Appendages of the Cyanobacterial Cell
Abstract
:1. Introduction
2. Appendages of Bacteria: Classification and Nomenclature
Appendages of Cyanobacteria
3. Type IV Pili of Cyanobacteria
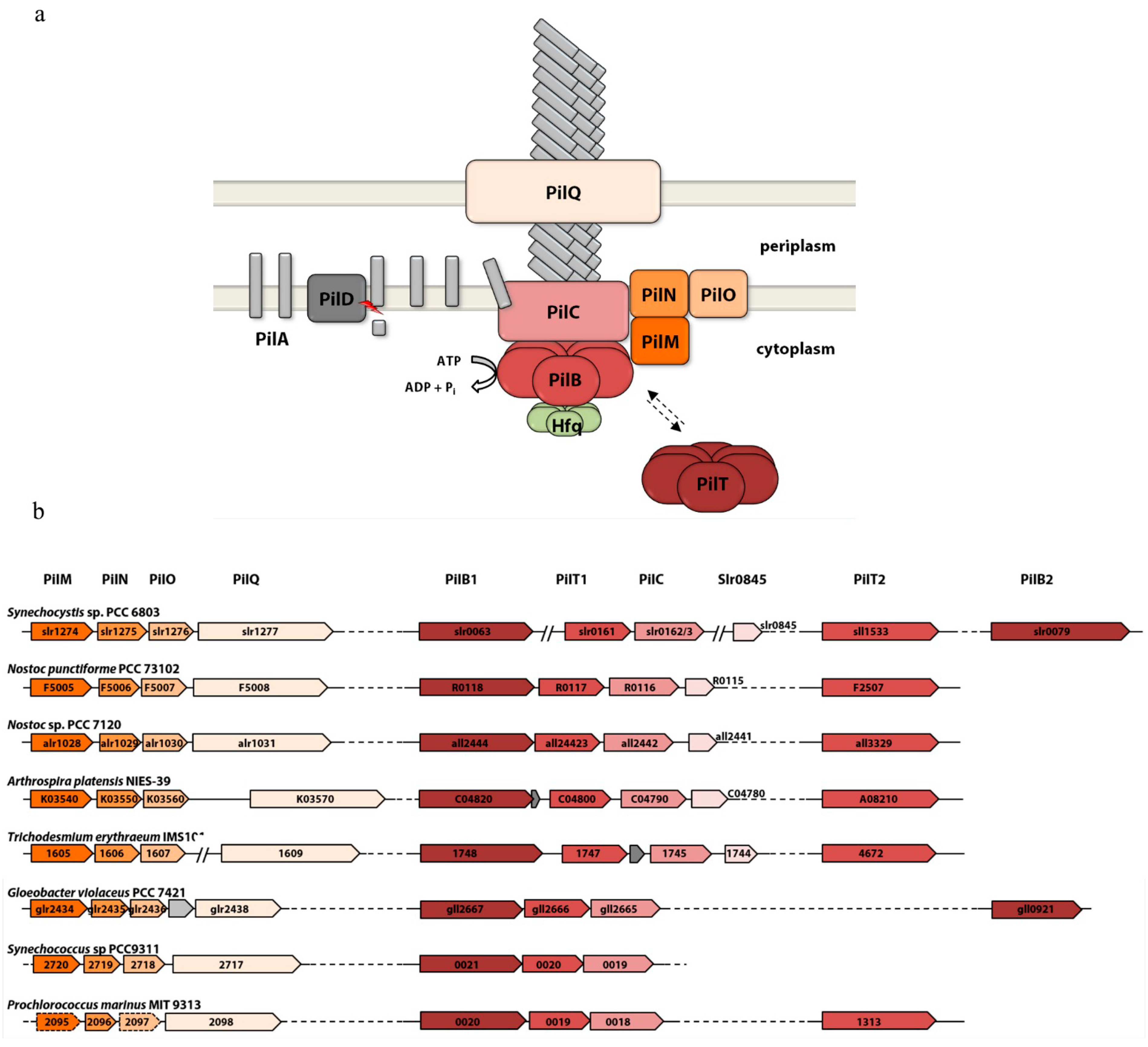
3.1. The Membrane Complexes
3.2. The Secretion ATPases
3.3. The Pilus Rod
3.4. Distribution of Pili Genes in Cyanobacterial Genomes
4. Function of Type IV Pili
5. Pili Assembled by the Chaperone-Usher Pathway (CU pili)

6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Terashima, H.; Kojima, S.; Homma, M. Flagellar motility in bacteria structure and function of flagellar motor. Int. Rev. Cell Mol. Biol. 2008, 270, 39–85. [Google Scholar] [PubMed]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and bacterial pathogenicity. J. Basic Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.C.; Gibiansky, M.L.; Jin, F.; Gordon, V.D.; Motto, D.A.; Mathewson, M.A.; Stopka, W.G.; Zelasko, D.C.; Shrout, J.D.; Wong, G.C.L. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys. J. 2011, 100, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Vaara, T. The outermost surface structures in chroococcacean cyanobacteria. Can. J. Microbiol. 1982, 28, 929–941. [Google Scholar] [CrossRef]
- Bhaya, D.; Watanabe, N.; Ogawa, T.; Grossman, A.R. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc. Natl. Acad. Sci USA 1999, 96, 3188–3193. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Geng, X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fronzes, R.; Remaut, H.; Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 2008, 27, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Yu, X.; Silverman, P.M.; Harris, R.L.; Egelman, E.H. The structure of F-pili. J. Mol. Biol. 2009, 385, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zechner, E.L.; Lang, S.; Schildbach, J.F. Assembly and mechanisms of bacterial type IV secretion machines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Lindner, A.B.; Vulic, M.; Stewart, E.J.; Radman, M. Direct visualization of horizontal gene transfer. Science 2008, 319, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol. Cell 2006, 23, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Pelicic, V. Type IV pili: E pluribus unum? Mol. Microbiol. 2008, 68, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Li, J. Type IV pili: Paradoxes in form and function. Curr. Opin. Struct. Biol. 2008, 18, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Chapman, M.R. Curli biogenesis: Order out of disorder. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Nuccio, S.-P.; Bäumler, A.J. Evolution of the chaperone/usher assembly pathway: Fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 2007, 71, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Lounatmaa, K.; Vaara, T.; Osterlund, K.; Vaara, M. Ultrastructure of the cell wall of a Synechocystis strain. Can. J. Microbiol. 1980, 26, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.E.; Sicko, L.M. The fine structure of the cell wall of Gloeocapsa alpicola, a blue-green alga. Cytobiologie 1972, 6, 439–446. [Google Scholar]
- Perkins, F.O.; Haas, L.W.; Phillips, D.E.; Webb, K.L. Ultrastructure of a marine Synechococcus possessing spinae. Can. J. Microbiol. 1981, 27, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Dick, H.; Stewart, W.D.P. The occurrence of fimbriae on a N2-fixing cyanobacterium which occurs in lichen symbiosis. Arch. Microbiol. 1980, 124, 107–109. [Google Scholar] [CrossRef]
