu, S.; Shetty, V.; Shetty, P.; Devegowda, D.; Ramesh, P.; Natarajan, S. Human papillomavirus in oral squamous cell carcinoma: An institutional study. Clin. Cancer Investig. J. 2021, 10, 102–107. [Google Scholar] [CrossRef]
- Rebello, N.E.; Spadigam, A.E.; Dhupar, A. Burden of High-Risk Human Papillomavirus 16- and 18-Associated Oral Squamous Cell Carcinoma in the Indian Population: A Multiplex Polymerase Chain Reaction Study. Cureus 2024, 16, e73427. [Google Scholar] [CrossRef]
- de Menezes, S.A.F.; Miranda, Y.M.S.; da Silva, Y.M.; Carvalho, T.R.B.; Alves, F.R.S.; Silvestre, R.V.D.; Oliveira-Filho, A.B.; de Alencar Menezes, T.O.; de Souza Fonseca, R.R.; Laurentino, R.V.; et al. Prevalence and Genotyping of HPV in Oral Squamous Cell Carcinoma in Northern Brazil. Pathogens 2022, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; Lynch, C.F.; Chan, O.T.M.; Goodman, M.T.; Unger, E.R.; Steinau, M.; Thompson, T.D.; Gillison, M.; Lyu, C.; Saraiya, M. HPV Typing of Cancer Workgroup. Human papillomavirus DNA detection, p16INK4a, and oral cavity cancer in a U.S. population. Oral Oncol. 2019, 91, 92–96. [Google Scholar] [CrossRef]
- Ibieta, B.R.; Lizano, M.; Fras-Mendivil, M.; Barrera, J.L.; Carrillo, A.; Ma Ruz-Godoy, L.; Mohar, A. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 311–315. [Google Scholar] [CrossRef]
- Das, R.; Kumar, R.; Rai, A.K.; Sarma, A.; Kakoti, L.; Kataki, A.C.; Bhattacharyya, M. HPV and p16 expression association with 5-year survival in oral squamous cell carcinoma patients of North-East India. Adv. Cancer Biol. Metastasis 2024, 10, 100115. [Google Scholar] [CrossRef]
- Giraldi, L.; Collatuzzo, G.; Hashim, D.; Franceschi, S.; Herrero, R.; Chen, C.; Schwartz, S.M.; Smith, E.; Kelsey, K.; McClean, M.; et al. Infection with Human Papilloma Virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol. 2021, 75, 102020. [Google Scholar] [CrossRef]
- Pimolbutr, K.; Poomsawat, S.; Na-Ek, N.; Warnakulasuriya, S.; Buajeeb, W. Prevalence of human papillomavirus in oral cancer in Asia: A systematic review and meta-analysis. Oral Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.Y.; Huang, C.H.; Roden, R.B.S.; Wu, T.C.; Chang, Y.N.; Hung, C.F. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio 2021, 12, e03224-20. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, X.; Tang, Y.; Lao, Z.; Li, X.; Lei, J.; Wei, G. Deciphering the mechanisms of HPV E6 mutations in the destabilization of E6/E6AP/p53 complex. Biophys. J. 2022, 121, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Magal, S.S.; Jackman, A.; Ish-Shalom, S.; Botzer, L.E.; Gonen, P.; Schlegel, R.; Sherman, L. Downregulation of Bax mRNA expression and protein stability by the E6 protein of human papillomavirus 16. J. Gen. Virol. 2005, 86 Pt 3, 611–621. [Google Scholar] [CrossRef]
- Simmonds, M.; Storey, A. Identification of the regions of the HPV 5 E6 protein involved in Bak degradation and inhibition of apoptosis. Int. J. Cancer 2008, 123, 2260–2266. [Google Scholar] [CrossRef]
- Caldeira, S.; Dong, W.; Tommasino, M. Analysis of E7/Rb associations. Methods Mol. Med. 2005, 119, 363–379. [Google Scholar] [CrossRef]
