On an RNA-Membrane Protogenome
Abstract
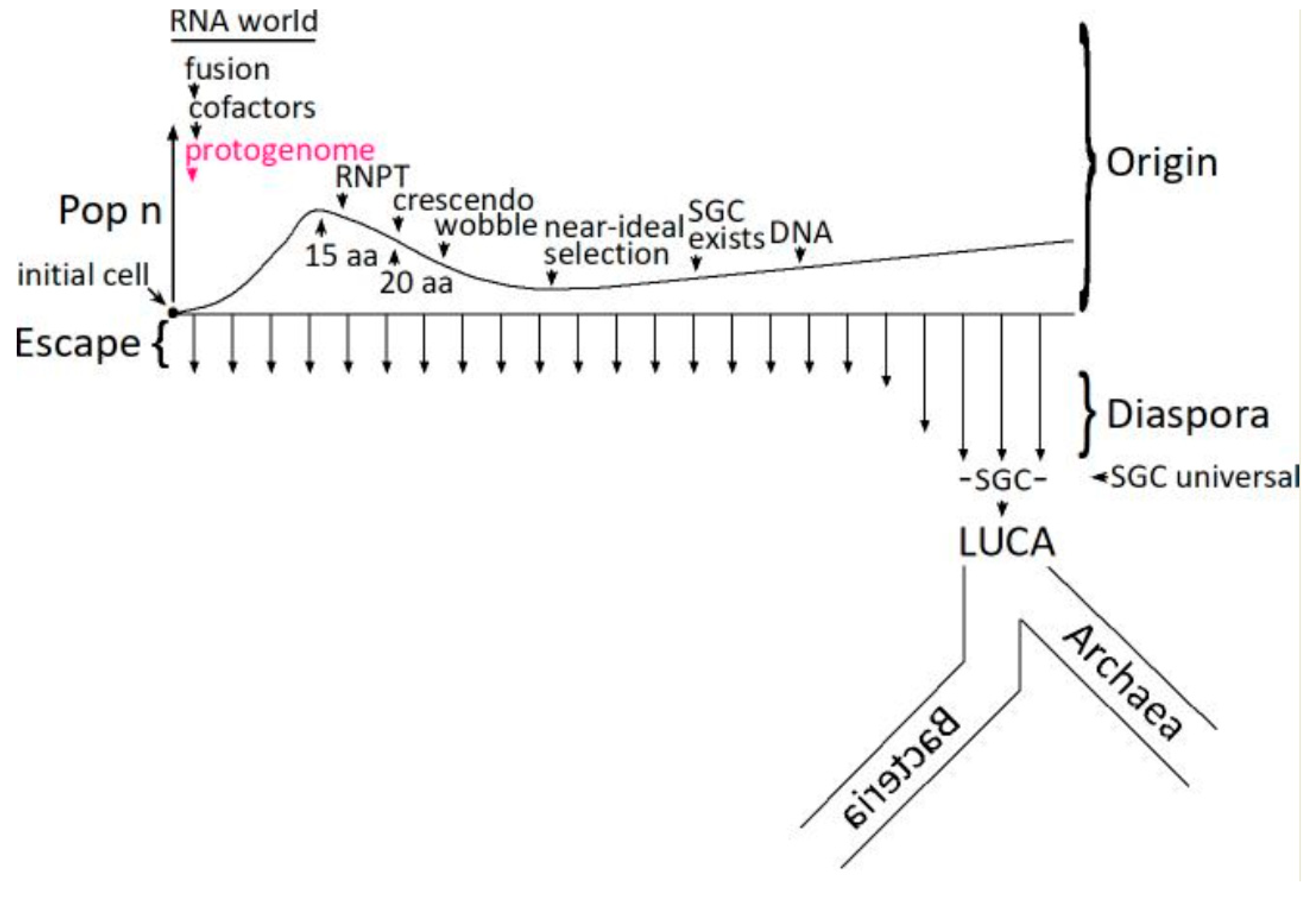
1. Introduction
1.1. Membrane-Binding RNA Selection
1.2. Multiple Lipid Forms
1.3. Molecular Dynamics on RNA-Membranes
1.4. Fluid Membrane Affinity
1.5. RNA9:RNA10
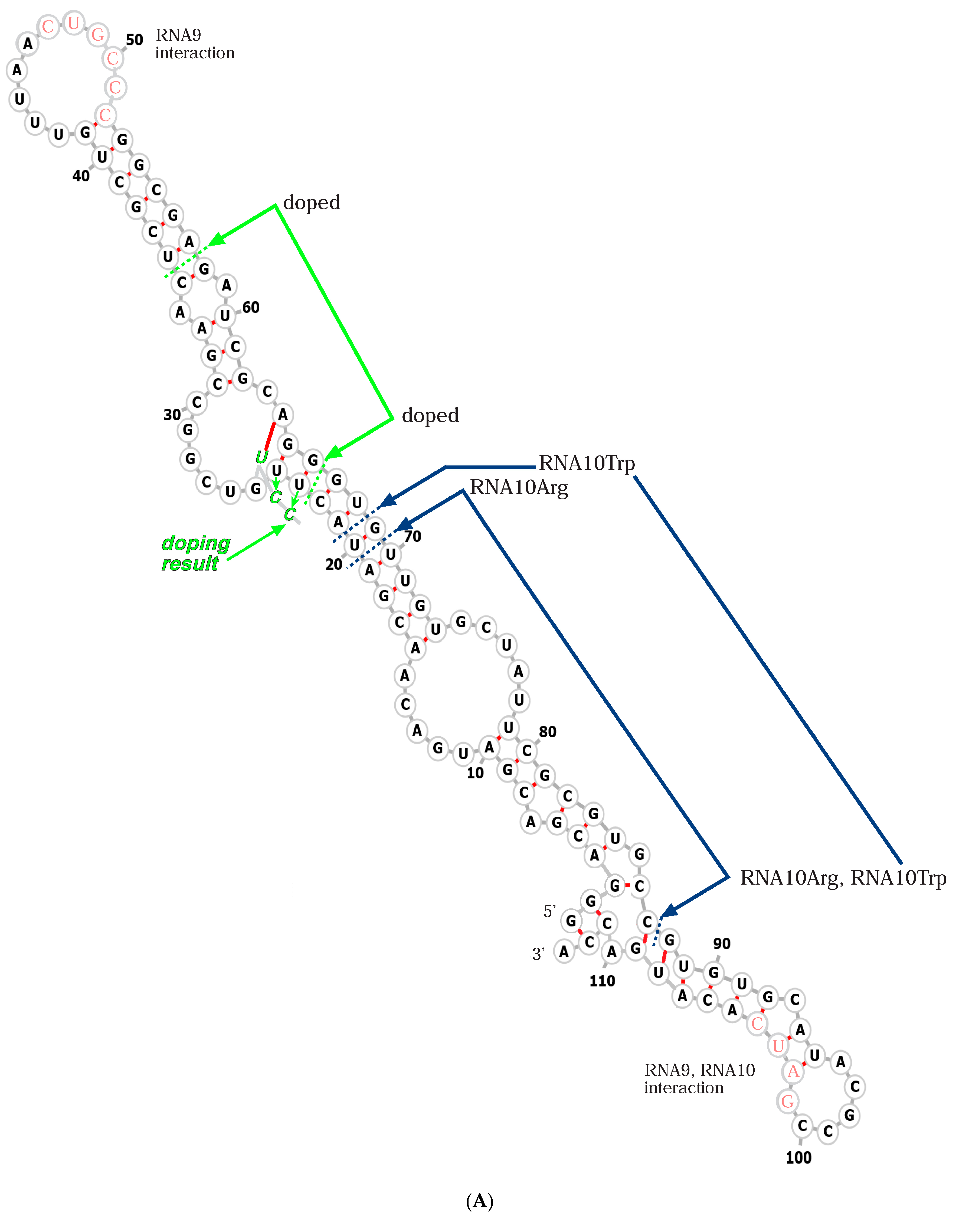
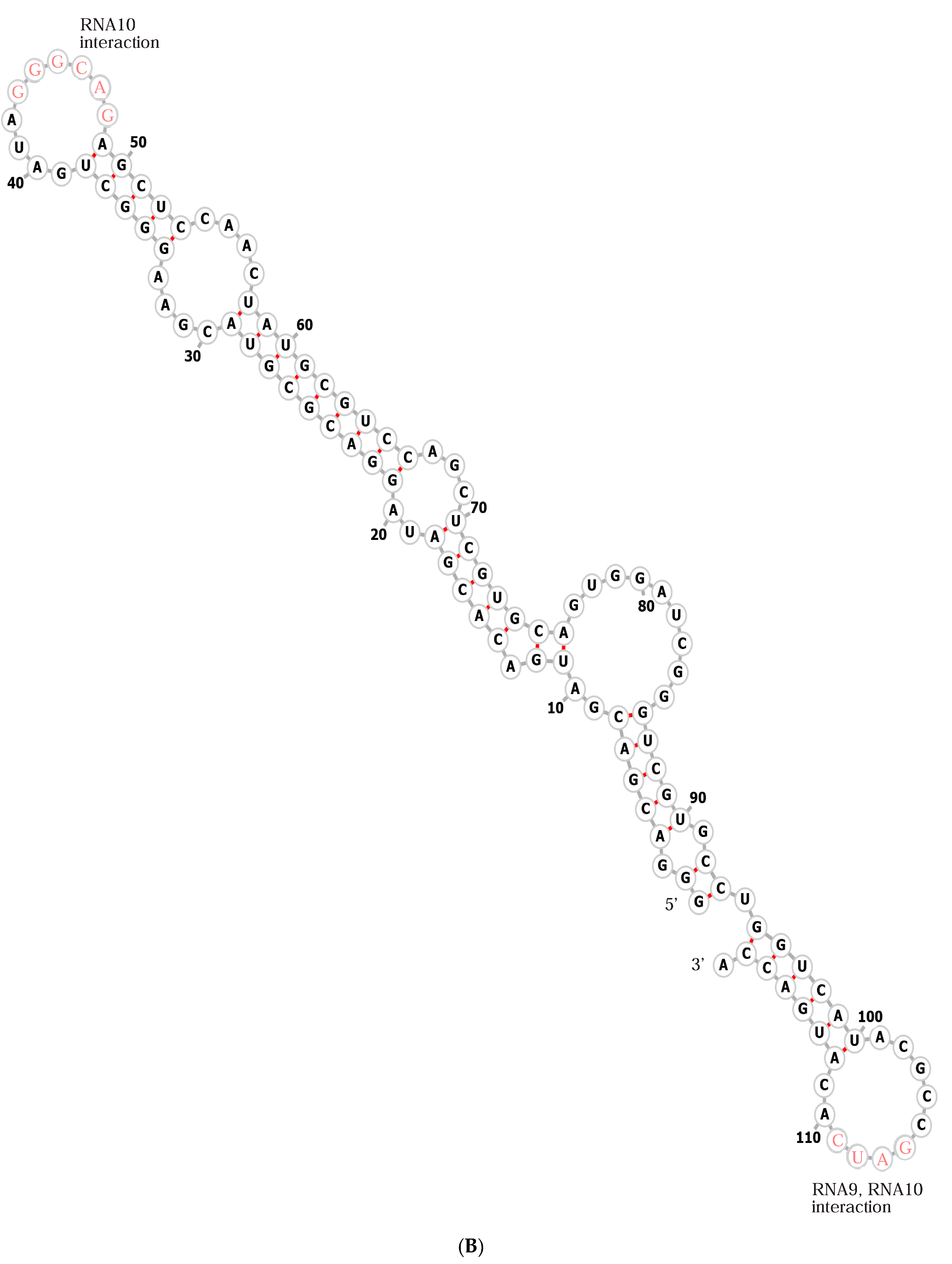

1.6. Bound RNAs Also Retain Complex Solution Functions
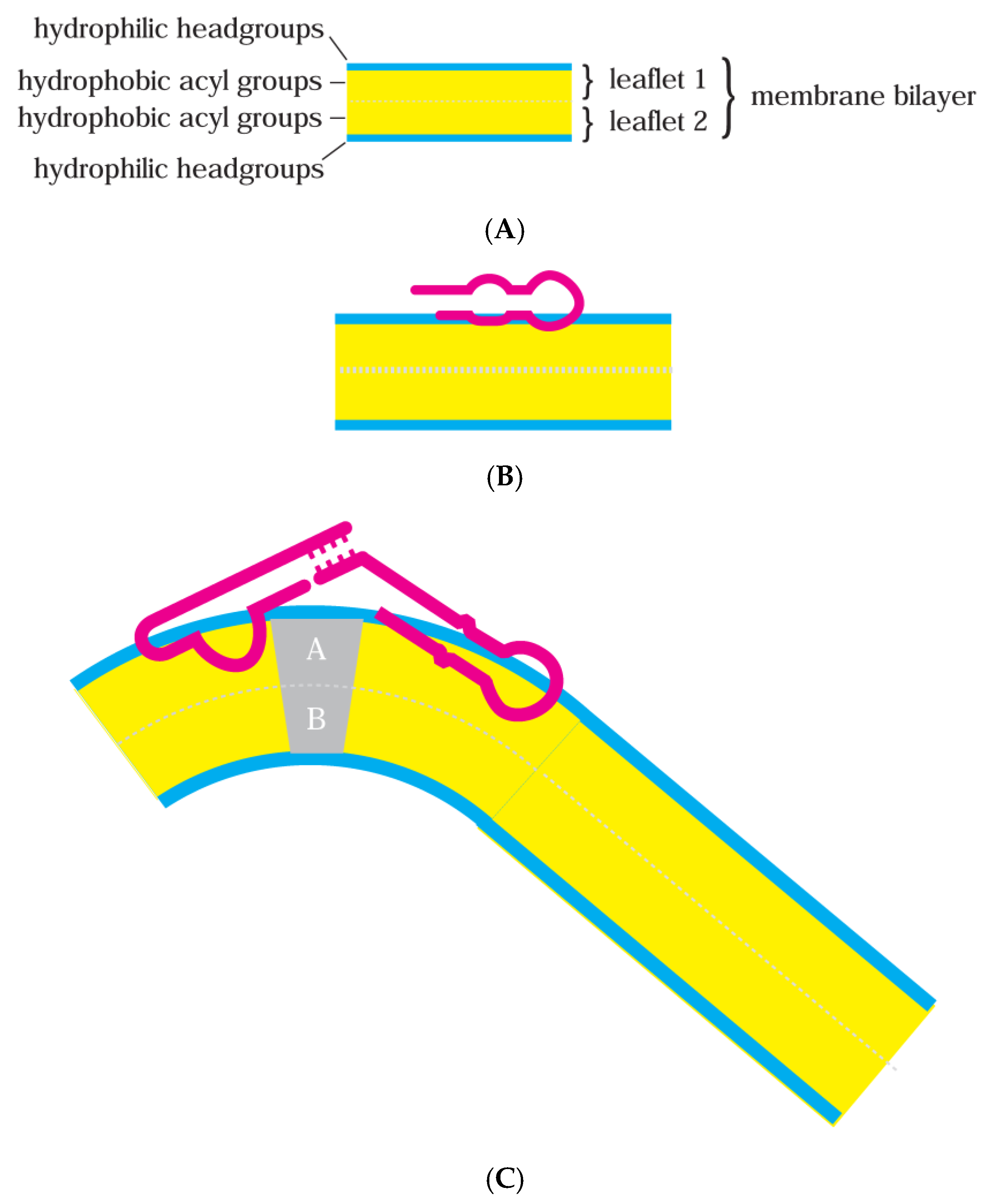
1.7. Bound RNAs Shape Bilayers
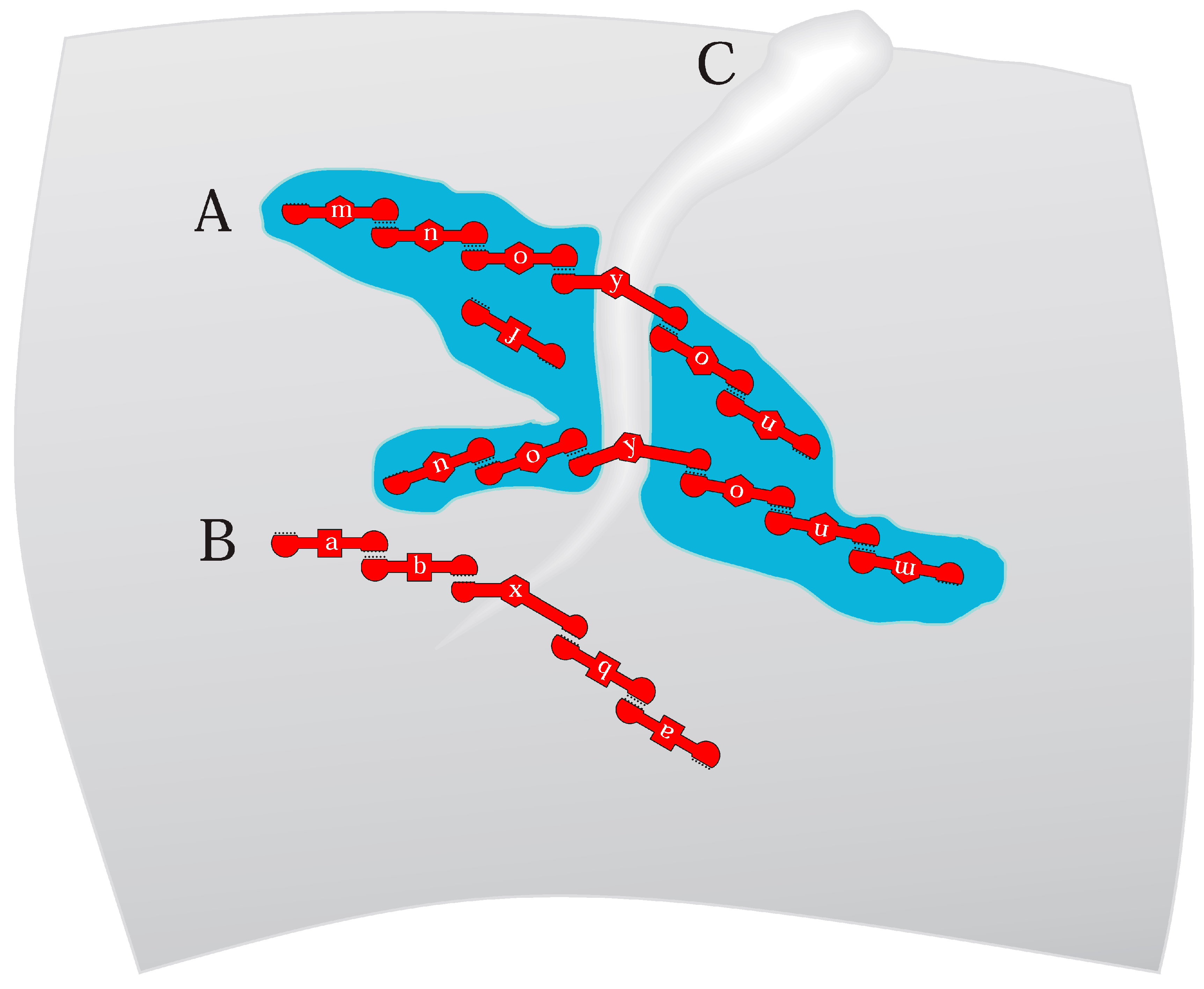
1.8. Rafts Uniquely Distinguish RNA Sequences
1.9. Singly Charged Lipid Headgroups, Including Fatty Acids, Also Form RNA-Membranes
2. Discussion
2.1. A Unified Binding Mechanism
2.2. Rafts Organize RNAs
2.3. Facilitating Replication
2.4. Coordination with Cell Division
2.5. Coordination of Expression
2.6. Reproducing RNA Groups
2.7. Rapid Evolution
2.8. Fatty Acid RNA-Membranes
2.9. Biology as Anthology
2.10. Relevance to Coding
2.11. RNA-Membranes Supply Indispensable Division Functions
2.12. Modern Membrane RNAs?
Funding
Conflicts of Interest
Abbreviations
References
- Khvorova, A.; Kwak, Y.G.; Tamkun, M.; Majerfeld, I.; Yarus, M. RNAs That Bind and Change the Permeability of Phospholipid Membranes. Proc. Natl. Acad. Sci. USA 1999, 96, 10649–10654. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, T.; Saenz, J.P. Lipid Membranes Modulate the Activity of RNA through Sequence-Dependent Interactions. Proc. Natl. Acad. Sci. USA 2022, 119, e2119235119. [Google Scholar] [CrossRef]
- Mańka, R.; Janas, P.; Sapoń, K.; Janas, T.; Janas, T. Role of RNA Motifs in RNA Interaction with Membrane Lipid Rafts: Implications for Therapeutic Applications of Exosomal RNAs. Int. J. Mol. Sci. 2021, 22, 9416. [Google Scholar] [CrossRef]