- Duggan, P.S.; Gottardello, P.; Adams, D.G. Molecular analysis of genes in Nostoc punctiforme involved in pilus biogenesis and plant infection. J. Bacteriol. 2007, 189, 4547–4551. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Chew, W.G.; Meeks, J.C. Genetic characterization of the hmp locus, a chemotaxis-like gene cluster that regulates hormogonium development and motility in Nostoc punctiforme. Mol. Microbiol. 2014, 92, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Hoiczyk, E.; Baumeister, W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 1998, 8, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, K.; Neilan, B.A. Identification of pilus-like structures and genes in Microcystis aeruginosa PCC7806. Appl. Environ. Microbiol. 2005, 71, 7621–7625. [Google Scholar] [CrossRef] [PubMed]
- Peabody, C.R.; Chung, Y.J.; Yen, M.-R.; Vidal-Ingigliardi, D.; Pugsley, A.P.; Saier, M.H. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 2003, 149, 3051–3072. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.; Mattick, J.S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: A general system for the formation of surface-associated protein complexes. Mol. Microbiol. 1993, 10, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wallace, R.A.; Black, W.P.; Li, Y.; Yang, Z. Type IV pilus proteins form an integrated structure extending from the cytoplasm to the outer membrane. PLoS One 2013, 8, e70144. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, M.; Castagnini, M.; Karimova, G.; Ladant, D.; Pelicic, V. Large-scale study of the interactions between proteins involved in type IV pilus biology in Neisseria meningitidis: Characterization of a subcomplex involved in pilus assembly. Mol. Microbiol. 2012, 84, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Sampaleanu, L.M.; Tammam, S.; Koo, J.; Harvey, H.; Howell, P.L.; Burrows, L.L. PilM/N/O/P proteins form an inner membrane complex that affects the stability of the Pseudomonas aeruginosa type IV pilus secretin. J. Mol. Biol. 2009, 394, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Tammam, S.; Sampaleanu, L.M.; Koo, J.; Manoharan, K.; Daubaras, M.; Burrows, L.L.; Howell, P.L. PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. J. Bacteriol. 2013, 195, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Burrows, L.L.; Lynne Howell, P. Decoding the roles of pilotins and accessory proteins in secretin escort services. FEMS Microbiol. Lett. 2012, 328, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Planet, P.J.; Kachlany, S.C.; DeSalle, R.; Figurski, D.H. Phylogeny of genes for secretion NTPases: Identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. USA 2001, 98, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Savvides, S.N.; Herr, A.B.; Lanka, E.; Waksman, G. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 2000, 6, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Robien, M.A.; Krumm, B.E.; Sandkvist, M.; Hol, W.G. Crystal structure of the extracellular protein secretion NTPase EpsE of Vibrio cholerae. J. Mol. Biol. 2003, 333, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Tainer, J.A. Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism. EMBO J. 2007, 26, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, K.; Alexova, R.; Svenson, C.J.; Neilan, B.A. Functional analysis of PilT from the toxic cyanobacterium Microcystis aeruginosa PCC 7806. J. Bacteriol. 