Effects of Inspiratory Muscle Training on Respiratory Muscle Strength, Lactate Accumulation and Exercise Tolerance in Amateur Runners: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Respiratory Muscle Strength Test
2.4. Pulmonary Function Test
2.5. Exercise Test
2.6. Inspiratory Muscle Training
2.7. Data and Statistical Analysis
3. Results
3.1. Respiratory Muscle Strength
3.2. Pulmonary Function
3.3. VO2 Max Data
3.4. Exercise Tolerance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Y.; Tian, H.; Zhou, W.; Lin, Y.; Gao, J. ‘Why Do People Commit to Long Distance Running’: Serious Leisure Qualities and Leisure Motivation of Marathon Runners. Sport Soc. 2020, 23, 1256–1272. [Google Scholar] [CrossRef]
- Predel, H.-G. Marathon Run: Cardiovascular Adaptation and Cardiovascular Risk. Eur. Heart J. 2014, 35, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, B.R.; Mujika, I. Optimizing Strength Training for Running and Cycling Endurance Performance: A Review. Scand. Med. Sci. Sports 2014, 24, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.R.; Kilding, A.E. Strategies to Improve Running Economy. Sports Med. 2015, 45, 37–56. [Google Scholar] [CrossRef]
- Rowe, G.C.; Safdar, A.; Arany, Z. Running Forward: New Frontiers in Endurance Exercise Biology. Circulation 2014, 129, 798–810. [Google Scholar] [CrossRef]
- Wüthrich, T.U.; Marty, J.; Kerherve, H.; Millet, G.Y.; Verges, S.; Spengler, C.M. Aspects of Respiratory Muscle Fatigue in a Mountain Ultramarathon Race. Med. Sci. Sports Exerc. 2015, 47, 519–527. [Google Scholar] [CrossRef]
- Frischhut, C.; Kennedy, M.D.; Niedermeier, M.; Faulhaber, M. Effects of a Heat and Moisture Exchanger on Respiratory Function and Symptoms Post–Cold Air Exercise. Scand. J. Med. Sci. Sports 2020, 30, 591–601. [Google Scholar] [CrossRef]
- Amann, M. Pulmonary System Limitations to Endurance Exercise Performance in Humans: Pulmonary System and Exercise Performance. Exp. Physiol. 2012, 97, 311–318. [Google Scholar] [CrossRef]
- Pałac, M.; Sikora, D.; Wolny, T.; Linek, P. Relationship between Respiratory Muscles Ultrasound Parameters and Running Tests Performance in Adolescent Football Players. A Pilot Study. PeerJ 2023, 11, e15214. [Google Scholar] [CrossRef]
- Welch, J.F.; Kipp, S.; Sheel, A.W. Respiratory Muscles during Exercise: Mechanics, Energetics, and Fatigue. Curr. Opin. Physiol. 2019, 10, 102–109. [Google Scholar] [CrossRef]