- Janas, T.; Janas, P.; Sapoń, K.; Janas, T. Binding of RNA Aptamers to Membrane Lipid Rafts: Implications for Exosomal miRNAs Transfer from Cancer to Immune Cells. Int. J. Mol. Sci. 2020, 21, 8503. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 Controls the Sorting of miRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Sapoń, K.; Janas, T.; Yarus, M. Specific Binding of VegT mRNA Localization Signal to Membranes in Xenopus Oocytes. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118952. [Google Scholar] [CrossRef]
- Janas, T.; Janas, T.; Yarus, M. Human tRNA(Sec) Associates with HeLa Membranes, Cell Lipid Liposomes, and Synthetic Lipid Bilayers. RNA 2012, 18, 2260–2268. [Google Scholar] [CrossRef]
- Michanek, A.; Kristen, N.; Höök, F.; Nylander, T.; Sparr, E. RNA and DNA Interactions with Zwitterionic and Charged Lipid Membranes—A DSC and QCM-D Study. Biochim. Biophys. Acta 2010, 1798, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L.C. Model Systems, Lipid Rafts, and Cell Membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and Phase Transitions of the Phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Davies, M.; Reyes-Figueroa, A.D.; Gurtovenko, A.A.; Frankel, D.; Karttunen, M. Elucidating Lipid Conformations in the Ripple Phase: Machine Learning Reveals Four Lipid Populations. Biophys. J. 2023, 122, 442–450. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.M.; Borst, J.; Fedorov, A.; Prieto, M.; Visser, A.J.W.G. Complexity of Lipid Domains and Rafts in Giant Unilamellar Vesicles Revealed by Combining Imaging and Microscopic and Macroscopic Time-Resolved Fluorescence. Biophys. J. 2007, 93, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Yarus, M. Visualization of Membrane RNAs. RNA 2003, 9, 1353–1361. [Google Scholar] [CrossRef]
