Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Collection of Seaweed Biomass
2.2. Morphological Identification of Natural Seaweeds
2.3. Taxonomic Identification
2.4. Data Documentation
2.5. Sample Preparation and Storage
2.6. Dry Weight and Ash Content Determination
2.7. Elemental Analysis
2.8. Extraction of Lipids
2.9. Direct Methylation of FAs and Analysis by GC–MS
2.10. Lipid Extraction Optimization
2.11. Functional Food Properties Determination
Swelling Capacity (SC)
2.12. Statistical Analysis
3. Results
3.1. Natural Field Seaweeds
3.2. Species Identification
3.3. Fatty Acid Composition
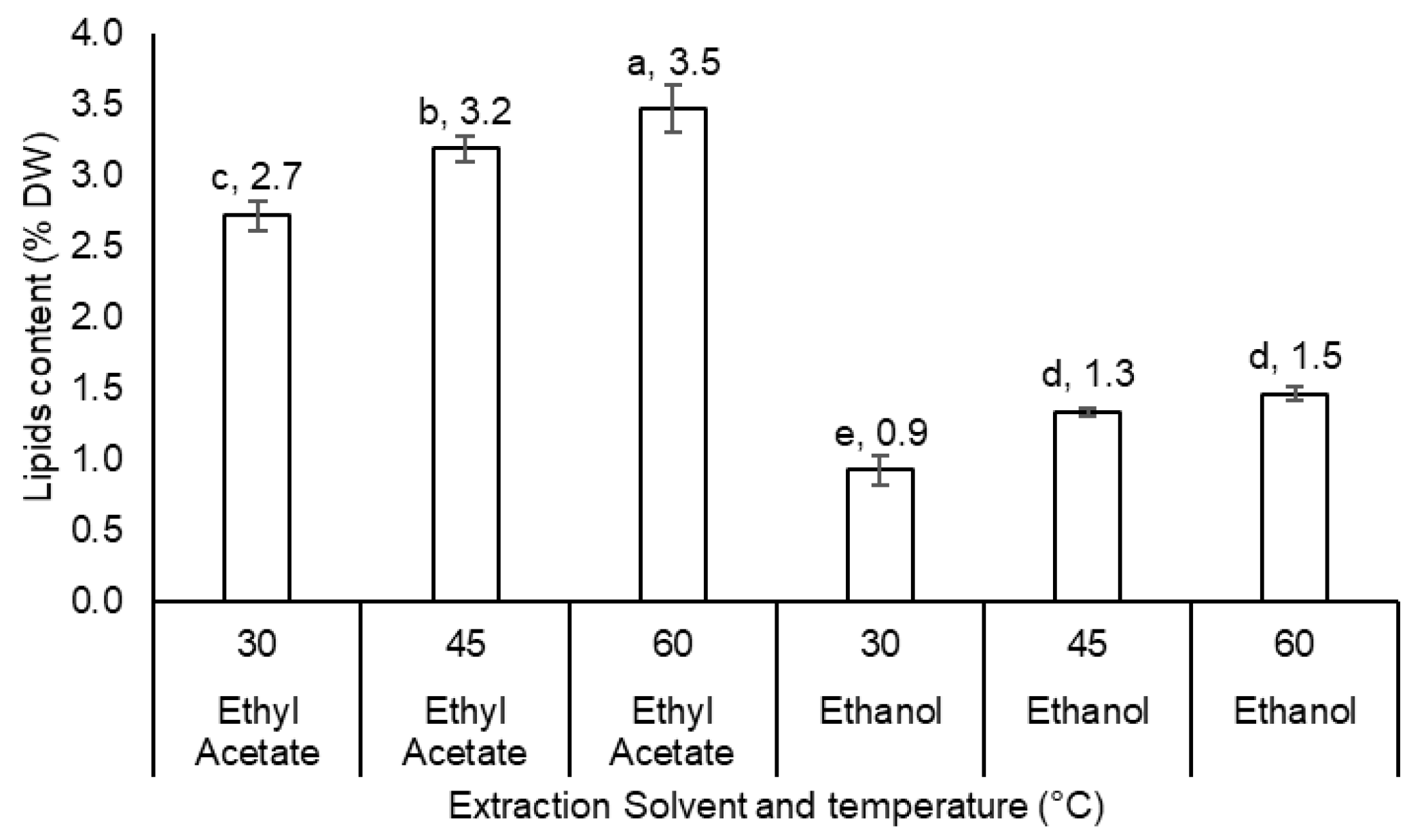
3.4. Extraction Optimization
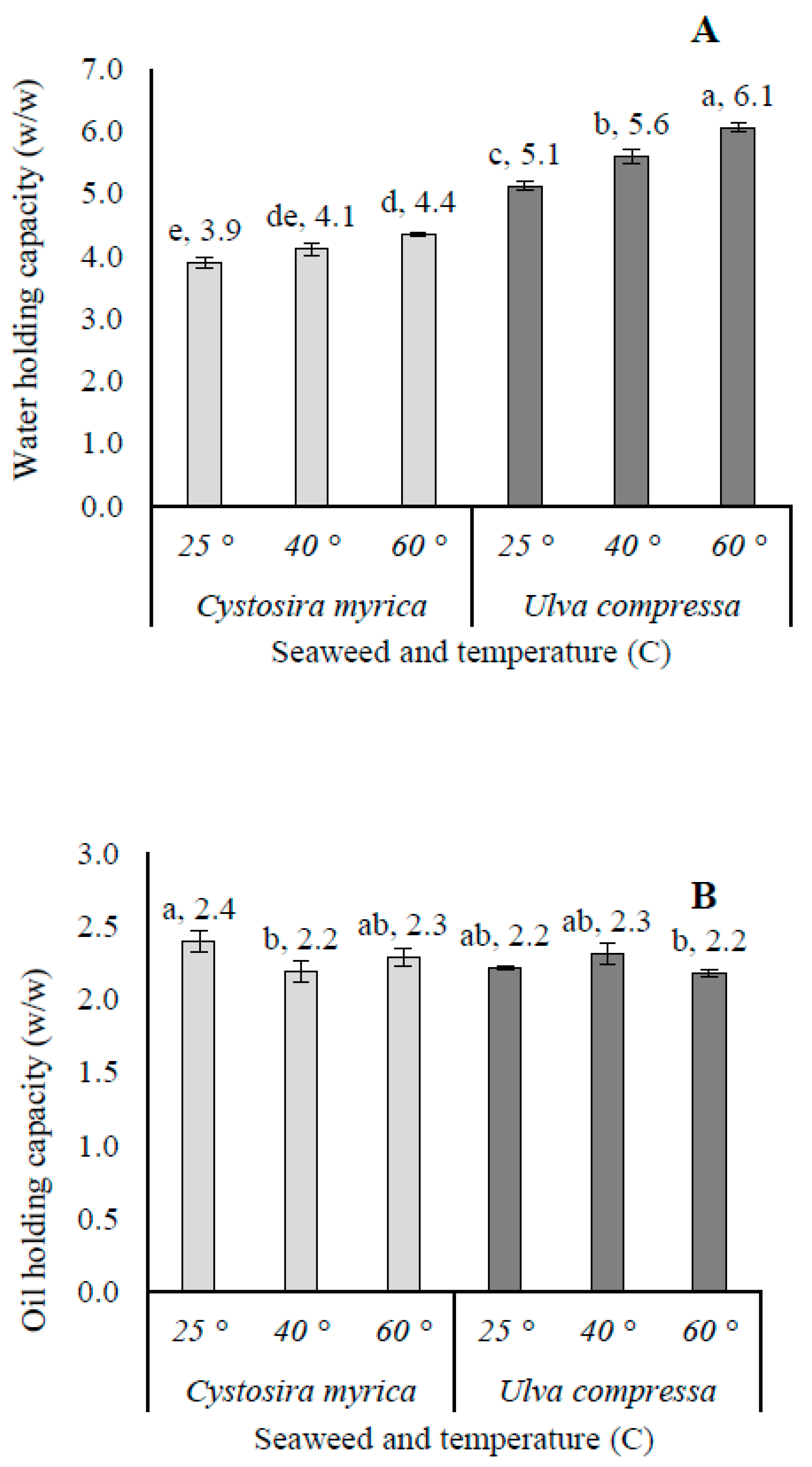
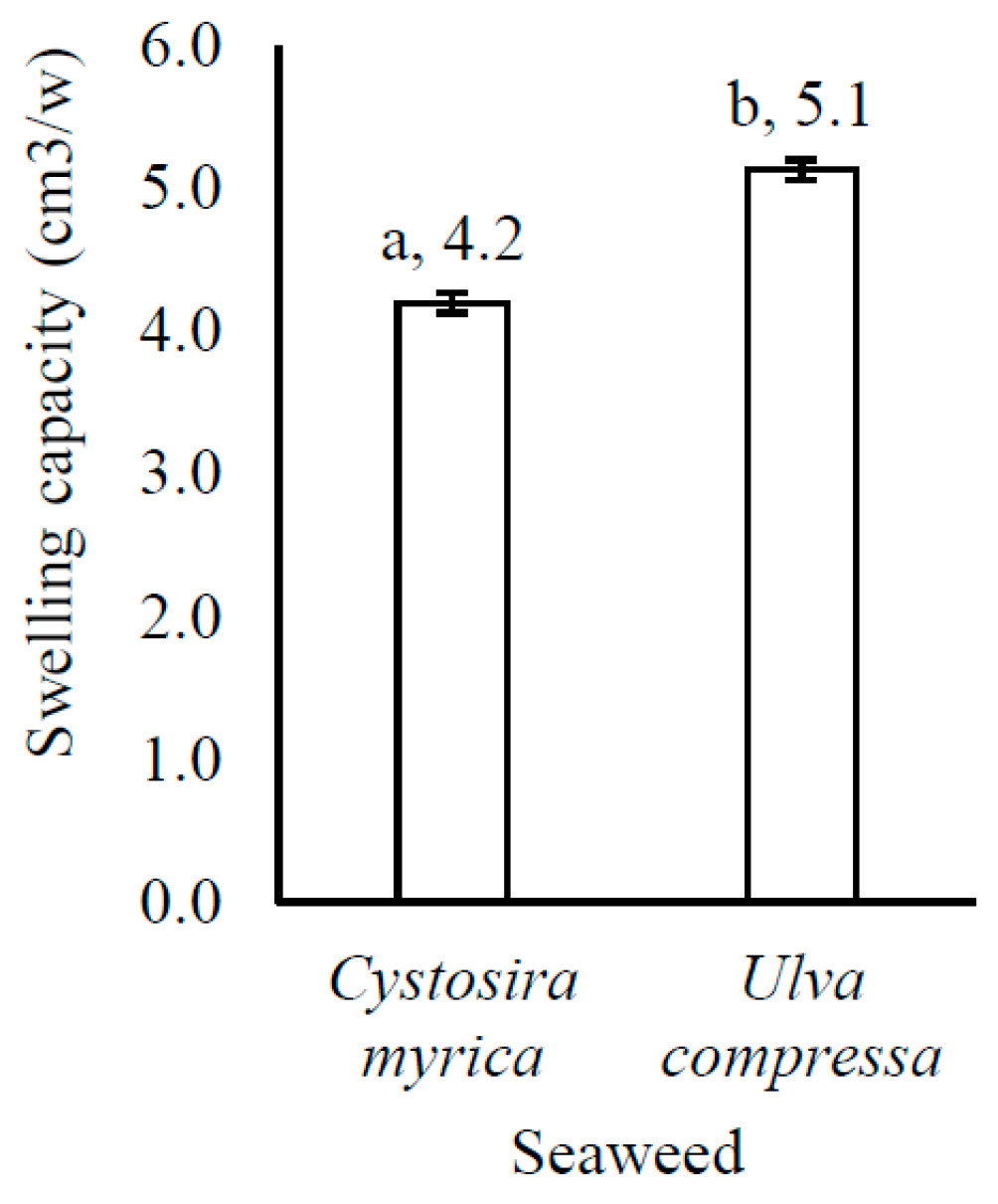
3.5. Functional Food Properties
4. Discussion
4.1. Extraction Optimization
4.2. Functional Food Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 13193. [Google Scholar] [CrossRef]
- Pathak, M.S.; Bhadra, S. Exploring Seaweed as Sustainable Green Aquafeed: Opportunities and Challenges. In Sustainable Feed Ingredients and Additives for Aquaculture Farming: Perspectives from Africa and Asia; Springer: Berlin/Heidelberg, Germany, 2024; pp. 95–113. [Google Scholar]
- Costa, S.P.; Cotas, J.; Pereira, L. Laminar Ulva Species: A Multi-Tool for Humankind? Appl. Sci. 2024, 14, 3448. [Google Scholar] [CrossRef]
- Wirenfeldt, C.B.; Hermund, D.B.; Feyissa, A.H.; Hyldig, G.; Holdt, S.L. Nutritional Value, Bioactive Composition, Physico-Chemical and Sensory Properties of Ulva sp. and Fucus vesiculosus Depending on Post-Harvest Processing: A Drying Comparison Study. J. Appl. Phycol. 2024, 36, 2795–2805. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Matos, J.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M.; Afonso, C. Seaweed Bioactives Potential as Nutraceuticals and Functional Ingredients: A Review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Tyagi, R.; Rastogi, R.P.; Babich, O.; Awasthi, M.K.; Tiwari, A. New Perspectives of Omega-3 Fatty Acids from Diatoms. Syst. Microbiol. Biomanuf. 2024, 4, 528–541. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Subbiah, V.; Agar, O.T.; Bringloe, T.T.; Legione, A.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds from Victorian Shorebound Red Seaweeds and Their Antioxidant Capacity. Algal Res. 2024, 82, 103609. [Google Scholar] [CrossRef]