- Huang, L.W.; Seow, K.M.; Lee, C.C.; Lin, Y.H.; Pan, H.S.; Chen, H.J. Decreased p21 expression in HPV-18 positive cervical carcinomas. Pathol. Oncol. Res. POR 2010, 16, 81–86. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Zuo, L.; Gao, S.; Jiang, X.; Han, Y.; Lin, J.; Peng, M.; Wu, N.; Tang, Y.; et al. HPV E6/E7: Insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024, 31, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, H.J. Glycosylation of glycolipids in the Golgi complex. J. Neurochem. 2007, 103, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Sun, Y.; Zhang, D.; Liu, T.; Yan, Q.; Yang, X. Mutation of N-linked glycosylation in EpCAM affected cell adhesion in breast cancer cells. Biol. Chem. 2017, 398, 1119–1126. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Wu, J.; Yuan, C.; Chen, S.; Liu, S.; Huo, M.; Zhang, C.; He, Y. GALNT1 Enhances Malignant Phenotype of Gastric Cancer via Modulating CD44 Glycosylation to Activate the Wnt/β-catenin Signaling Pathway. Int. J. Biol. Sci. 2022, 18, 6068–6083. [Google Scholar] [CrossRef]
- Lin, W.L.; Lin, Y.S.; Shi, G.Y.; Chang, C.F.; Wu, H.L. Lewisy promotes migration of oral cancer cells by glycosylation of epidermal growth factor receptor. PLoS ONE 2015, 10, e0120162. [Google Scholar] [CrossRef]
- Saitou, A.; Hasegawa, Y.; Fujitani, N.; Ariki, S.; Uehara, Y.; Hashimoto, U.; Saito, A.; Kuronuma, K.; Matsumoto, K.; Chiba, H.; et al. N-glycosylation regulates MET processing and signaling. Cancer Sci. 2022, 113, 1292–1304. [Google Scholar] [CrossRef]
- Li, H.W.; Liu, M.B.; Jiang, X.; Song, T.; Feng, S.X.; Wu, J.Y.; Deng, P.F.; Wang, X.Y. GALNT14 regulates ferroptosis and apoptosis of ovarian cancer through the EGFR/mTOR pathway. Future Oncol. 2022, 18, 149–161. [Google Scholar] [CrossRef]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, NY, USA, 2022; pp. 103–116. [Google Scholar]
- Brockhausen, I.; Wandall, H.H.; Hagen, K.G.T.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 117–128. [Google Scholar]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Krzewinski-Recchi, M.A.; Colomb, F.; Foulquier, F.; Groux-Degroote, S.; Delannoy, P. Sialyltransferases functions in cancers. Front. Biosci. 2012, 4, 499–515. [Google Scholar] [CrossRef]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [CrossRef]
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic Acids and Other Nonulosonic Acids. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; Chapter 15. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579976/ (accessed on 7 January 2025). [CrossRef]
- Raval, G.N.; Patel, D.D.; Parekh, L.J.; Patel, J.B.; Shah, M.H.; Patel, P.S. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral Dis. 2023, 9, 119–128. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Wuhrer, M.; Holst, S. Serum sialylation changes in cancer. Glycoconj. J. 2018, 35, 139–160. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic acid and biology of life: An introduction. In Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 1–61. [Google Scholar] [CrossRef]
