Exploring the Relationship Between Obstructive Sleep Apnea and Olfactory Function
Abstract
1. Introduction
2. Materials and Methods
3. What Do We Know About Sleep Apnea?
4. How Do Olfactory Disorders Affect Human Health?
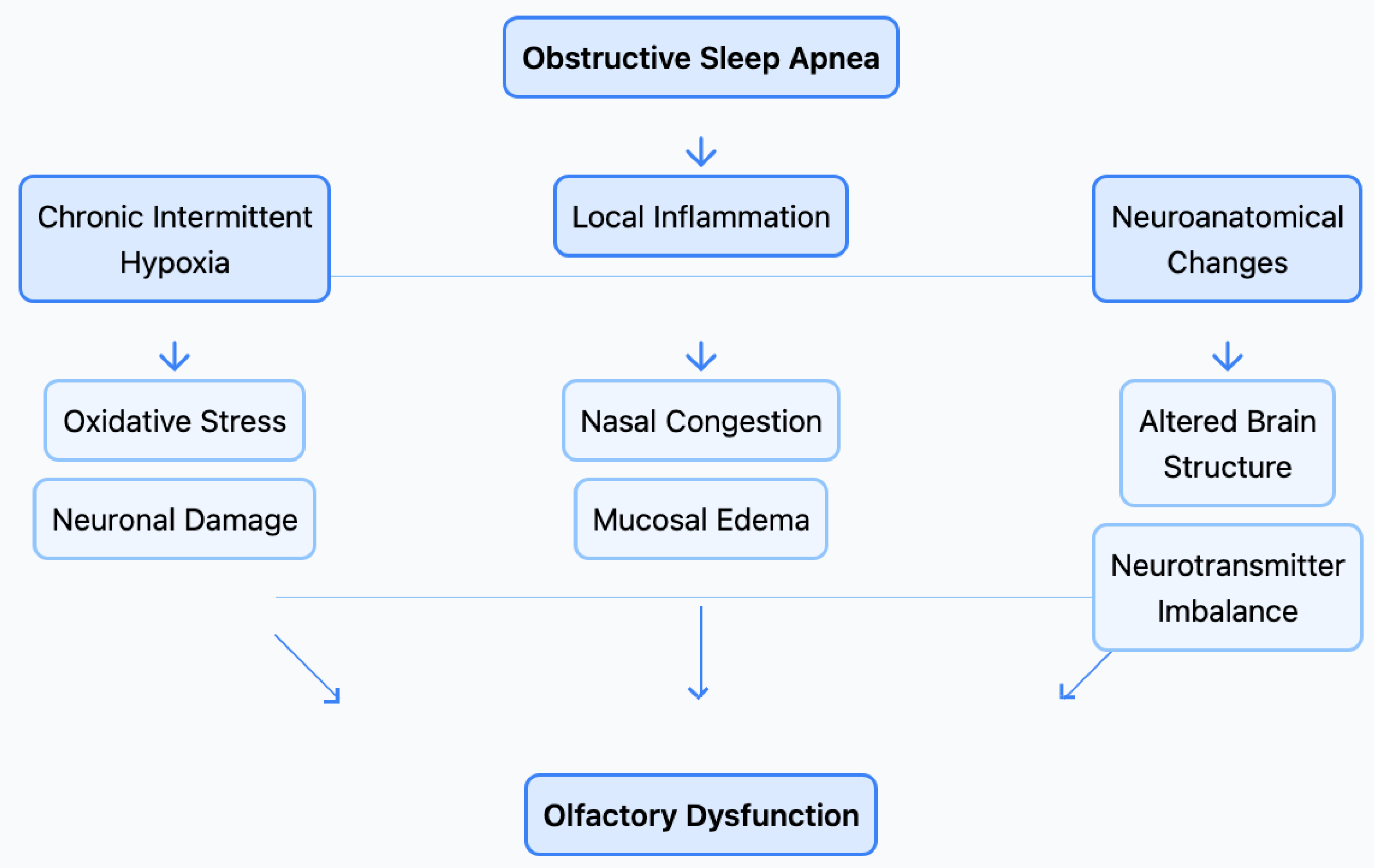
5. How Common Are Olfactory Disorders in Sleep Apnea Patients?
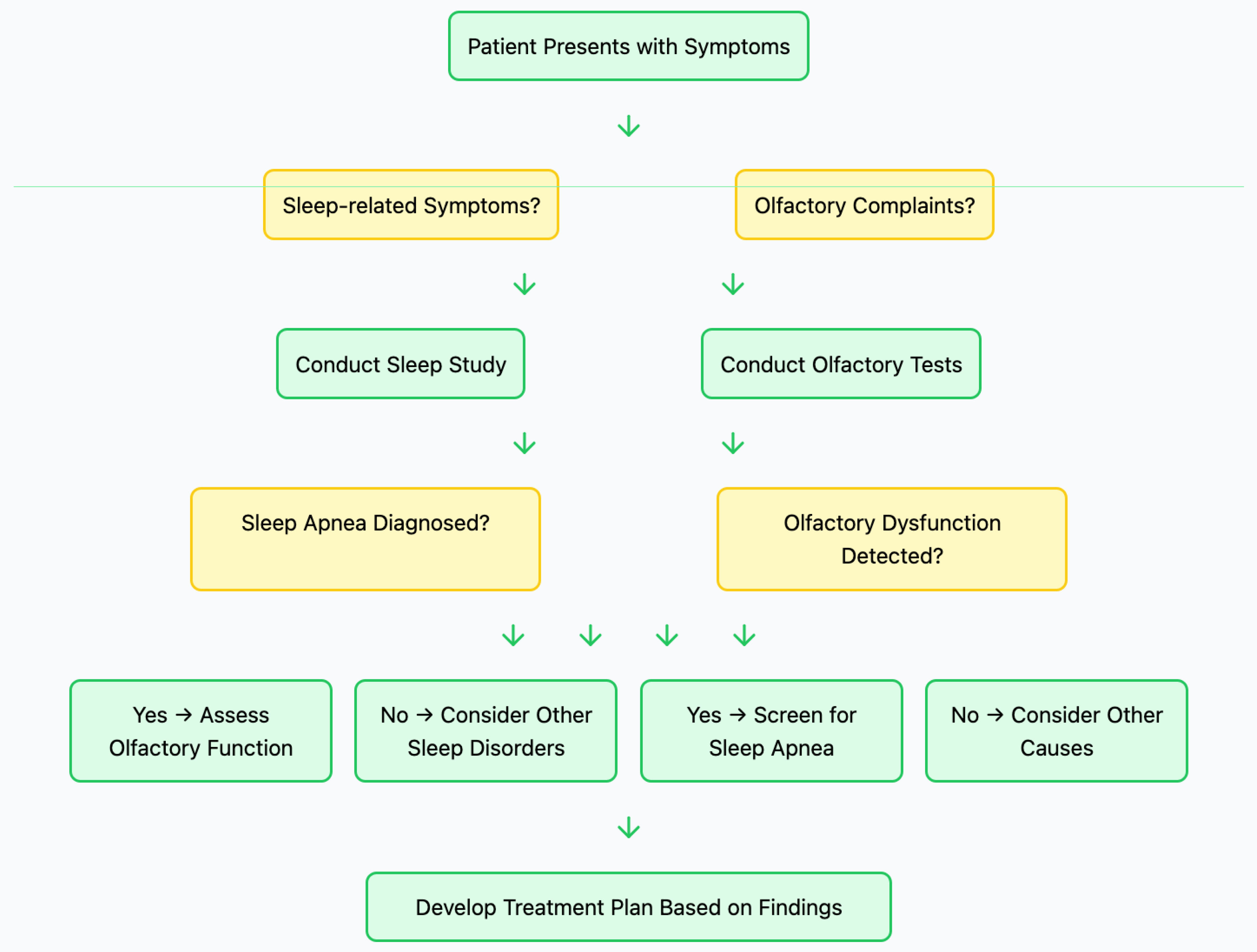
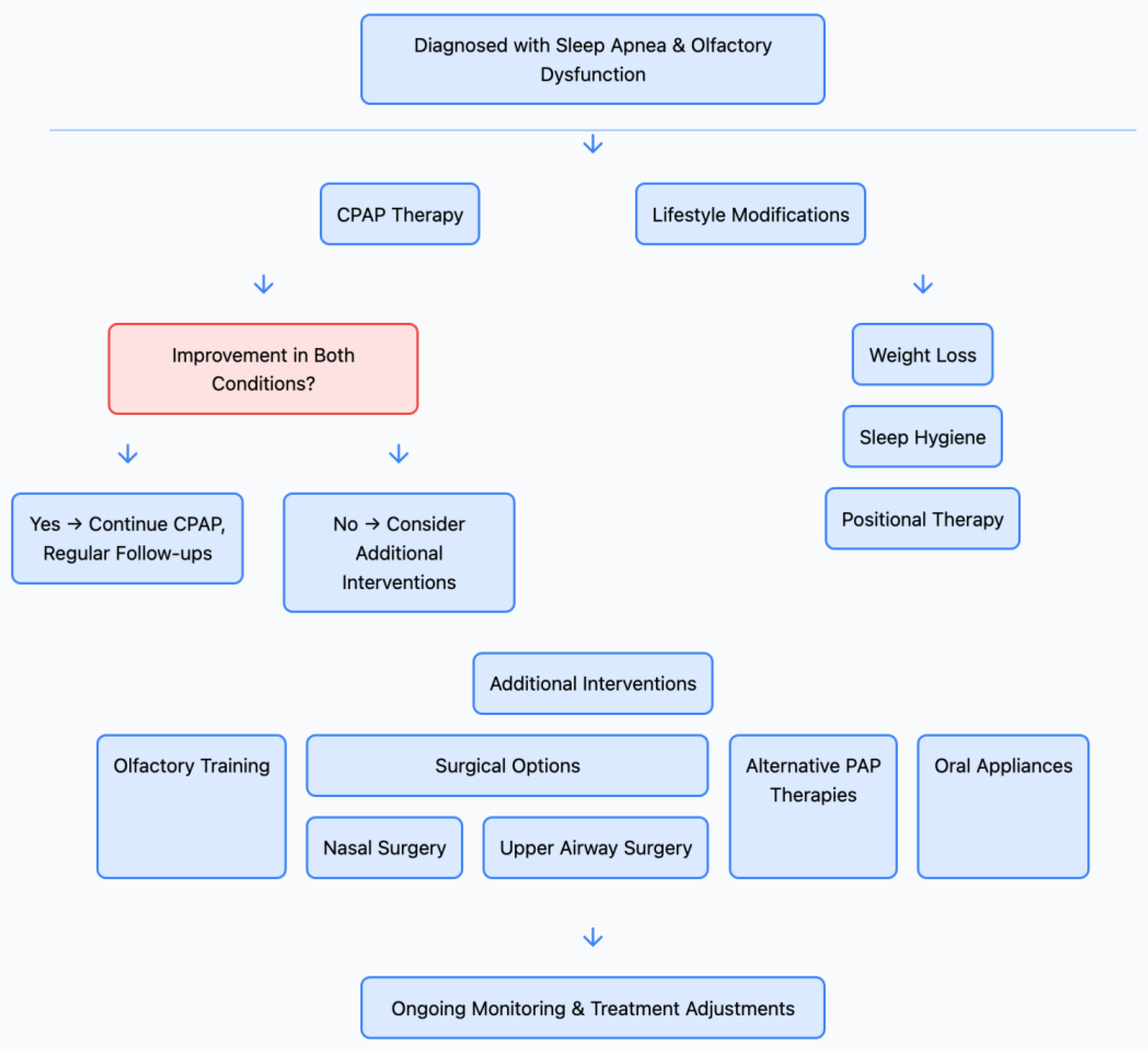
6. Clinical Implications
7. Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| CIH | Chronic Intermittent Hypoxia |
| CompSAS | Complex Sleep Apnea Syndrome |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CPAP | Continuous Positive Airway Pressure |
| CRS | Chronic Rhinosinusitis |
| CSA | Central Sleep Apnea |
| HSAT | Home Sleep Apnea Testing |
| MOIT | Monell Olfactory Image Test |
| OSA | Obstructive Sleep Apnea |
| PSG | Polysomnography |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| UPSIT | University of Pennsylvania Smell Identification Test |
References
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Malhotra, A. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Welge-Lüssen, A. Position paper on olfactory dysfunction. Rhinology 2017, 54, 1–30. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Saussez, S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Salihoglu, M.; Kendirli, M.T.; Altundag, A.; Tekeli, H.; Saglam, M.; Cayonu, M.; Kaya, A. The effect of obstructive sleep apnea on olfactory functions. Laryngoscope 2014, 124, 2190–2194. [Google Scholar] [CrossRef]
- Günbey, E.; Güzel, A.; Karlı, R.; Güzel, A.; Ünal, M. The relationship between the severity of obstructive sleep apnea and olfactory functions. Laryngoscope 2015, 125, 1450–1453. [Google Scholar]
- Bliwise, D.L.; Greer, S.A.; Scullin, M.K.; Phillips, L.S. Habitual and recent sleep duration in relation to olfactory function in older adults. J. Sleep Res. 2017, 26, 771–776. [Google Scholar]
- Nacher-Carda, V.; Oltra-Cucarella, J.; Piñeiro-Dieguez, B.; Lloret, M.A. Olfactory dysfunction in sleep apnea patients: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101446. [Google Scholar]
- Lötsch, J.; Hummel, T. Clinical usefulness of self-rated olfactory performance—A data science-based assessment of 6000 patients. Chem. Senses 2019, 44, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ishman, S.L.; Benke, J.R.; Cohen, A.P.; Stephen, M.J.; Ishii, L.E.; Gourin, C.G. Does surgery for obstructive sleep apnea improve olfaction? Laryngoscope 2018, 128, 1056–1063. [Google Scholar]