- Fernandez-Segovia, I.; Jesús Lerma-García, M.; Fuentes, A.; Barat, J.M. Characterization of Spanish Powdered Seaweeds: Composition, Antioxidant Capacity and Technological Properties. Food Res. 2018, 111, 212–219. [Google Scholar] [CrossRef]
- Ms, V.Q.; Miss, M.S.; Miss, G.G.V.; Ms, P.J. Seaweeds in Bakery and Farinaceous Foods: A Mini-Review. Int. J. Gastron. Food Sci. 2022, 28, 100403. [Google Scholar]
- Peñalver, R.; Martínez-Zamora, L.; Lorenzo, J.M.; Ros, G.; Nieto Martínez, G. Effect of Hydroxytyrosol, Moringa, and Spirulina on the Physicochemical Properties and Nutritional Characteristics of Gluten-Free Brownies. Food Sci. Nutr. 2024, 12, 385–398. [Google Scholar] [CrossRef]
- Jouhet, J.; Alves, E.; Boutté, Y.; Darnet, S.; Domergue, F.; Durand, T.; Fischer, P.; Fouillen, L.; Grube, M.; Joubès, J.; et al. Plant and Algal Lipidomes: Analysis, Composition, and Their Societal Significance. Prog. Lipid Res. 2024, 96, 101290. [Google Scholar] [CrossRef]
- Holm, H.C.; Fredricks, H.F.; Bent, S.M.; Lowenstein, D.P.; Ossolinski, J.E.; Becker, K.W.; Van Mooy, B.A. Global Ocean Lipidomes Show a Universal Relationship Between Temperature and Lipid Unsaturation. Science 2022, 376, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 Fatty Acids Eicosapentaenoic (EPA) and Docosahexaenoic (DHA) as Modulatory and Anti-Inflammatory Agents in Noncommunicable Diet-Related Diseases–Reports from the Last 10 Years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, E.; Pasdar, Y.; Asoudeh, F.; Najafi, F.; Saber, A.; Shakiba, M.H.; Bagheri, A. The Relationship of Dietary Omega-3 Fatty Acid and Omega-6 to Omega-3 Ratio Intake and Likelihood of Type 2 Diabetes in a Cross-Sectional Study. BMC Endocr. Disord. 2024, 24, 259. [Google Scholar] [CrossRef]
- Savatinova, M.; Ivanova, M. Functional Dairy Products Enriched with Omega-3 Fatty Acids. Food Sci. Appl. Biotechnol. 2024, 7, 1–13. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Liu, D. Ultrasound-Assisted Extraction of Lipids as Food Components: Mechanism, Solvent, Feedstock, Quality Evaluation and Coupled Technologies–A Review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Marques, F.; Pinho, M.; Guerra, I.M.; Conde, T.A.; Silva, J.; Cardoso, H.; Domingues, M.R. Unlocking Functional Lipid Ingredients from Algae by Food-Grade Biosolvents and Ultrasound-Assisted Extraction for Nutritional Applications. LWT 2024, 200, 116136. [Google Scholar] [CrossRef]