2007, 189, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Ohmori, M. The cyanobacterial PilT protein responsible for cell motility and transformation hydrolyzes ATP. Plant Cell Physiol. 2002, 43, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Dühring, U.; Mollenkopf, H.J.; Vogel, J.; Golecki, J.; Hess, W.R.; Wilde, A. The cyanobacterial homologue of the RNA chaperone Hfq is essential for motility of Synechocystis sp. PCC 6803. Microbiology 2008, 154, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Ruppert, U.; Watanabe, S.; Nürnberg, D.J.; Lochnit, G.; Dienst, D.; Mullineaux, C.W.; Wilde, A. Binding of the RNA chaperone Hfq to the type IV pilus base is crucial for its function in Synechocystis sp. PCC 6803. Mol. Microbiol. 2014, 92, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Mathews, D.W. Signature proteins for the major clades of Cyanobacteria. BMC Evol. Biol. 2010, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shimoda, Y.; Muraki, A.; Kohara, M.; Nakamura, Y.; Tabata, S. A large-scale protein protein interaction analysis in Synechocystis sp. PCC6803. DNA Res. 2007, 14, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Geng, X.; Ikeuchi, M. pilG Gene cluster and split pilL genes involved in pilus biogenesis, motility and genetic transformation in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Linhartová, M.; Bučinská, L.; Halada, P.; Ječmen, T.; Šetlík, J.; Komenda, J.; Sobotka, R. Accumulation of the Type IV prepilin triggers degradation of SecY and YidC and inhibits synthesis of Photosystem II proteins in the cyanobacterium Synechocystis PCC 6803. Mol. Microbiol. 2014, 93, 1207–1223. [Google Scholar] [PubMed]
- Williams, J.G.K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766–778. [Google Scholar]
- Bhaya, D.; Takahashi, A.; Shahi, P.; Grossman, A.R. Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 2001, 183, 6140–6143. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Ikeuchi, M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004, 3, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, C.; Spence, E.; Kirkilionis, M.A.; Frigerio, L.; Robinson, C. Tat-dependent targeting of Rieske iron-sulphur proteins to both the plasma and thylakoid membranes in the cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 2008, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, K.H.; Kim, S.-Y.; Ji, E.S.; Kim, J.Y.; Lee, S.K.; Yoo, J.S.; Kim, H.S.; Park, Y.M. Identification of trimethylation at C-terminal lysine of pilin in the cyanobacterium Synechocystis PCC 6803. Biochem. Biophys. Res. Commun. 2011, 404, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.Y.; Kim, S.-Y.; Lee, J.H.; Lee, J.S.; Chung, Y.-H.; Yoo, J.S.; Park, Y.M. Alteration in the glycan pattern of pilin in a nonmotile mutant of Synechocystis sp. PCC 6803. Proteomics 2009, 9, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, Y.M.; Kim, S.-J.; Park, Y.-I.; Choi, J.-S.; Chung, Y.-H. The role of Slr1443 in pilus biogenesis in Synechocystis sp. PCC 6803: Involvement in post-translational modification of pilins. Biochem. Biophys. Res. Commun. 2004, 315, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P. Photosensory behavior in procaryotes. Microbiol. Rev. 1987, 51, 1–21. [Google Scholar] [PubMed]