- İliklerden, Ü.H.; Peksen, C.; Kalayci, T.; Kemik, O. Evaluation of preoperative and postoperative total serum sialic acid levels in patients with colon cancer. Ann. Ital. Chir. 2020, 91, 649–657. [Google Scholar]
- Sata, T.; Roth, J.; Zuber, C.; Stamm, B.; Heitz, P.U. Expression of alpha 2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. A lectin-gold cytochemical study with Sambucus nigra and Maackia amurensis lectins. Am. J. Pathol. 1991, 139, 1435–1448. [Google Scholar]
- Cao, Y.; Karsten, U.; Otto, G.; Bannasch, P. Expression of MUC1, Thomsen-Friedenreich antigen, Tn, sialosyl-Tn, and alpha2,6-linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Arch. Int. J. Pathol. 1999, 434, 503–509. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Y.; Wang, L.; Chen, X.; Su, Z.; Zhang, H.; Yuan, Q.; Wang, S. Sialylated β1, 6 branched N-glycans modulate the adhesion, invasion and metastasis of hepatocarcinoma cells. Biomed. Pharmacother. = Biomed. Pharmacother. 2016, 84, 1654–1661. [Google Scholar] [CrossRef]
- Pally, D.; Pramanik, D.; Hussain, S.; Verma, S.; Srinivas, A.; Kumar, R.V.; Everest-Dass, A.; Bhat, R. Heterogeneity in 2,6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia. ACS Cent. Sci. 2021, 7, 110–125. [Google Scholar] [CrossRef]
- Zamudio Cañas, R.; Jaramillo Flores, M.E.; Vallejo Ruiz, V.; Delgado Macuil, R.J.; López Gayou, V. Detection of Sialic Acid to Differentiate Cervical Cancer Cell Lines Using a Sambucus nigra Lectin Biosensor. Biosensors 2024, 14, 34. [Google Scholar] [CrossRef]
- López-Morales, D.; Reyes-Leyva, J.; Santos-López, G.; Zenteno, E.; Vallejo-Ruiz, V. Increased expression of sialic acid in cervical biopsies with squamous intraepithelial lesions. Diagn. Pathol. 2010, 5, 74. [Google Scholar] [CrossRef]
- Daniel, D.; Jose, J.; Harish Kumar, A. Is Salivary Sialic Acid a Reliable Biomarker in the Detection of Oral Potentially Malignant Disorder and Oral Squamous Cell Carcinoma. J. Maxillofac. Oral Surg. 2021, 20, 83–89. [Google Scholar] [CrossRef]
- Chaudhari, V.; Pradeep, G.L.; Prakash, N.; Mahajan, A.M. Estimation of salivary sialic acid in oral premalignancy and oral squamous cell carcinoma. Contemp. Clin. Dent. 2016, 7, 451–456. [Google Scholar] [CrossRef]
- Jacob, T.V.; Ramesh, M.; Murali, S.; Ramesh, K.; Sanjay, P.R.; Abraham, P. A non-invasive study to estimate and compare salivary sialic acid level as tumor marker in patients with pre-cancer and oral cancer. J. Cancer Res. Ther. 2016, 12, 634–639. [Google Scholar] [CrossRef]
- Guruaribam, V.D.; Sarumathi, T. Relevance of serum and salivary sialic acid in oral cancer diagnostics. J. Cancer Res. Ther. 2020, 16, 401–404. [Google Scholar] [CrossRef]
- Dhakar, N.; Astekar, M.; Jain, M.; Saawarn, S.; Saawarn, N. Total sialic acid, total protein and total sugar levels in serum and saliva of oral squamous cell carcinoma patients: A case control study. Dent. Res. J. 2013, 10, 343–347. [Google Scholar] [CrossRef]
- Rajaram, S.; Danasekaran, B.P.; Venkatachalapathy, R.; Prashad, K.V.; Rajaram, S. N-acetylneuraminic acid: A scrutinizing tool in oral squamous cell carcinoma diagnosis. Dent. Res. J. 2017, 14, 267–271. [Google Scholar] [CrossRef]