- González-Meza, G.M.; Rubio-Hernández, C.C.; López-Pacheco, I.Y.; López-Pacheco, L.D.; Marín-Santibáñez, B.M.; Medina-Bañuelos, E.F.; Farfan-Cabrera, L.I. Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations. J. Mar. Sci. Eng. 2024, 12, 2242. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Marques, F.; Lopes, D.; da Costa, E.; Conde, T.; Rego, A.; Ribeiro, A.I.; Domingues, M.R. Seaweed blends as a valuable source of polyunsaturated and healthy fats for nutritional and food applications. Mar. Drugs 2021, 19, 684. [Google Scholar] [CrossRef]
- Golani, D.; Reef-Motro, R.; Ekshtein, S.; Baranes, A.; Diamant, A. Ichthyofauna of the rocky coastal littoral of the Israeli Mediterranean, with reference to the paucity of Red Sea (Lessepsian) migrants in this habitat. Mar. Biol. Res. 2007, 3, 333–341. [Google Scholar] [CrossRef]
- Abbott, I.A.; Hollenberg, G.J. Marine Algae of California; Stanford University Press: Stanford, CA, USA, 1976. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase: Seaweeds of the World; National University of Ireland: Galway, Ireland, 2020. [Google Scholar]
- Israel, A.; Einav, R.; Silva, P.C. Seaweeds of the Eastern Mediterranean Coast: Taxonomy and Morphology; Israel Academy of Sciences and Humanities: Galway, Ireland, 2020. [Google Scholar]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Costa, E.; Melo, T.; Domingues, P.; Fort, A.; Domingues, M.R.; Sulpice, R. Lipidome in-depth characterization highlights the nutritional value and species-specific idiosyncrasies of different Ulva species. Algal Res. 2022, 64, 102694. [Google Scholar] [CrossRef]
- Gnayem, N.; Unis, R.; Gnaim, R.; Chemodanov, A.; Israel, Á.; Gnaim, J.; Golberg, A. Seasonal and culture period variations in the lipid and fatty acid content of Ulva lactuca cultivated in Mikhmoret onshore (Israel). Bot. Mar. 2024, 67, 101–114. [Google Scholar] [CrossRef]
- Lawton, R.J.; Sutherland, J.E.; Glasson, C.R.; Magnusson, M.E. Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Res. 2021, 56, 102320. [Google Scholar] [CrossRef]
- Gnayem, N.; Unis, R.; Gnaim, R.; Chemodanov, A.; Israel, Á.; Gnaim, J.; Golberg, A. Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors. Life 2025, 15, 57. [Google Scholar] [CrossRef]