- Janas, T.; Janas, T.; Yarus, M. Specific RNA Binding to Ordered Phospholipid Bilayers. Nucleic Acids Res. 2006, 34, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Budker, V.G.; Kazatchkov, Y.A.; Naumova, L.P. Polynucleotides Adsorb on Mitochondrial and Model Lipid Membranes in the Presence of Bivalent Cations. FEBS Lett. 1978, 95, 143–146. [Google Scholar] [CrossRef]
- Di Marco, S.; Aupič, J.; Bussi, G.; Magistrato, A. All-Atom Simulations Elucidate the Molecular Mechanism Underlying RNA-Membrane Interactions. Nano Lett. 2025, 25, 4628–4635. [Google Scholar] [CrossRef]
- Singh, A.P.; Prabhu, J.; Vanni, S. RNA Order Regulates Its Interactions with Zwitterionic Lipid Bilayers. Nano Lett. 2025, 25, 77–83. [Google Scholar] [CrossRef]
- Michanek, A.; Yanez, M.; Wacklin, H.; Hughes, A.; Nylander, T.; Sparr, E. RNA and DNA Association to Zwitterionic and Charged Monolayers at the Air-Liquid Interface. Langmuir ACS J. Surf. Colloids 2012, 28, 9621–9633. [Google Scholar] [CrossRef]
- Vlassov, A.; Khvorova, A.; Yarus, M. Binding and Disruption of Phospholipid Bilayers by Supramolecular RNA Complexes. Proc. Natl. Acad. Sci. USA 2001, 98, 7706–7711. [Google Scholar] [CrossRef]
- Tomizawa, J. Control of ColE1 Plasmid Replication: The Process of Binding of RNA I to the Primer Transcript. Cell 1984, 38, 861–870. [Google Scholar] [CrossRef]
- Janas, T.; Janas, T.; Yarus, M. A Membrane Transporter for Tryptophan Composed of RNA. RNA 2004, 10, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Sapoń, K.; Janas, T. Selection of Bifunctional RNAs with Specificity for Arginine and Lipid Membranes. FEBS Lett. 2024, 598, 1061–1079. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA Websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, C.; Han, R.; Wang, Z.; Ye, L.; Du, Z.; Wei, H.; Zhang, F.; Peng, Z.; Yang, J. trRosettaRNA: Automated Prediction of RNA 3D Structure with Transformer Network. Nat. Commun. 2023, 14, 7266. [Google Scholar] [CrossRef]
- Majerfeld, I.; Yarus, M. A Diminutive and Specific RNA Binding Site for L-Tryptophan. Nucleic Acids Res. 2005, 33, 5482–5493. [Google Scholar] [CrossRef]
- Suga, K.; Tanaka, S.; Umakoshi, H. Liposome Membrane Can Induce Self-Cleavage of RNA That Models the Core Fragments of Hammerhead Ribozyme. Eur. Biophys. J. 2016, 45, 55–62. [Google Scholar] [CrossRef]
- Rogers, J.; Joyce, G.F. The Effect of Cytidine on the Structure and Function of an RNA Ligase Ribozyme. RNA 2001, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef]
- Dabkowska, A.P.; Michanek, A.; Jaeger, L.; Rabe, M.; Chworos, A.; Höök, F.; Nylander, T.; Sparr, E. Assembly of RNA Nanostructures on Supported Lipid Bilayers. Nanoscale 2015, 7, 583–596. [Google Scholar] [CrossRef]
- Suga, K.; Tanabe, T.; Tomita, H.; Shimanouchi, T.; Umakoshi, H. Conformational Change of Single-Stranded RNAs Induced by Liposome Binding. Nucleic Acids Res. 2011, 39, 8891–8900. [Google Scholar] [CrossRef]
- Pannwitt, S.; Slama, K.; Depoix, F.; Helm, M.; Schneider, D. Against Expectations: Unassisted RNA Adsorption onto Negatively Charged Lipid Bilayers. Langmuir 2019, 35, 14704–14711. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Simoneit, B.R. Lipid Formation by Aqueous Fischer-Tropsch-Type Synthesis over a Temperature Range of 100 to 400 Degrees C. Orig. Life Evol. Biosph. J. Int. Soc. Study Orig. Life 2001, 31, 103–118. [Google Scholar] [CrossRef]