- Shah, M.H.; Telang, S.D.; Shah, P.M.; Patel, P.S. Tissue and serum alpha 2-3- and alpha 2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj. J. 2008, 25, 279–290. [Google Scholar] [CrossRef]
- Elgendi, M.; Lyzwinski, L.; Kübler, E.; Shokurov, A.V.; Howard, N.; Menon, C. Advancing cancer detection with portable salivary sialic acid testing. Npj Biosens. 2024, 1, 3. [Google Scholar] [CrossRef]
- Arthisri, A.S.; Sathiyamoorthy, A.; Meenakshi, B.; Chandran, C.R. Ratio of Salivary Sialic Acid to Fucose as Tumor Markers in Potentially Malignant Disorders and Oral Cancer. Contemp Clin Dent. 2020, 11, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.C.; Coelho, C.B.; Campos, A.R.; de Azevedo Moreira, R.; de Oliveira Monteiro-Moreira, A.C. Animal Galectins and Plant Lectins as Tools for Studies in Neurosciences. Curr. Neuropharmacol. 2020, 18, 202–215. [Google Scholar] [CrossRef]
- Singh, K.; Agrawal, L.; Gupta, R.; Singh, D.; Kathpalia, M.; Kaur, N. Lectins as a promising therapeutic agent for breast cancer: A review. Breast Dis. 2024, 43, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.P.E.; de Jong, H.; Boltje, T.J.; Büll, C. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mandal, C. O-acetylated sialic acids: Multifaceted role in childhood acute lymphoblastic leukaemia. Biotechnol. J. 2009, 4, 361–374. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Reisfeld, R.A.; Varki, A.P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science 1984, 225, 844–846. [Google Scholar] [CrossRef]
- Fahr, C.; Schauer, R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J. Investig. Dermatol. 2001, 116, 254–260. [Google Scholar] [CrossRef]
- Cavdarli, S.; Dewald, J.H.; Yamakawa, N.; Guérardel, Y.; Terme, M.; Le Doussal, J.M.; Delannoy, P.; Groux-Degroote, S. Identification of 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acid species of GD2 in breast cancer cells. Glycoconj. J. 2019, 36, 79–90. [Google Scholar] [CrossRef]
- Zenteno, R.; Vazquez, L.; Sierra, C.; Pereyra, A.; Slomianny, M.C.; Bouquelet, S.; Zenteno, E. Chemical characterization of the lectin from the freshwater prawn Macrobrachium rosenbergii (De Man) by MALDI-TOF. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 127, 243–250. [Google Scholar] [CrossRef]
- Zenteno, R.; Vázquez, L.; Martínez-Cairo, S.; Bouquelet, S.; Agundis, C.; Zenteno, E. Identification of lectin isoforms in juvenile freshwater prawns Macrobrachium rosenbergii (DeMan, 1879). Glycoconj. J. 2000, 17, 339–347. [Google Scholar] [CrossRef]
- Pérez-Campos-Mayoral, L.; Ruiz-Argüelles, A.; Pérez-Romano, B.; Zenteno, E.; Hernández-Cruz, P.; Martínez-Cruz, R.; Martínez-Cruz, M.; Pina-Canseco, S.; Pérez-Campos, E. Potential use of the Macrobrachium rosenbergii lectin for diagnosis of T-cell acute lymphoblastic leukemia. Tohoku J. Exp. Med. 2008, 214, 11–16. [Google Scholar] [CrossRef] [PubMed]
- American Type Culture Collection. Available online: https://www.atcc.org/products/crl-3240 (accessed on 7 January 2025).
- Cellosaurus UPCI-SCC-152. Available online: https://www.cellosaurus.org/CVCL_C058 (accessed on 7 January 2025).