- Einav, R.; Israel, A. Checklist of seaweeds from the Israeli Mediterranean: Taxonomical and ecological approaches. Isr. J. Plant Sci. 2008, 56, 91–127. [Google Scholar] [CrossRef]
- Einav, R.; Guiry, M.D.; Israel, Á. A revised list of seaweeds from the Red Sea (1756–2020). Isr. J. Plant Sci. 2021, 68, 175–247. [Google Scholar] [CrossRef]
- Oucif, H.; Benaissa, M.; Ali Mehidi, S.; Prego, R.; Aubourg, S.P.; Abi-Ayad, S.M.E.A. Chemical Composition and Nutritional Value of Different Seaweeds from the West Algerian Coast. J. Aquat. Food Prod. Technol. 2020, 29, 90–104. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- Nunes, N.; Ferreira-Santos, P.; Moreira, C.; Teixeira, C.A.; Rocha, C.M.R. Tuning the extraction methodology targeting protein-enriched fractions from red algae. Future Foods 2024, 9, 100335. [Google Scholar] [CrossRef]
- Ramírez-Partida, R.A.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, M.; Fakhry, E.M. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef]
- Limiñana, V.A.; Benoist, T.; Sempere, S.A.; Pérez, S.E.M.; Moya, M.S.P. Chemical composition of sustainable Mediterranean macroalgae obtained from land-based and sea-based aquaculture systems. Food Biosci. 2023, 54, 102902. [Google Scholar]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public. Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Osman, N.A.; Nafie, M.S.; Kang, H.K.; Hyun, J.W.; Koh, Y.S. Chemical composition, antimicrobial activity, and molecular docking of the main phytoconstituents from three rarely investigated marine macroalgae. Nat. Prod. Commun. 2024, 19, 1934578X241261016. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Marçal, C.; Cotas, J.; Varela, J.; Barreira, L. Fatty acid profile of different species of algae of the Cystoseira genus: A nutraceutical perspective. Nat. Prod. Res. 2015, 29, 1264–1270. [Google Scholar] [CrossRef]
- Méndez, F.; Rivero, A.; Bahamonde, F.; Gallardo, P.; Frangopulos, M.; Zolezzi, J.; Inestrosa, N.C.; Mansilla, A. Fatty acid composition of different morphological structures in the sub-Antarctic kelps Macrocystis pyrifera (L.) C. Agardh and Lessonia flavicans Bory of the Magellan Ecoregion: Nutritional and biomedical potentials. J. Appl. Phycol. 2023, 36, 831–844. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids, and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.Z.; Baran, T.B.M.; Hayashi, L. Protocol for scaling up biomass production of the green seaweed Ulva ohnoi from germling clusters. J. Appl. Phycol. 2024, 36, 579–589. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green. Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure, and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols, and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Dhaouafi, J.; Nedjar, N.; Jridi, M.; Romdhani, M.; Balti, R. Extraction of protein and bioactive compounds from Mediterranean red algae (Sphaerococcus coronopifolius and Gelidium spinosum) using various innovative pretreatment strategies. Foods 2024, 13, 1362. [Google Scholar] [CrossRef]
- Santana, I.; Felix, M.; Bengoechea, C. Feasibility of Invasive Brown Seaweed Rugulopteryx okamurae as Source of Alginate: Characterization of Products and Evaluation of Derived Gels. Polymers 2024, 16, 702. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Performance evaluation of green and integrated extraction approaches for the recovery of fatty acids, polysaccharides, and proteins from brown macroalgae for a sustainable biorefinery. J. Appl. Phycol. 2024, 36, 341–357. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2015, 73, 204–210. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the green seaweed Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]


| Seaweed Group | Species | Harvest Location | ||
|---|---|---|---|---|
| Rosh Hanikra | Atlit | Jisir Azarqah | ||
| Rhodophyta (Red) | Gracilaria sp. | + * | + | + |
| Ballia callitriche | + | |||
| Gelidium coulteri | + | |||
| Ochrophyta (Brown) | Padina pavonia | + | ||
| Sargassum vulgare | + | |||
| Cystoseira myrica | + | + | + | |
| Dictyota dichotoma | + | |||
| Chlorophyta (Green) | Ulva compressa | + | + | + |
| Site | GPS Location | Sea Water PH | Seawater Salinity % | Seawater Temperature (°C) | Harvesting Time |
|---|---|---|---|---|---|
| Rosh Hanikra | 33°05′19″ E 35°07′02″ N | 8.1 | 3.45 | 24.0 | 10:10 |
| Atlit | 34°56′25″ E 32°40′59″ N | 8.1 | 3.46 | 24.6 | 13:00 |
| Jisir Alzarqah | 32°32′15″ E 34° 54′07″ N | 8.1 | 3.51 | 24.5 | 16:30 |
| Percentage of Fatty Acid (%) of Total Fatty Acids in Brown Seaweeds Collected from 3 Locations | ||||||
|---|---|---|---|---|---|---|
| JA 11 | JA | RH | AT | JA | JA | |