- Mansy, S.S.; Szostak, J.W. Reconstructing the Emergence of Cellular Life through the Synthesis of Model Protocells. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, M.G.; Szostak, J.W. Semipermeable Lipid Bilayers Exhibit Diastereoselectivity Favoring Ribose. Proc. Natl. Acad. Sci. USA 2005, 102, 6004–6008. [Google Scholar] [CrossRef]
- Adamala, K.; Szostak, J.W. Nonenzymatic Template-Directed RNA Synthesis inside Model Protocells. Science 2013, 342, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Kamat, N.P.; Jena, S.; Szostak, J.W. Fatty Acid/Phospholipid Blended Membranes: A Potential Intermediate State in Protocellular Evolution. Small Weinh. Bergstr. Ger. 2018, 14, e1704077. [Google Scholar] [CrossRef]
- Cohen, Z.R.; Ding, D.; Zhou, L.; DasGupta, S.; Haas, S.; Sinclair, K.P.; Todd, Z.R.; Black, R.A.; Szostak, J.W.; Catling, D.C. Natural Soda Lakes Provide Compatible Conditions for RNA and Membrane Function That Could Have Enabled the Origin of Life. PNAS Nexus 2024, 3, page084. [Google Scholar] [CrossRef]
- Gilbert, W. The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Ekland, E.H.; Szostak, J.W.; Bartel, D.P. Structurally Complex and Highly Active RNA Ligases Derived from Random RNA Sequences. Science 1995, 269, 364–370. [Google Scholar] [CrossRef]
- Papastavrou, N.; Horning, D.P.; Joyce, G.F. RNA-Catalyzed Evolution of Catalytic RNA. Proc. Natl. Acad. Sci. USA 2024, 121, e2321592121. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hill, A.R.; Liu, R.; Orgel, L.E. Synthesis of Long Prebiotic Oligomers on Mineral Surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.M.; Illangasekare, M.; Yarus, M. Catalyzed and Spontaneous Reactions on Ribozyme Ribose. J. Am. Chem. Soc. 2011, 133, 6044–6050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Yarus, M. RNA-Catalyzed Amino Acid Activation. Biochemistry 2001, 40, 6998–7004. [Google Scholar] [CrossRef]