- Walliczek-Dworschak, U.; Cassel, W.; Mittendorf, L.; Pellegrino, R.; Koehler, U.; Guldner, C.; Hummel, T. Continuous positive air pressure improves orthonasal olfactory function of patients with obstructive sleep apnea. Sleep Med. 2017, 34, 24–29. [Google Scholar] [CrossRef]
- Rosenzweig, I.; Glasser, M.; Polsek, D.; Leschziner, G.D.; Williams, S.C.; Morrell, M.J. Sleep apnoea and the brain: A complex relationship. Lancet Respir. Med. 2015, 3, 404–414. [Google Scholar] [CrossRef]
- Gagnon, K.; Baril, A.A.; Gagnon, J.F.; Fortin, M.; Décary, A.; Lafond, C.; Gosselin, N. Cognitive impairment in obstructive sleep apnea. Pathol. Biol. 2018, 66, 63–70. [Google Scholar] [CrossRef]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 2014, 9, e107541. [Google Scholar] [CrossRef]
- Stuck, B.A.; Hummel, T. Olfaction in allergic rhinitis: A systematic review. J. Allergy Clin. Immunol. 2015, 136, 1460–1470. [Google Scholar] [CrossRef]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef]
- Seo, H.S.; Hummel, T. Olfactory dysfunction and its consequences on quality of life. Auris Nasus Larynx 2020, 47, 153–158. [Google Scholar]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Somers, V.K. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Oliven, A. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr. Opin. Pulm. Med. 2011, 17, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Pack, A.I. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Terrill, P.I.; Edwards, B.A.; Nemati, S.; Butler, J.P.; Owens, R.L.; Eckert, D.J.; Wellman, A. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur. Respir. J. 2015, 45, 408–418. [Google Scholar] [CrossRef]
- Edwards, B.A.; Eckert, D.J.; McSharry, D.G.; Sands, S.A.; Desai, A.; Kehlmann, G.; White, D.P. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1293–1300. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Haba-Rubio, J. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med. Rev. 2008, 12, 481–496. [Google Scholar] [CrossRef]
- Redline, S.; Tishler, P.V. The genetics of sleep apnea. Sleep Med. Rev. 2000, 4, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.E.; Chen, H.; Stilp, A.M.; Gleason, K.J.; Sofer, T.; Ancoli-Israel, S.; Saxena, R. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am. J. Respir. Crit. Care Med. 2016, 194, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Schwab, R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar]