| Fatty Acid | Padina pavoina 9 (B) 10 | Sargassum vulgare (B) | Cystoseira myrica (B) | Cystoseira myrica (B) | Cystoseira myrica (B) | Dictyota dichotoma (B) |
| C14:0 | 6.73 ± 0.40 b | 8.36 ± 0.25 a | 1.29 ± 0.05 c | 1.35 ± 0.09 c | 1.34 ± 0.06 c | 0.78 ± 0.16 c |
| C16:4 n-4 | 0.42 ± 0.08 a | 0.47 ± 0.10 a | 0.13 ± 0.01 c | 0.12 ± 0.01 c | 0.13 ± 0.01 c | 0.13 ± 0.01 c |
| C16:2 n-6 | 0.32 ± 0.03 a | 0.13 ± 0.03 b | 0.01 ± 0.00 c | 0.02 ± 0.00 c | 0.01 ± 0.00 c | 0.01 ± 0.00 c |
| C16:1 n-5 | 0.06 ± 0.01 a | 0.02 ± 0.01 a | 0.00 ± 0.00 a | 0.02 ± 0.00 a | 0.00 ± 0.00 a | 0.01 ± 0.00 a |
| C16:1 n-9 | 5.39 ± 0.28 b | 8.84 ± 0.25 a | 0.39 ± 0.05 c | 0.37 ± 0.02 c | 0.41 ± 0.05 c | 0.27 ± 0.05 c |
| C16:0 | 45.07 ± 0.30 c | 54.63 ± 2.12 b | 86.82 ± 0.39 a | 84.64 ± 0.10 a | 85.72 ± 0.25 a | 88.98 ± 0.26 a |
| C18:4 n-3 | 4.63 ± 0.82 a | 0.42 ± 0.08 b | 0.02 ± 0.00 c | 0.03 ± 0.00 c | 0.02 ± 0.00 c | 0.02 ± 0.00 c |
| C18:2 n-6 | 4.94 ± 0.23 a | 2.51 ± 0.36 b | 0.38 ± 0.00 c | 0.28 ± 0.01 c | 0.31 ± 0.00 c | 0.06 ± 0.01 d |
| C18:3 n-3 | 21.90 ± 0.76 a | 12.53 ± 1.02 b | 2.47 ± 0.18 c | 2.44 ± 0.11 c | 2.33 ± 0.15 c | 0.76 ± 0.10 d |
| C18:1 n-7 | 1.42 ± 0.07 a | 1.89 ± 0.01 a | 0.26 ± 0.04 b | 0.31 ± 0.04 b | 0.28 ± 0.03 b | 1.24 ± 0.07 a |
| C22:0 | 2.29 ± 0.09 a | 1.85 ± 0.04 b | 0.41 ± 0.04 c | 0.42 ± 0.02 c | 0.44 ± 0.03 c | 2.37 ± 0.14 a |
| C20:4 n-6 | 6.84 ± 0.26 b | 8.35 ± 0.30 a | 1.91 ± 0.04 c | 1.93 ± 0.11 c | 1.85 ± 0.05 c | 2.60 ± 0.17 c |
| C20:5 n-3 (EPA) | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 5.88 ± 0.44 a | 6.05 ± 0.32 a | 5.88 ± 0.37 a | 2.78 ± 0.03 b |
| TFA 1 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| SFA 2 | 54.09 ± 1.59 c | 64.84 ± 1.84 b | 88.52 ± 0.00 a | 86.41 ± 0.31 a | 87.33 ± 0.36 a | 92.14 ± 0.20 a |
| MUSFA 3 | 6.87 ± 0.33 b | 10.75 ± 0.25 a | 0.66 ± 0.05 d | 0.70 ± 0.25 d | 0.61 ± 0.05 d | 1.51 ± 0.02 c |
| PUFA 4 | 45.91 ± 1.59 a | 35.16 ± 1.84 b | 11.48 ± 0.36 c | 10.87 ± 0.31 c | 10.62 ± 0.36 c | 7.86 ± 0.20 d |
| n-3 5 | 26.53 ± 1.58 a | 12.96 ± 1.08 b | 8.38 ± 0.36 c | 8.52 ± 0.62 c | 8.23 ± 0.36 c | 3.56 ± 0.12 d |
| n-6 6 | 12.09 ± 0.38 a | 10.99 ± 0.67 b | 2.31 ± 0.04 c | 2.23 ± 0.10 c | 2.26 ± 0.04 c | 2.67 ± 0.17 c |
| n6/n3 7 | 0.46 ± 0.02 b | 0.85 ± 0.04 a | 0.28 ± 0.02 c | 0.26 ± 0.03 c | 0.27 ± 0.02 c | 0.75 ± 0.06 a |