- Ramos-Barrera, G.E.; DeLucia, C.M.; Bailey, E.F. Inspiratory Muscle Strength Training Lowers Blood Pressure and Sympathetic Activity in Older Adults with OSA: A Randomized Controlled Pilot Trial. J. Appl. Physiol. 2020, 129, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Klusiewicz, A.; Starczewski, M.; Sadowska, D.; Ładyga, M. Effect of High- and Low-Resistance Inspiratory Muscle Training on Physiological Response to Exercise in Cross-Country Skiers. J. Sports Med. Phys. Fit. 2019, 59, 1156–1161. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández Zoppino, D.; González-Bernal, J.J.; García Gómez, B.; Mielgo-Ayuso, J. Inspiratory Muscle Training Program Using the Powerbreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6703. [Google Scholar] [CrossRef]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of Respiratory Muscle Training on Performance in Athletes: A Systematic Review with Meta-Analyses. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef]
- de Sousa, M.M.; dos Santos Pimentel, M.; de Andrade Sobreira, I.; de Jesus Barros, R.; Borghi-Silva, A.; Mazzoli-Rocha, F. Inspiratory Muscle Training Improves Aerobic Capacity in Amateur Indoor Football Players. Int. J. Sports Med. 2021, 42, 456–463. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, X.; Huang, Y.; Chen, L. Internet of Things-Based Home Respiratory Muscle Training for Patients with Chronic Obstructive Pulmonary Disease: A Randomized Clinical Trial. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 1093–1103. [Google Scholar] [CrossRef]
- Carlos de Medeiros, A.I.; Bastos Fuzari, H.K.; Rattes, C.; Brandao, D.C.; de Melo Marinho, P.E. Inspiratory Muscle Training Improves Respiratory Muscle Strength, Functional Capacity and Quality of Life in Patients with Chronic Kidney Disease: A Systematic Review. J. Physiother. 2017, 63, 76–83. [Google Scholar] [CrossRef]
- Enright, S.; Chatham, K.; Ionescu, A.A.; Unnithan, V.B.; Shale, D.J. Inspiratory Muscle Training Improves Lung Function and Exercise Capacity in Adults With Cystic Fibrosis. Chest 2004, 126, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, X.L. Physiological Determinants of VO2max and the Methods to Evaluate It: A Critical Review. Sci. Sports 2021, 36, 259–271. [Google Scholar] [CrossRef]