- Xu, C.; Yan, T.; Yang, J. OVOL1 inhibits oral squamous cell carcinoma growth and metastasis by suppressing zinc finger E-box binding homeobox 1. Int. J. Clin. Exp. Pathol. 2019, 12, 2801–2808. [Google Scholar]
- Ziemann, F.; Arenz, A.; Preising, S.; Wittekindt, C.; Klussmann, J.P.; Engenhart-Cabillic, R.; Wittig, A. Increased sensitivity of HPV-positive head and neck cancer cell lines to x-irradiation ± Cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis. Am. J. Cancer Res. 2015, 5, 1017–1031. [Google Scholar]
- Pekarek, L.; GarridoGil, M.J.; SánchezCendra, A.; Cassinello, J.; Pekarek, T.; FraileMartinez, O.; García-Montero, C.; López-González, L.; Rios-Parra, A.; Álvarez-Mon, M.; et al. Emerging histological and serological biomarkers in oral squamous cell carcinoma: Applications in diagnosis, prognosis evaluation and personalized therapeutics (Review). Oncol. Rep. 2023, 50, 213. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Sethy, M.; Bhue, S.; Mohanta, B.K.; Dixit, A. Identification of therapeutically potential targets and their ligands for the treatment of OSCC. Front. Oncol. 2022, 12, 910494. [Google Scholar] [CrossRef]
- He, K.; Baniasad, M.; Kwon, H.; Caval, T.; Xu, G.; Lebrilla, C.; Hommes, D.W.; Bertozzi, C. Decoding the glycoproteome: A new frontier for biomarker discovery in cancer. J. Hematol. Oncol. 2024, 17, 12. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, W.; Yang, J.; Zhan, X. Cancer glycomics offers potential biomarkers and therapeutic targets in the framework of 3P medicine. Front. Endocrinol. 2022, 13, 970489. [Google Scholar] [CrossRef]
- Mayoral, M.A.; Mayoral, C.; Meneses, A.; Villalvazo, L.; Guzman, A.; Espinosa, B.; Ochoa, J.L.; Zenteno, E.; Guevara, J. Identification of galectin-3 and mucin-type O-glycans in breast cancer and its metastasis to brain. Cancer Investig. 2008, 26, 615–623. [Google Scholar] [CrossRef]
- Hernández-Maqueda, J.G.; Luna-Ulloa, L.B.; Santoyo-Ramos, P.; Castañeda-Patlán, M.C.; Robles-Flores, M. Protein kinase C delta negatively modulates canonical Wnt pathway and cell proliferation in colon tumor cell lines. PLoS ONE 2013, 8, e58540. [Google Scholar] [CrossRef]
- Katz, D.; Ito, E.; Lau, K.S.; Mocanu, J.D.; Bastianutto, C.; Schimmer, A.D.; Liu, F.F. Increased efficiency for performing colony formation assays in 96-well plates: Novel applications to combination therapies and high-throughput screening. BioTechniques 2008, 44, ix–xiv. [Google Scholar] [CrossRef]
- Josic, D.; Martinovic, T.; Pavelic, K. Glycosylation and metastases. Electrophoresis 2019, 40, 140–150. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, P.S. Sialylation: An Avenue to Target Cancer Cells. Pathol. Oncol. Res. POR 2016, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Holíková, Z.; Hrdlicková-Cela, E.; Plzák, J.; Smetana, K., Jr.; Betka, J.; Dvoránková, B.; Esner, M.; Wasano, K.; André, S.; Kaltner, H.; et al. Defining the glycophenotype of squamous epithelia using plant and mammalian lectins. Differentiation-dependent expression of alpha2,6- and alpha2,3-linked N-acetylneuraminic acid in squamous epithelia and carcinomas, and its differential effect on binding of the endogenous lectins galectins-1 and -3. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2002, 110, 845–856. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, J.B.; Shah, F.D.; Joshi, G.M.; Patel, P.S. Salivary glyco-sialylation changes monitors oral carcinogenesis. Glycoconj. J. 2014, 31, 649–659. [Google Scholar] [CrossRef]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. J. Tech. Methods Pathol. 2007, 87, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Schröter, L.; Albers, M.; Baumann, A.M.; Vicogne, D.; Le Doussal, J.M.; Mühlenhoff, M.; Delannoy, P.; Groux-Degroote, S. Role of Sialyl-O-Acetyltransferase CASD1 on GD2 Ganglioside O-Acetylation in Breast Cancer Cells. Cells 2021, 10, 1468. [Google Scholar] [CrossRef]