- Mendonça, F.; Mostafa, S.S.; Ravelo-García, A.G.; Morgado-Dias, F.; Penzel, T. A review of obstructive sleep apnea detection approaches. IEEE J. Biomed. Health Inform. 2018, 23, 825–837. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of adult obstructive sleep apnea with positive airway pressure: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Ravesloot, M.J.; White, D.; Heinzer, R.; Oksenberg, A.; Pépin, J.L. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: A systematic review of the literature and meta-analysis. J. Clin. Sleep Med. 2017, 13, 813–824. [Google Scholar] [CrossRef]
- Strollo, P.J., Jr.; Soose, R.J.; Maurer, J.T.; De Vries, N.; Cornelius, J.; Froymovich, O.; Strohl, K.P. Upper-airway stimulation for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Holty, J.E.C.; Guilleminault, C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2010, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Vroegop, A.V.; Braem, M.J.; De Vries, N. Drug-induced sleep endoscopy: Evaluation of a selection tool for treatment modalities for obstructive sleep apnea. Respir. Med. 2017, 126, 9–14. [Google Scholar]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S. Sleep apnea and cardiovascular disease: Lessons from recent trials and need for team science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- Pamidi, S.; Tasali, E. Obstructive sleep apnea and type 2 diabetes: Is there a link? Front. Neurol. 2012, 3, 126. [Google Scholar] [CrossRef]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: A systematic review and meta-analysis. JAMA Neurol. 2017, 74, 1237–1245. [Google Scholar] [CrossRef]
- Abuyassin, B.; Sharma, K.; Ayas, N.T.; Laher, I. Obstructive sleep apnea and kidney disease: A potential bidirectional relationship? J. Clin. Sleep Med. 2015, 11, 915–924. [Google Scholar] [CrossRef]
- Javaheri, S.; Dempsey, J.A. Central sleep apnea. Compr. Physiol. 2013, 3, 141–163. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef]
- Eckert, D.J. Phenotypic approaches to obstructive sleep apnoea–New pathways for targeted therapy. Sleep Med. Rev. 2018, 37, 45–59. [Google Scholar] [CrossRef]
- Patel, R.M.; Pinto, J.M. Olfaction: Anatomy, physiology, and disease. Clin. Anat. 2014, 27, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Matsunami, H. The missense of smell: Functional variability in the human odorant receptor repertoire. Nat. Neurosci. 2014, 17, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Konnerth, C.G.; Hummel, T. A study on the frequency of olfactory dysfunction. Laryngoscope 2004, 114, 1764–1769. [Google Scholar] [CrossRef]
- Murphy, C.; Schubert, C.R.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Nondahl, D.M. Prevalence of olfactory impairment in older adults. JAMA 2002, 288, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Postma, E.M.; Boak, D.; Welge-Luessen, A.; Schöpf, V.; Mainland, J.D.; Duffy, V.B. Anosmia—A clinical review. Chem. Senses 2017, 42, 513–523. [Google Scholar] [CrossRef]