- Lorenzo, S.; Babb, T.G. Oxygen Cost of Breathing and Breathlessness during Exercise in Nonobese Women and Men. Med. Sci. Sports Exerc. 2012, 44, 1043–1048. [Google Scholar] [CrossRef]
- Bernhardt, V.; Stickford, J.L.; Bhammar, D.M.; Balmain, B.N.; Babb, T.G. Repeatability of Dyspnea Measurements during Exercise in Women with Obesity. Respir. Physiol. Neurobiol. 2022, 297, 103831. [Google Scholar] [CrossRef] [PubMed]
- Port, I.; Kwakkel, G.; Wittink, H. Systematic Review of Cardiopulmonary Exercise Testing Post Stroke: Are We Adhering to Practice Recommendations? J. Rehabil. Med. 2015, 47, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, W.; Hu, Y.; Huang, Y.; Dai, Q.; Yang, Y.; Fu, C.; Zeng, Z.; Li, L.; Yang, B.; et al. Effectiveness of High-Intensity Inspiratory Muscle Training, and Resistance and Aerobic Exercise for Cardiovascular Health in Chronic Obstructive Pulmonary Disease (HIRAC-COPD): A Randomized Controlled Trial Protocol. BMC Pulm. Med. 2024, 24, 627. [Google Scholar] [CrossRef]
- Witt, J.D.; Guenette, J.A.; Rupert, J.L.; McKenzie, D.C.; Sheel, A.W. Inspiratory Muscle Training Attenuates the Human Respiratory Muscle Metaboreflex: Cardiovascular Effects of Training Respiratory Muscle. J. Physiol. 2007, 584, 1019–1028. [Google Scholar] [CrossRef]
- Vural, M.; Özdal, M.; Pancar, Z. Effects of Inspiratory Muscle Training on Respiratory Functions and Respiratory Muscle Strength in Down Syndrome: A Preliminary Study. Isokinet. Exerc. Sci. 2019, 27, 283–288. [Google Scholar] [CrossRef]
- Nicks, C.R.; Morgan, D.W.; Fuller, D.K.; Caputo, J.L. The Influence of Respiratory Muscle Training upon Intermittent Exercise Performance. Int. J. Sports Med. 2009, 30, 16–21. [Google Scholar] [CrossRef]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C.; Oliveira, C.R.D.; Ricci, P.A.; Amaral, A.C.D.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of Inspiratory Muscle Training in Professional Women Football Players: A Randomized Sham-Controlled Trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef]
- Zeren, M.; Demir, R.; Yigit, Z.; Gurses, H.N. Effects of Inspiratory Muscle Training on Pulmonary Function, Respiratory Muscle Strength and Functional Capacity in Patients with Atrial Fibrillation: A Randomized Controlled Trial. Clin. Rehabil. 2016, 30, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- MccONNELL, A.K.; Griffiths, L.A. Acute Cardiorespiratory Responses to Inspiratory Pressure Threshold Loading. Med. Sci. Sports Exerc. 2010, 42, 1696–1703. [Google Scholar] [CrossRef]