- Xu, J.; Appel, B.; Balke, D.; Wichert, C.; Muller, S. RNA Aminoacylation Mediated by Sequential Action of Two Ribozymes and a Nonactivated Amino Acid. Chembiochem 2014, 15, 1200–1209. [Google Scholar] [CrossRef]
- Illangasekare, M.; Yarus, M. Small Aminoacyl Transfer Centers at GU within a Larger RNA. RNA Biol. 2012, 9, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, T.; Saenz, J.P. Effects of Lipid Membranes on RNA Catalytic Activity and Stability. Biol. Cell 2025, 117, e202400115. [Google Scholar] [CrossRef]
- Vetsigian, K.; Woese, C.; Goldenfeld, N. Collective Evolution and the Genetic Code. Proc. Natl. Acad. Sci. USA 2006, 103, 10696–10701. [Google Scholar] [CrossRef]
- Yarus, M. The Genetic Code Assembles via Division and Fusion, Basic Cellular Events. Life 2023, 13, 2069. [Google Scholar] [CrossRef]
- Peng, H.; Lelievre, A.; Landenfeld, K.; Müller, S.; Chen, I.A. Vesicle Encapsulation Stabilizes Intermolecular Association and Structure Formation of Functional RNA and DNA. Curr. Biol. 2022, 32, 86–96.e6. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Liu, Z.; Chen, I.A. Encapsulation of Ribozymes inside Model Protocells Leads to Faster Evolutionary Adaptation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025054118. [Google Scholar] [CrossRef]
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of Archaeal and Bacterial Membranes to Variations in Temperature, pH and Pressure. Extrem. Life Extrem. Cond. 2017, 21, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. Amino Acids as RNA Ligands: A Direct-RNA-Template Theory for the Code’s Origin. J. Mol. Evol. 1998, 47, 109–117. [Google Scholar] [CrossRef]