- Soler, Z.M.; Patel, Z.M.; Turner, J.H.; Holbrook, E.H. A primer on viral-associated olfactory loss in the era of COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 814–820. [Google Scholar] [CrossRef]
- Costanzo, R.M.; Miwa, T. Posttraumatic olfactory loss. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 82–84. [Google Scholar]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of ambient air pollution exposure on olfaction: A review. Environ. Health Perspect. 2016, 124, 1683–1693. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Lapid, H.; Hummel, T. Recording odor-evoked response potentials at the human olfactory epithelium. Chem. Senses 2013, 38, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Rissom, K.; Reden, J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Pollak, M.; Trattnig, S.; Schöpf, V. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014, 2014, 140419. [Google Scholar] [CrossRef]
- Seo, B.S.; Lee, H.J.; Mo, J.H.; Lee, C.H.; Rhee, C.S.; Kim, J.W. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1000–1004. [Google Scholar] [CrossRef]
- Kurtenbach, S.; Goss, G.M.; Goncalves, S.; Choi, R.; Hare, J.M.; Chaudhari, N.; Goldstein, B.J. Cell-based therapy restores olfactory function in an inducible model of hyposmia. Stem Cell Rep. 2019, 12, 1354–1365. [Google Scholar] [CrossRef]
- Spence, C. Just how much of what we taste derives from the sense of smell? Flavour 2015, 4, 30. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Zervakis, J. Taste and smell perception in the elderly: Effect of medications and disease. Adv. Food Nutr. Res. 2002, 44, 247–346. [Google Scholar]
- Santos, D.V.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Hazardous events associated with impaired olfactory function. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 317–319. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life—An updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kadohisa, M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The association between olfaction and depression: A systematic review. Chem. Senses 2016, 41, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Whitcroft, K.L.; Rueter, G.; Haehner, A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 2819–2825. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Hayes, J.E. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Hummel, T. Olfactory dysfunction in COVID-19: Diagnosis and management. JAMA 2020, 323, 2512–2514. [Google Scholar] [CrossRef]