| TFA 8 g kg−1 | 15.30 ± 1.12 b | 8.28 ± 0.67 d | 19.93 ± 1.49 a | 19.49 ± 0.86 a | 19.05 ± 1.49 a | 13.94 ± 0.59 c |
| Percentage of Fatty Acid (%) of Total Fatty Acids in Green Seaweed Collected from 3 Locations | |||
|---|---|---|---|
| RH 11 | AT | JA | |
| Fatty Acid | Ulva compressa 9 (G 10) | Ulva compressa (G) | Ulva compressa (G) |
| C14:0 | 0.13 ± 0.00 a | 0.10 ± 0.00 a | 0.12 ± 0.00 a |
| C16:4 n-4 | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.06 ± 0.00 a |
| C16:2 n-6 | 0.23 ± 0.03 a | 0.21 ± 0.02 a | 0.22 ± 0.01 a |
| C16:1 n-5 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| C16:1 n-9 | 0.26 ± 0.05 a | 0.25 ± 0.08 a | 0.22 ± 0.07 a |
| C16:0 | 96.18 ± 0.10 a | 95.12 ± 0.14 a | 97.15 ± 0.12 a |
| C18:4 n-3 | 0.11 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a |
| C18:2 n-6 | 0.04 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| C18:3 n-3 | 0.25 ± 0.02 a | 0.22 ± 0.02 a | 0.23 ± 0.02 a |
| C18:1 n-7 | 1.50 ± 0.03 a | 1.60 ± 0.02 a | 1.40 ± 0.04 a |
| C22:0 | 0.30 ± 0.05 a | 0.20 ± 0.05 a | 0.40 ± 0.05 a |
| C20:4 n-6 | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| C20:5 n-3 (EPA) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| TFA 1 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| SFA 2 | 97.66 ± 0.12 a | 95.42 ± 0.12 a | 97.66 ± 0.12 a |
| MUSFA 3 | 1.77 ± 0.11 a | 1.86 ± 0.11 a | 1.64 ± 0.11 a |
| PUFA 4 | 0.74 ± 0.12 a | 0.70 ± 0.12 a | 2.34 ± 0.12 a |
| n-3 5 | 0.37 ± 0.04 a | 0.37 ± 0.04 a | 0.37 ± 0.04 a |
| n-6 6 | 0.29 ± 0.02 a | 0.25 ± 0.02 a | 0.26 ± 0.02 a |
| n6/n3 7 | 0.78 ± 0.10 a | 0.68 ± 0.10 a | 0.71 ± 0.10 a |
| TFA 8 g kg−1 | 19.53 ± 0.52 a | 19.81 ± 0.61 a | 20.03 ± 0.84 a |
| Percentage of Fatty Acid (%) of Total Fatty Acids in Red Seaweeds Collected from 3 Locations | |||||
|---|---|---|---|---|---|
| JA 11 | AT | RH | AT | JA | |
| Fatty Acid | Ballia callitricha 9 (R) | Gelidium coulteri (R) | Gracilaria sp. (R) 10 | Gracilaria sp. (R) | Gracilaria sp. (R) |
| C14:0 | 0.54 ± 0.11 c | 0.78 ± 0.09 c | 2.00 ± 0.39 b | 4.03 ± 0.10 a | 3.41 ± 0.21 a |
| C16:4 n-4 | 0.29 ± 0.03 b | 0.34 ± 0.04 b | 0.05 ± 0.06 c | 0.13 ± 0.01 c | 0.46 ± 0.04 a |
| C16:2 n-6 | 0.67 ± 0.09 a | 0.04 ± 0.03 c | 0.19 ± 0.11 b | 0.01 ± 0.00 c | 0.11 ± 0.01 b |
| C16:1 n-5 | 0.20 ± 0.02 a | 0.04 ± 0.03 b | 0.01 ± 0.01 b | 0.01 ± 0.00 b | 0.01 ± 0.01 b |
| C16:1 n-9 | 0.20 ± 0.02 c | 4.75 ± 0.04 b | 0.32 ± 0.21 c | 0.27 ± 0.05 c | 6.91 ± 0.52 a |