- Kawashima, I.; Nagata, I.; Tai, T. Immunocytochemical analysis of gangliosides in rat primary cerebellar cultures using specific monoclonal antibodies. Brain Res. 1996, 732, 75–86. [Google Scholar] [CrossRef]
- Wang, Y.H. Sialidases From Clostridium perfringens and Their Inhibitors. Front. Cell. Infect. Microbiol. 2020, 9, 462. [Google Scholar] [CrossRef]
- Joo, E.J.; Wasik, B.R.; Parrish, C.; Paz, H.; Mϋhlenhoff, M.; Abdel-Azim, H.; Groffen, J.; Heisterkamp, N. Pre-B acute lymphoblastic leukemia expresses cell surface nucleolin as a 9-O-acetylated sialoglycoprotein. Sci. Rep. 2018, 8, 17174. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bandyopadhyay, S.; Mandal, C.; Chandra, S.; Mandal, C. Flow-cytometric monitoring of disease-associated expression of 9-O-acetylated sialoglycoproteins in combination with known CD antigens, as an index for MRD in children with acute lymphoblastic leukaemia: A two-year longitudinal follow-up study. BMC Cancer 2008, 8, 40. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, C.; Zhou, L.; Cai, Z.; Zhao, S.; Yu, D. Knockdown of ST6Gal-I increases cisplatin sensitivity in cervical cancer cells. BMC Cancer 2016, 16, 949. [Google Scholar] [CrossRef]
- Wei, A.; Fan, B.; Zhao, Y.; Zhang, H.; Wang, L.; Yu, X.; Yuan, Q.; Yang, D.; Wang, S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget 2016, 7, 65374–65388. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bandyopadhyay, S.; Pal, S.; Das, B.; Bhattacharya, D.K.; Mandal, C. Increased interferon gamma production by peripheral blood mononuclear cells in response to stimulation of overexpressed disease-specific 9-O-acetylated sialoglycoconjugates in children suffering from acute lymphoblastic leukaemia. Br. J. Haematol. 2005, 128, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, M.; Wipfler, D.; Merling, A.; Cao, Y.; Claus, C.; Kniep, B.; Sadick, H.; Bergler, W.; Vlasak, R.; Schwartz-Albiez, R. Differential surface expression and possible function of 9-O- and 7-O-acetylated GD3 (CD60 b and c) during activation and apoptosis of human tonsillar B and T lymphocytes. Glycoconj. J. 2006, 23, 627–638. [Google Scholar] [CrossRef]
- Tonelli, Q.; Meints, R.H. Sialic acid: A specific role in hematopoietic spleen colony formation. J. Supramol. Struct. 1978, 8, 67–78. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martínez, H.; Jiménez-Castillo, V.; Illescas-Barbosa, D.; Ávila-Curiel, B.X.; Hernández-Huerta, M.T.; Díaz-Castillejos, R.; Torres-Rosas, R.; Zenteno, E.; Pereyra-Morales, M.A.; Solórzano-Mata, C.J. Expression of 9-O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells. Life 2025, 15, 663. https://doi.org/10.3390/life15040663
Sánchez-Martínez H, Jiménez-Castillo V, Illescas-Barbosa D, Ávila-Curiel BX, Hernández-Huerta MT, Díaz-Castillejos R, Torres-Rosas R, Zenteno E, Pereyra-Morales MA, Solórzano-Mata CJ. Expression of 9-O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells. Life. 2025; 15(4):663. https://doi.org/10.3390/life15040663
Chicago/Turabian StyleSánchez-Martínez, Hugo, Victoria Jiménez-Castillo, Daniela Illescas-Barbosa, Beatriz Xochitl Ávila-Curiel, María Teresa Hernández-Huerta, Risk Díaz-Castillejos, Rafael Torres-Rosas, Edgar Zenteno, Mohamed Alí Pereyra-Morales, and Carlos Josué Solórzano-Mata. 2025. "Expression of 9-O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells" Life 15, no. 4: 663. https://doi.org/10.3390/life15040663
APA StyleSánchez-Martínez, H., Jiménez-Castillo, V., Illescas-Barbosa, D., Ávila-Curiel, B. X., Hernández-Huerta, M. T., Díaz-Castillejos, R., Torres-Rosas, R., Zenteno, E., Pereyra-Morales, M. A., & Solórzano-Mata, C. J. (2025). Expression of 9-O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells. Life, 15(4), 663. https://doi.org/10.3390/life15040663