- Pinto, J.M. Olfaction. Proc. Am. Thorac. Soc. 2011, 8, 46–52. [Google Scholar] [CrossRef]
- Fu, D.; Pinto, J.M.; Wang, L.; Chen, G.; Zhan, X.; Wei, Y. The effect of nasal structure on olfactory function in patients with obstructive sleep apnea. Am. J. Rhinol. Allergy 2015, 29, 437–443. [Google Scholar]
- Chen, X.W.; Liu, Y.N.; Wei, S.Q.; Li, H.B. Correlation between obstructive sleep apnea and olfactory function: Assessment using the Sniffin’ Sticks test and chemosensory event-related potentials. Sleep Breath. 2016, 20, 917–924. [Google Scholar]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V.; et al. Unraveling the complexities of oxidative stress and inflammation biomarkers in obstructive sleep apnea syndrome: A comprehensive review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Ma, J.; Wang, Q.; Yang, W. Micro-RNA profiling analysis reveals altered expression patterns in olfactory epithelium of sleep apnea patients. Respir. Res. 2019, 20, 1–12. [Google Scholar]
- Devouassoux, G.; Lévy, P.; Rossini, E.; Pin, I.; Fior-Gozlan, M.; Henry, M.; Pépin, J.L. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J. Allergy Clin. Immunol. 2007, 119, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Lian, G.; Huang, Y.; Ye, J. Effects of sleep apnea-related intermittent hypoxia on olfactory epithelium. Laryngoscope 2020, 130, E375–E380. [Google Scholar]
- Maniaci, A.; La Via, L.; Lechien, J.R.; Sangiorgio, G.; Iannella, G.; Magliulo, G.; Pace, A.; Mat, Q.; Lavalle, S.; Lentini, M. Hearing loss and oxidative stress: A comprehensive review. Antioxidants 2024, 13, 842. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R.S.; Hillman, D.R.; Eastwood, P.R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018, 38, 39–49. [Google Scholar] [CrossRef]
- Shankar, V.; Vengalil, S.; Shenkutie, A.; Rook, W.; Henson, M.; Rothstein, R. Olfactory dysfunction and sleep-disordered breathing: A systematic review and meta-analysis. Sleep Med. 2020, 75, 294–303. [Google Scholar]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher body mass index is associated with subjective olfactory dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef]
- Cavaliere, M.; Russo, F.; Iemma, M. Olfactory and gustatory impairment in patients with chronic rhinosinusitis: A case-control study. Auris Nasus Larynx 2018, 45, 1237–1241. [Google Scholar]
- Jiang, R.S.; Liang, K.L.; Hsin, C.H.; Su, M.C. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology 2019, 57, 440–445. [Google Scholar] [CrossRef]