| C16:0 | 82.21 ± 0.15 b | 82.17 ± 0.10 b | 92.08 ± 0.94 a | 88.98 ± 0.26 a | 69.09 ± 1.04 c |
| C18:4 n-3 | 0.14 ± 0.02 a | 0.03 ± 0.02 b | 0.04 ± 0.02 b | 0.02 ± 0.00 b | 0.07 ± 0.01 b |
| C18:2 n-6 | 0.77 ± 0.06 a | 0.11 ± 0.08 b | 0.05 ± 0.01 b | 0.06 ± 0.01 b | 0.15 ± 0.12 b |
| C18:3 n-3 | 0.13 ± 0.01 d | 0.65 ± 0.04 c | 1.42 ± 0.09 b | 0.76 ± 0.10 c | 4.65 ± 0.21 a |
| C18:1 n-7 | 0.14 ± 0.02 d | 4.96 ± 0.18 b | 1.67 ± 0.37 c | 1.24 ± 0.07 c | 11.08 ± 0.50 a |
| C22:0 | 14.06 ± 0.03 a | 1.68 ± 0.11 b | 2.07 ± 0.17 b | 2.37 ± 0.14 b | 2.31 ± 0.07 b |
| C20:4 n-6 | 0.53 ± 0.03 b | 1.27 ± 0.18 b | 0.02 ± 0.01 c | 2.60 ± 0.17 a | 1.68 ± 0.04 b |
| C20:5 n-3 (EPA) | 0.12 ± 0.01 c | 3.18 ± 0.09 a | 0.09 ± 0.02 c | 2.78 ± 0.03 b | 0.07 ± 0.01 c |
| TFA 1 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| SFA 2 | 96.81 ± 0.09 a | 84.63 ± 0.18 b | 96.15 ± 0.53 a | 95.38 ± 0.29 a | 74.81 ± 1.06 c |
| MUSFA 3 | 0.55 ± 0.03 d | 9.75 ± 0.12 b | 2.00 ± 0.56 c | 1.51 ± 0.02 c | 18.00 ± 1.01 a |
| PUFA 4 | 3.19 ± 0.09 d | 15.37 ± 0.18 b | 3.85 ± 0.53 d | 7.86 ± 0.20 c | 25.19 ± 1.06 a |
| n-3 5 | 0.39 ± 0.01 d | 3.86 ± 0.03 b | 1.54 ± 0.09 c | 3.56 ± 0.12 b | 4.79 ± 0.22 a |
| n-6 6 | 1.96 ± 0.09 b | 1.42 ± 0.09 b | 0.26 ± 0.12 c | 2.67 ± 0.17 a | 1.94 ± 0.15 b |
| n6/n3 7 | 5.02 ± 0.28 a | 0.37 ± 0.02 b | 0.17 ± 0.08 c | 0.75 ± 0.06 b | 0.40 ± 0.03 b |
| TFA 8 g kg−1 | 0.33 ± 0.02 c | 5.27 ± 0.14 a | 3.57 ± 0.14 b | 3.48 ± 0.06 b | 3.32 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnayem, N.; Unis, R.; Gnaim, R.; Israel, Á.; Gnaim, J.; Golberg, A. Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties. Life 2025, 15, 710. https://doi.org/10.3390/life15050710
Gnayem N, Unis R, Gnaim R, Israel Á, Gnaim J, Golberg A. Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties. Life. 2025; 15(5):710. https://doi.org/10.3390/life15050710
Chicago/Turabian StyleGnayem, Nabeel, Razan Unis, Rima Gnaim, Álvaro Israel, Jallal Gnaim, and Alexander Golberg. 2025. "Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties" Life 15, no. 5: 710. https://doi.org/10.3390/life15050710
APA StyleGnayem, N., Unis, R., Gnaim, R., Israel, Á., Gnaim, J., & Golberg, A. (2025). Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties. Life, 15(5), 710. https://doi.org/10.3390/life15050710