- Turk-Macleod, R.M.; Puthenvedu, D.; Majerfeld, I.; Yarus, M. The Plausibility of RNA-Templated Peptides: Simultaneous RNA Affinity for Adjacent Peptide Side Chains. J. Mol. Evol. 2012, 74, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. Ordering Events in a Developing Genetic Code. RNA Biol. 2024, 21, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. Familiar Biological, Chemical and Physical Events Credibly Evolve the Standard Genetic Code. arXiv 2024, arXiv:2406.08302. [Google Scholar] [CrossRef]
- Yarus, M. Fitting the Standard Genetic Code into Its Triplet Table. Proc. Natl. Acad. Sci. USA 2021, 118, e2021103118. [Google Scholar] [CrossRef]
- Yarus, M. A Crescendo of Competent Coding (C3) Contains the Standard Genetic Code. RNA 2022, 28, 1337–1347. [Google Scholar] [CrossRef]
- Yarus, M. Near-Ideal Selection for the Standard Genetic Code. arXiv 2024, arXiv:2410.07814. [Google Scholar] [CrossRef]
- White, H.B. Coenzymes as Fossils of an Earlier Metabolic State. J. Mol. Evol. 1976, 7, 101–104. [Google Scholar] [CrossRef]
- Yarus, M. Getting Past the RNA World: The Initial Darwinian Ancestor. Cold Spring Harb. Perspect. Biol. 2011, 3, a003590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-Splicing RNA: Autoexcision and Autocyclization of the Ribosomal RNA Intervening Sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA Moiety of Ribonuclease P Is the Catalytic Subunit of the Enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Jadhav, V.R.; Yarus, M. Coenzymes as Coribozymes. Biochimie 2002, 84, 877–888. [Google Scholar] [CrossRef]
- Huang, F.; Bugg, C.W.; Yarus, M. RNA-Catalyzed CoA, NAD, and FAD Synthesis from Phosphopantetheine, NMN, and FMN. Biochemistry 2000, 39, 15548–15555. [Google Scholar] [CrossRef]