- Maniaci, A.; La Via, L.; Pecorino, B.; Chiofalo, B.; Scibilia, G.; Lavalle, S.; Scollo, P. Obstructive sleep apnea in pregnancy: A comprehensive review of maternal and fetal implications. Neurol. Int. 2024, 16, 522–532. [Google Scholar] [CrossRef]
- Köseoğlu, S.; Derin, S.; Derin, A.T.; Şahin, M. The effect of positive airway pressure therapy on olfactory function in obstructive sleep apnea. Eur. Arch. Otorhinolaryngol. 2019, 276, 1355–1360. [Google Scholar]
- La Via, L.; Cuttone, G.; Misseri, G.; Sorbello, M.; Pappalardo, F.; Maniaci, A.; Duarte-Medrano, G.; Nuño-Lámbarri, N.; Zanza, C.; Gregoretti, C. The use of noninvasive positive pressure ventilation for severe asthma: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Expert Rev. Respir. Med. 2025, 19, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, P.C.; Chen, Y.P.; Lee, L.A.; Fang, T.J.; Lin, H.C. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am. J. Rhinol. Allergy 2019, 33, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Salihoğlu, M.; Altundağ, A.; Tekeli, H.; Kendirli, M.T.; Çayönü, M. The relationship between obstructive sleep apnea and olfactory sensitivity: A structural and functional neuroimaging study. Eur. Arch. Otorhinolaryngol. 2017, 274, 2259–2266. [Google Scholar]
- Liu, B.; Luo, Z.; Pinto, J.M.; Shiroma, E.J.; Tranah, G.J.; Wirdefeldt, K.; Harris, T.B. Relationship between poor olfaction and mortality among community-dwelling older adults: A cohort study. Ann. Intern. Med. 2019, 170, 673–681. [Google Scholar] [CrossRef]
- Nakata, Y.; Yoshimura, A.; Yamamoto, K.; Nakayama, H.; Fujita, M. Impact of continuous positive airway pressure therapy on the olfactory function of patients with obstructive sleep apnea: A longitudinal study. J. Clin. Sleep Med. 2019, 15, 279–284. [Google Scholar]
- Patel, Z.M.; Wise, S.K.; DelGaudio, J.M. Randomized controlled trial demonstrating cost-effective method of olfactory training in clinical practice: Essential oils at uncontrolled concentration. Laryngoscope 2017, 127, 294–300. [Google Scholar] [CrossRef]
- Landis, B.N.; Hummel, T.; Hugentobler, M.; Giger, R.; Lacroix, J.S. Ratings of overall olfactory function. Chem. Senses 2003, 28, 691–694. [Google Scholar] [CrossRef]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Cohen, N.A. The Monell Olfactory Image Test (MOIT): A novel quantitative test of olfactory dysfunction. Laryngoscope 2018, 128, 1759–1764. [Google Scholar]
- Park, J.G.; Kim, K.S.; Kim, S.T.; Cho, S.W.; Shin, C. Changes in olfactory function and sleep respiratory parameters following six months of continuous positive airway pressure therapy in patients with obstructive sleep apnea. Sleep Med. 2020, 76, 111–117. [Google Scholar]