- Hernández-Álvarez, E.D.; Guzmán-David, C.A.; Ruiz-González, J.C.; Ortega-Hernández, A.M.; Ortiz-González, D.C. Effect of a Respiratory Muscle Training Program on Lung Function, Respiratory Muscle Strength and Resting Oxygen Consumption in Sedentary Young People. Rev. Fac. Med. 2018, 66, 605–610. [Google Scholar] [CrossRef]
- Lee, K. Correlation between Respiratory Muscle Strength and Pulmonary Function with Respiratory Muscle Length Increase in Healthy Adults. Phys. Ther. Rehabil. Sci. 2021, 10, 398–405. [Google Scholar] [CrossRef]
- Bağiran, Y.; Dağlioğlu, Ö.; Bostanci, Ö. The Effect of Respiratory Muscle Training on Aerobic Power and Respiratory Parameters in Swimmers. Int. J. Sport Exerc. Train. Sci. 2019, 5, 214–220. [Google Scholar] [CrossRef]
- Romer, L.M.; Polkey, M.I. Exercise-Induced Respiratory Muscle Fatigue: Implications for Performance. J. Appl. Physiol. 2008, 104, 879–888. [Google Scholar] [CrossRef]
- Niemeyer, M.; Knaier, R.; Beneke, R. The Oxygen Uptake Plateau—A Critical Review of the Frequently Misunderstood Phenomenon. Sports Med. 2021, 51, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Karsten, M.; Ribeiro, G.S.; Esquivel, M.S.; Matte, D.L. Maximizing the Effectiveness of Inspiratory Muscle Training in Sports Performance: A Current Challenge. Phys. Ther. Sport 2019, 36, 68–69. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Saquetto, M.B.; Silva, C.M.; Carvalho, V.O.; Ribeiro, N.; Conceição, C.S. Effects of Respiratory Muscle Training on Respiratory Function, Respiratory Muscle Strength, and Exercise Tolerance in Patients Poststroke: A Systematic Review with Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 1994–2001. [Google Scholar] [CrossRef]
- Guy, J.H.; Edwards, A.M.; Deakin, G.B. Inspiratory Muscle Training Improves Exercise Tolerance in Recreational Soccer Players Without Concomitant Gain in Soccer-Specific Fitness. J. Strength Cond. Res. 2014, 28, 483–491. [Google Scholar] [CrossRef]
- Najafi, A.; Ebrahim, K.; Ahmadizad, S.; Jahani Ghaeh Ghashlagh, G.R.; Javidi, M.; Hackett, D. Improvements in Soccer-Specific Fitness and Exercise Tolerance Following 8 Weeks of Inspiratory Muscle Training in Adolescent Males. J. Sports Med. Phys. Fit. 2020, 59, 1975–1984. [Google Scholar] [CrossRef]