- Sapkota, K.; Lucas, J.K.; Faulkner, J.W.; Lichte, M.F.; Guo, Y.-L.; Burke, D.H.; Huang, F. Post-Transcriptional Capping Generates Coenzyme A-Linked RNA. RNA Biol. 2024, 21, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.-F. A Co-Evolution Theory of the Genetic Code. Proc. Natl. Acad. Sci. USA 1975, 72, 1909–1912. [Google Scholar] [CrossRef]
- Di Giulio, M. An Extension of the Coevolution Theory of the Origin of the Genetic Code. Biol. Direct 2008, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. Evolution of the Standard Genetic Code. J. Mol. Evol. 2021, 89, 19–44. [Google Scholar] [CrossRef]
- Kolberg, M.; Strand, K.R.; Graff, P.; Andersson, K.K. Structure, Function, and Mechanism of Ribonucleotide Reductases. Biochim. Biophys. Acta 2004, 1699, 1–34. [Google Scholar] [CrossRef]
- Yarus, M. From Initial RNA Encoding to the Standard Genetic Code. bioRxiv 2023. bioRxiv:2023.11.07.566042. [Google Scholar]
- Wehbi, S.; Wheeler, A.; Morel, B.; Manepalli, N.; Minh, B.Q.; Lauretta, D.S.; Masel, J. Order of Amino Acid Recruitment into the Genetic Code Resolved by Last Universal Common Ancestor’s Protein Domains. Proc. Natl. Acad. Sci. USA 2024, 121, e2410311121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarus, M. On an RNA-Membrane Protogenome. Life 2025, 15, 692. https://doi.org/10.3390/life15050692
Yarus M. On an RNA-Membrane Protogenome. Life. 2025; 15(5):692. https://doi.org/10.3390/life15050692
Chicago/Turabian StyleYarus, Michael. 2025. "On an RNA-Membrane Protogenome" Life 15, no. 5: 692. https://doi.org/10.3390/life15050692
APA StyleYarus, M. (2025). On an RNA-Membrane Protogenome. Life, 15(5), 692. https://doi.org/10.3390/life15050692