- Wu, J.; Zang, H.R.; Wang, T.; Zhou, B.; Ye, J.Y.; Li, Y.C.; Han, D.M. Evaluation of the subjective and objective outcome of maxillomandibular advancement for the treatment of obstructive sleep apnea syndrome. J. Craniofac. Surg. 2017, 28, 371–375. [Google Scholar]
- Konstantinidis, I.; Tsakiropoulou, E.; Bekiaridou, P.; Kazantzidou, C.; Constantinidis, J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 2016, 126, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Banglawala, S.M.; Schlosser, R.J.; Morella, K.; Chandra, R.; Khetani, J.; Poetker, D.M.; Soler, Z.M. Qualitative development of the sinus control test: A survey-based clinical endpoint for sinusitis. Int. Forum Allergy Rhinol. 2017, 7, 480–487. [Google Scholar]
- Bhattacharyya, N.; Kepnes, L.J.; Alam, S. Quality of life and symptom profiles of elderly patients with chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 2017, 118, 452–456. [Google Scholar]
- Ottaviano, G.; Savietto, E.; Scarpa, B.; Bertocco, A.; Maculan, P.; Sergi, G.; Martini, A. Influence of body composition and nasal and systemic inflammation on olfactory function in elderly subjects. Rhinology 2018, 56, 336–342. [Google Scholar]
- Lavalle, S.; Caruso, S.; Foti, R.; Gagliano, C.; Cocuzza, S.; La Via, L.; Parisi, F.M.; Calvo-Henriquez, C.; Maniaci, A. Behçet’s Disease, pathogenesis, clinical features, and treatment approaches: A comprehensive review. Medicina 2024, 60, 562. [Google Scholar] [CrossRef]
- Poletti, S.C.; Michel, E.; Hummel, T.; Stuck, B.A. Olfactory training in patients with olfactory loss: A systematic review and meta-analysis. Laryngoscope 2020, 130, 2532–2539. [Google Scholar]
- Trotier, D.; Bensimon, J.L.; Herman, P.; Tran Ba Huy, P.; Døving, K.B.; Eloit, C. Inflammatory obstruction of the olfactory clefts and olfactory loss in humans: A new pattern of olfactory dysfunction. Eur. Arch. Otorhinolaryngol. 2018, 275, 1553–1562. [Google Scholar]
- Iannilli, E.; Noennig, N.; Hummel, T.; Schoenfeld, A.M. Spatio-temporal correlates of taste processing in the human primary gustatory cortex. Neuroscience 2019, 396, 46–57. [Google Scholar] [CrossRef]
- Lane, J.M.; Jones, S.E.; Dashti, H.S.; Wood, A.R.; Aragam, K.G.; van Hees, V.T.; Saxena, R. Biological and clinical insights from genetics of insomnia symptoms. Nat. Genet. 2019, 51, 387–393. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Parisi, F.M.; Barbanti, M.; Cocuzza, S.; Iannella, G.; Magliulo, G.; Pace, A.; Lentini, M.; Masiello, E.; et al. Impact of obstructive sleep apnea and sympathetic nervous system on cardiac health: A comprehensive review. J. Cardiovasc. Dev. Dis. 2024, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Sabha, M.; Damm, M.; Philpott, C.; Oleszkiewicz, A.; Hähner, A.; Hummel, T. Parosmia is Associated with Relevant Olfactory Recovery After Olfactory Training. Laryngoscope 2021, 131, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Deeb, W.; Giordano, J.J.; Rossi, P.J.; Mogilner, A.Y.; Gunduz, A.; Judy, J.W.; Okun, M.S. Proceedings of the Fourth Annual Deep Brain Stimulation Think Tank: A Review of Emerging Issues and Technologies. Front. Integr. Neurosci. 2019, 13, 50. [Google Scholar] [CrossRef]
- Liu, J.; Wu, T.; Liu, Q.; Wu, S.; Chen, J.C. Air pollution exposure and adverse sleep health across the life course: A systematic review. Environ. Pollut. 2020, 262, 114263. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, J.W.; Lee, K. Detection of sleep disordered breathing severity using acoustic biomarker and machine learning techniques. Biomed. Eng. Online 2020, 19, 16. [Google Scholar] [CrossRef]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Redline, S. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef]
- Damm, M.; Pikart, L.K.; Reimann, H.; Burkert, S.; Göktas, Ö.; Haxel, B.; Hüttenbrink, K.B. Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope 2014, 124, 826–831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniaci, A.; Lentini, M.; Bianco, M.R.; Paternò, D.S.; Lavalle, S.; Pace, A.; Iannella, G.; Boscolo-Rizzo, P.; Mayo-Yanez, M.; Calvo-Henriquez, C.; et al. Exploring the Relationship Between Obstructive Sleep Apnea and Olfactory Function. Life 2025, 15, 675. https://doi.org/10.3390/life15040675
Maniaci A, Lentini M, Bianco MR, Paternò DS, Lavalle S, Pace A, Iannella G, Boscolo-Rizzo P, Mayo-Yanez M, Calvo-Henriquez C, et al. Exploring the Relationship Between Obstructive Sleep Apnea and Olfactory Function. Life. 2025; 15(4):675. https://doi.org/10.3390/life15040675
Chicago/Turabian StyleManiaci, Antonino, Mario Lentini, Maria Rita Bianco, Daniele Salvatore Paternò, Salvatore Lavalle, Annalisa Pace, Giannicola Iannella, Paolo Boscolo-Rizzo, Miguel Mayo-Yanez, Christian Calvo-Henriquez, and et al. 2025. "Exploring the Relationship Between Obstructive Sleep Apnea and Olfactory Function" Life 15, no. 4: 675. https://doi.org/10.3390/life15040675
APA StyleManiaci, A., Lentini, M., Bianco, M. R., Paternò, D. S., Lavalle, S., Pace, A., Iannella, G., Boscolo-Rizzo, P., Mayo-Yanez, M., Calvo-Henriquez, C., Lechien, J. R., & La Via, L. (2025). Exploring the Relationship Between Obstructive Sleep Apnea and Olfactory Function. Life, 15(4), 675. https://doi.org/10.3390/life15040675