- Rożek-Piechura, K.; Kurzaj, M.; Okrzymowska, P.; Kucharski, W.; Stodółka, J.; Maćkała, K. Influence of Inspiratory Muscle Training of Various Intensities on The Physical Performance of Long-Distance Runners. J. Hum. Kinet. 2020, 75, 127–137. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, H.-Y.; Ho, C.-C.; Lee, P.-F.; Chou, Y.-C.; Tsai, M.-W.; Chou, L.-W. Effects of 4-Week Inspiratory Muscle Training on Sport Performance in College 800-Meter Track Runners. Medicina 2021, 57, 72. [Google Scholar] [CrossRef]
- Leddy, J.J.; Limprasertkul, A.; Patel, S.; Modlich, F.; Buyea, C.; Pendergast, D.R.; Lundgren, C.E.G. Isocapnic Hyperpnea Training Improves Performance in Competitive Male Runners. Eur. J. Appl. Physiol. 2007, 99, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.I.; Sharpe, G.R.; Johnson, M.A. Inspiratory Muscle Training Reduces Blood Lactate Concentration during Volitional Hyperpnoea. Eur. J. Appl. Physiol. 2008, 104, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.A.; McConnell, A.K. The Influence of Inspiratory and Expiratory Muscle Training upon Rowing Performance. Eur. J. Appl. Physiol. 2007, 99, 457–466. [Google Scholar] [CrossRef] [PubMed]
| Variable | HIMT | LIMT | Con | p |
|---|---|---|---|---|
| Age (yrs) | 22.29 ± 1.25 | 22.57 ± 1.51 | 21.86 ± 1.77 | 0.684 |
| Height (cm) | 175.63 ± 4.67 | 177.86 ± 5.34 | 178.20 ± 4.97 | 0.589 |
| Weight (kg) | 67.79 ± 5.21 | 67.30 ± 5.04 | 65.41 ± 4.11 | 0.630 |
| BMI (kg/m2) | 21.9 ± 1.21 | 21.2 ± 1.14 | 20.5 ± 1.05 | 0.625 |
| Running experience (yrs) | 5.29 ± 1.11 | 5.43 ± 1.40 | 5.14 ± 1.35 | 0.918 |
| HR (bpm) | 61.80 ± 3.74 | 61.50 ± 4.40 | 60.70 ± 4.30 | 0.830 |
| LA (mmol) | 1.73 ± 0.40 | 2.07 ± 0.63 | 1.90 ± 0.48 | 0.354 |
| Variable | Time | HIMT | LIMT | Con |
|---|---|---|---|---|
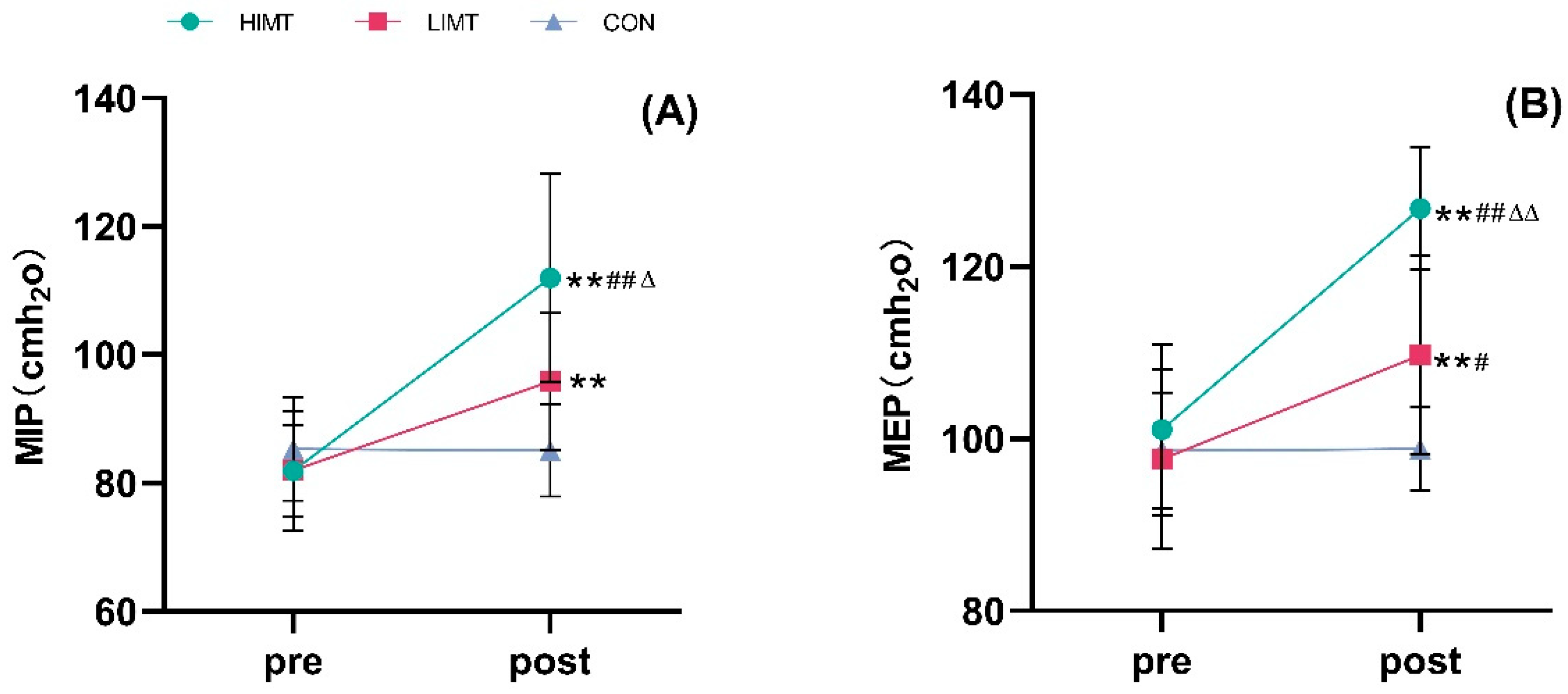
| MIP (cmH2o) | Pre | 81.90 ± 9.33 | 81.97 ± 7.12 | 85.27 ± 8.08 |
| Post | 111.99 ± 16.21 **##Δ | 95.86 ± 10.69 ** | 85.10 ± 7.21 | |
| MEP (cmH2o) | Pre | 101.12 ± 9.95 | 97.64 ± 10.47 | 98.64 ± 6.75 |
| Post | 126.78 ± 7.12 **##ΔΔ | 109.74 ± 11.57 **# | 98.82 ± 4.84 | |
| FVC (mL) | Pre | 3770.2 ± 306.7 | 3842.4 ± 452.7 | 3788.0 ± 264.0 |
| Post | 3800.6 ± 306.0 | 3839.1 ± 378.2 | 3472.0 ± 947.2 | |