- Read, N.; Connell, S.; Adams, D.G. Nanoscale visualization of a fibrillar array in the cell wall of filamentous cyanobacteria and its implications for gliding motility. J. Bacteriol. 2007, 189, 7361–7366. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Meeks, J.C. Comparative transcriptomics with a motility-deficient mutant leads to identification of a novel polysaccharide secretion system in Nostoc punctiforme. Mol. Microbiol. 2013, 87, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Ma, X.; Lu, A.; Lux, R.; Zusman, D.; Shi, W. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 2003, 100, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Burriesci, M.; Bhaya, D. Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC6803. J. Photochem. Photobiol. B 2008, 91, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Seitz, P.; Blokesch, M. DNA-uptake machinery of naturally competent Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2013, 110, 17987–17992. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.J.; Hill, R.E.; Eaton-Rye, J.J.; Hohmann-Marriott, M.F. Functional role of PilA in iron acquisition in the cyanobacterium Synechocystis sp. PCC 6803. PLoS One 2014, 9, e105761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, J.; Luisi, B.F. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011, 9, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, K.V.; Sandkvist, M.; Hol, W.G.J. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 2012, 10, 336–351. [Google Scholar] [PubMed]
- Kirn, T.J.; Bose, N.; Taylor, R.K. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 2003, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.D.; Petersen, J.D.; Reese, T.S. Envelope structure of Synechococcus sp. WH8113, a nonflagellated swimming cyanobacterium. BMC Microbiol. 2001, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palenik, B.; Brahamsha, B.; Larimer, F.W.; Land, M.; Hauser, L.; Chain, P.; Lamerdin, J.; Regala, W.; Allen, E.E.; McCarren, J.; et al. The genome of a motile marine Synechococcus. Nature 2003, 424, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Nagar, E.; Sendersky, E.; Parnasa, R.; Zilberman, S.; Carmeli, S.; Mastai, Y.; Shimoni, E.; Klein, E.; Yeger, O.; et al. Self-suppression of biofilm formation in the cyanobacterium Synechococcus elongatus. Environ. Microbiol. 2013, 15, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Kaneko, Y.; Ehira, S.; Yoshihara, S.; Ikeuchi, M.; Ohmori, M. CccS and CccP are involved in construction of cell surface components in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2010, 51, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Waksman, G.; Hultgren, S.J. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 2009, 7, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Kamei, A.; Yuasa, T.; Orikawa, K.; Geng, X.X.; Ikeuchi, M. A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2001, 183, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Kanesaki, Y.; Shiwa, Y.; Tajima, N.; Suzuki, M.; Watanabe, S.; Sato, N.; Ikeuchi, M.; Yoshikawa, H. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, D.; Voss, B.; Wilde, A.; Al-Babili, S.; Hess, W.R. Microevolution in cyanobacteria: Re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 435–448. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuergers, N.; Wilde, A. Appendages of the Cyanobacterial Cell. Life 2015, 5, 700-715. https://doi.org/10.3390/life5010700
Schuergers N, Wilde A. Appendages of the Cyanobacterial Cell. Life. 2015; 5(1):700-715. https://doi.org/10.3390/life5010700
Chicago/Turabian StyleSchuergers, Nils, and Annegret Wilde. 2015. "Appendages of the Cyanobacterial Cell" Life 5, no. 1: 700-715. https://doi.org/10.3390/life5010700