| FEV1 (mL) | Pre | 2856.0 ± 228.0 | 2937.8 ± 341.7 | 2885.4 ± 256.3 |
| Post | 2963.6 ± 244.7 | 2955.6 ± 296.6 | 2647.2 ± 727.4 | |
| FEV1/FVC | Pre | 0.75 ± 0.02 | 0.76 ± 0.03 | 0.76 ± 0.02 |
| Post | 0.78 ± 0.02 ** | 0.77 ± 0.02 | 0.76 ± 0.03 |
| Variable | Time | HIMT | LIMT | Con |
|---|---|---|---|---|
| VO2 max (ml/kg/min) | Pre | 44.60 ± 1.58 | 45.00 ± 2.61 | 44.80 ± 1.48 |
| Post | 45.00 ± 1.83 | 44.70 ± 1.77 | 44.60 ± 1.51 | |
| Power (W) | Pre | 204.3 ± 7.7 | 202.9 ± 5.26 | 202.1 ± 8.05 |
| Post | 209.6 ± 7.06 **# | 204.5 ± 5.13 * | 201.4 ± 7.86 | |
| HR peak (bpm) | Pre | 175.30 ± 6.95 | 176.40 ± 7.09 | 175.60 ± 8.76 |
| Post | 173.90 ± 6.40 | 175.10 ± 6.01 | 176.00 ± 7.63 | |
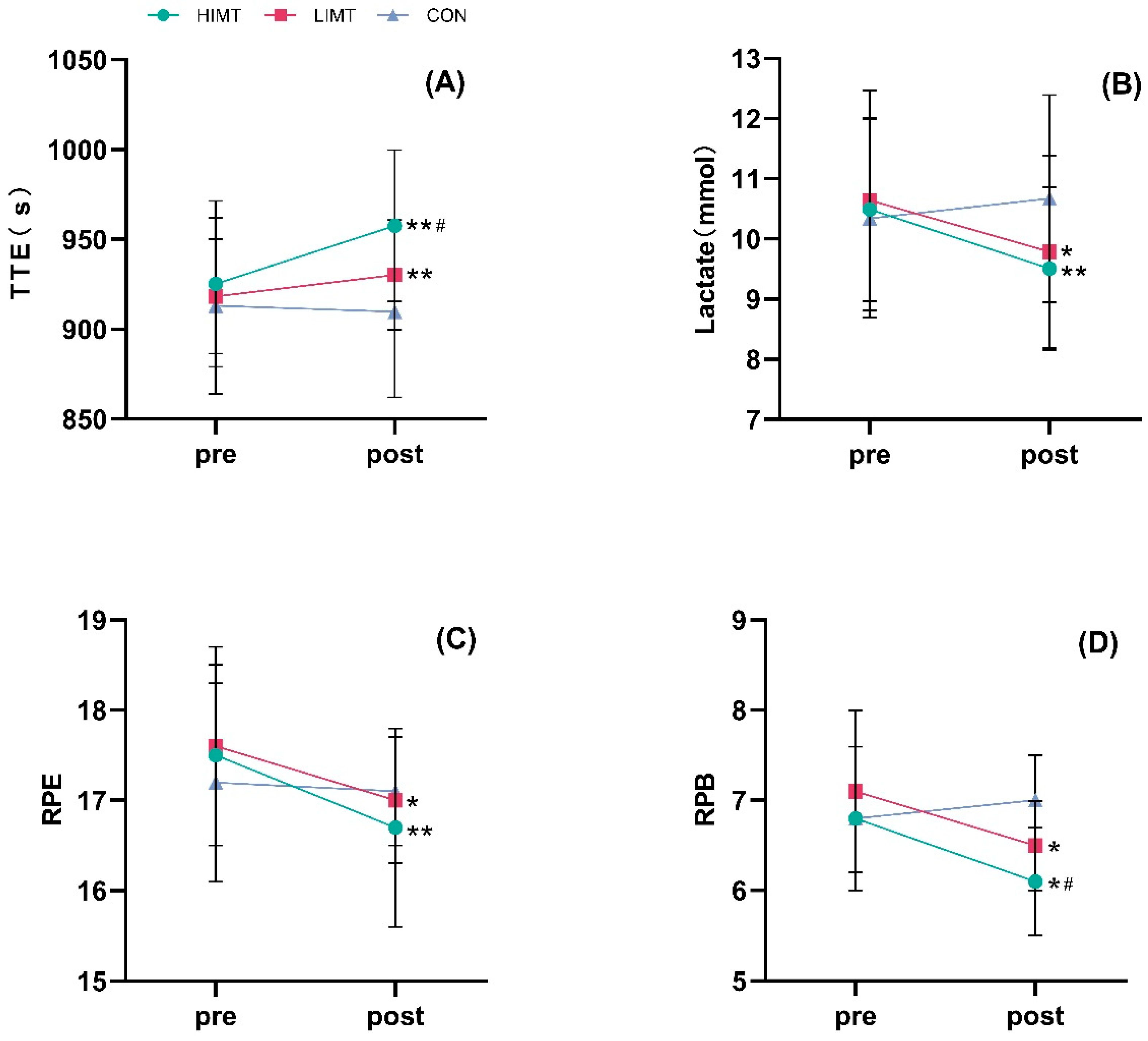
| TTE (s) | Pre | 925.30 ± 46.25 | 918.23 ± 31.90 | 913.06 ± 49.00 |
| Post | 957.55 ± 42.18 **# | 930.25 ± 30.55 ** | 909.78 ± 47.71 | |
| Lactate (mmol) | Pre | 10.49 ± 1.53 | 10.64 ± 1.83 | 10.34 ± 1.65 |
| Post | 9.51 ± 1.36 ** | 9.79 ± 1.60 * | 10.67 ± 1.72 | |
| RPE | Pre | 17.5 ± 1.0 | 17.6 ± 1.1 | 17.2 ± 1.1 |
| Post | 16.7 ± 1.1 ** | 17.0 ± 0.7 * | 17.1 ± 0.6 | |
| RPB | Pre | 6.8 ± 0.8 | 7.1 ± 0.9 | 6.8 ± 0.8 |
| Post | 6.1 ± 0.6 *# | 6.5 ± 0.5 * | 7.0 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Guo, J.; He, Y.; Luo, Y.; Wu, H. Effects of Inspiratory Muscle Training on Respiratory Muscle Strength, Lactate Accumulation and Exercise Tolerance in Amateur Runners: A Randomized Controlled Trial. Life 2025, 15, 705. https://doi.org/10.3390/life15050705
Ren Z, Guo J, He Y, Luo Y, Wu H. Effects of Inspiratory Muscle Training on Respiratory Muscle Strength, Lactate Accumulation and Exercise Tolerance in Amateur Runners: A Randomized Controlled Trial. Life. 2025; 15(5):705. https://doi.org/10.3390/life15050705
Chicago/Turabian StyleRen, Zhe, Junxia Guo, Yurong He, Yu Luo, and Hao Wu. 2025. "Effects of Inspiratory Muscle Training on Respiratory Muscle Strength, Lactate Accumulation and Exercise Tolerance in Amateur Runners: A Randomized Controlled Trial" Life 15, no. 5: 705. https://doi.org/10.3390/life15050705
APA StyleRen, Z., Guo, J., He, Y., Luo, Y., & Wu, H. (2025). Effects of Inspiratory Muscle Training on Respiratory Muscle Strength, Lactate Accumulation and Exercise Tolerance in Amateur Runners: A Randomized Controlled Trial. Life, 15(5), 705. https://doi.org/10.3390/life15050705






