Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plasmid Construction and Conjugation
3. Results
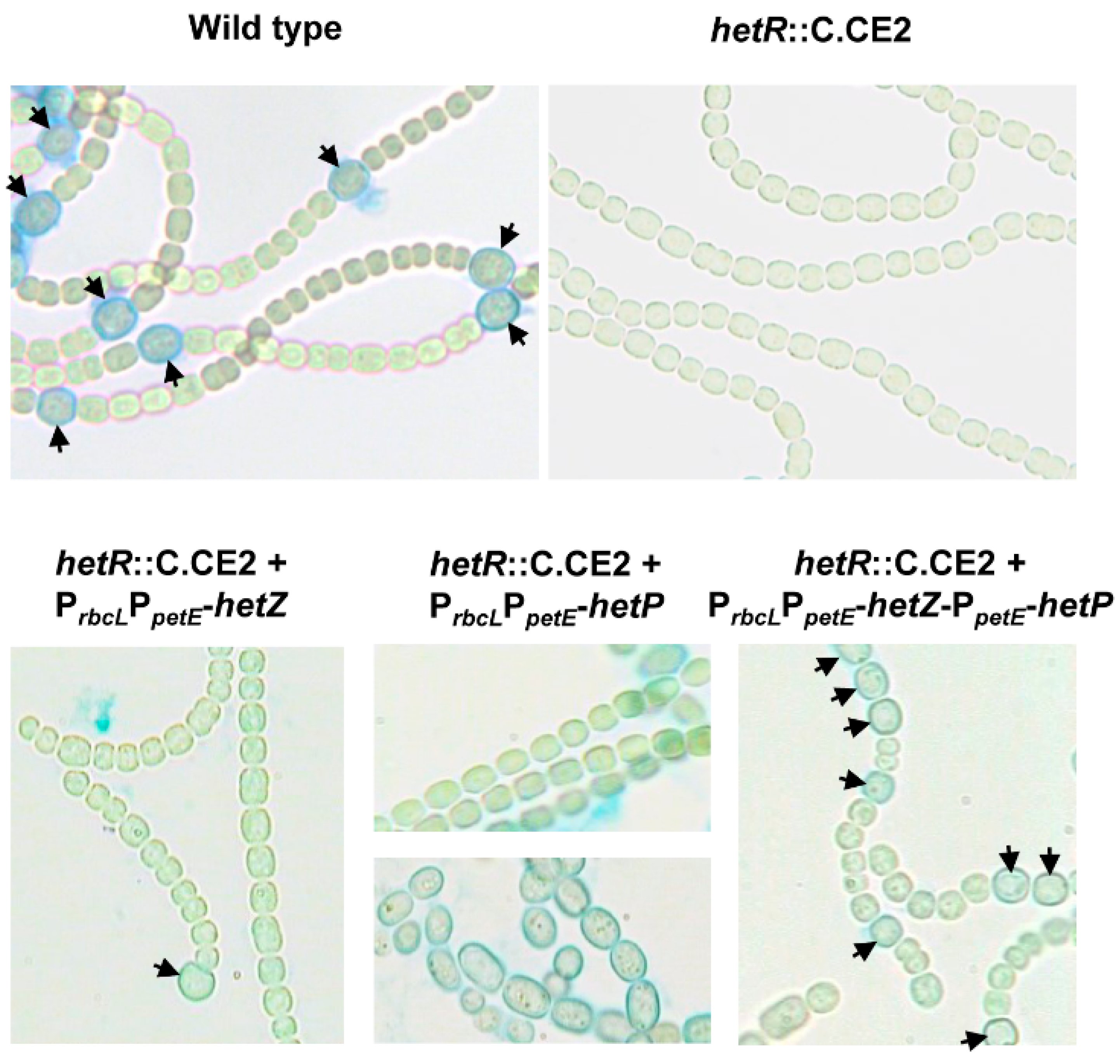
3.1. Formation of Mch in a hetR Mutant that Overexpresses hetZ and hetP
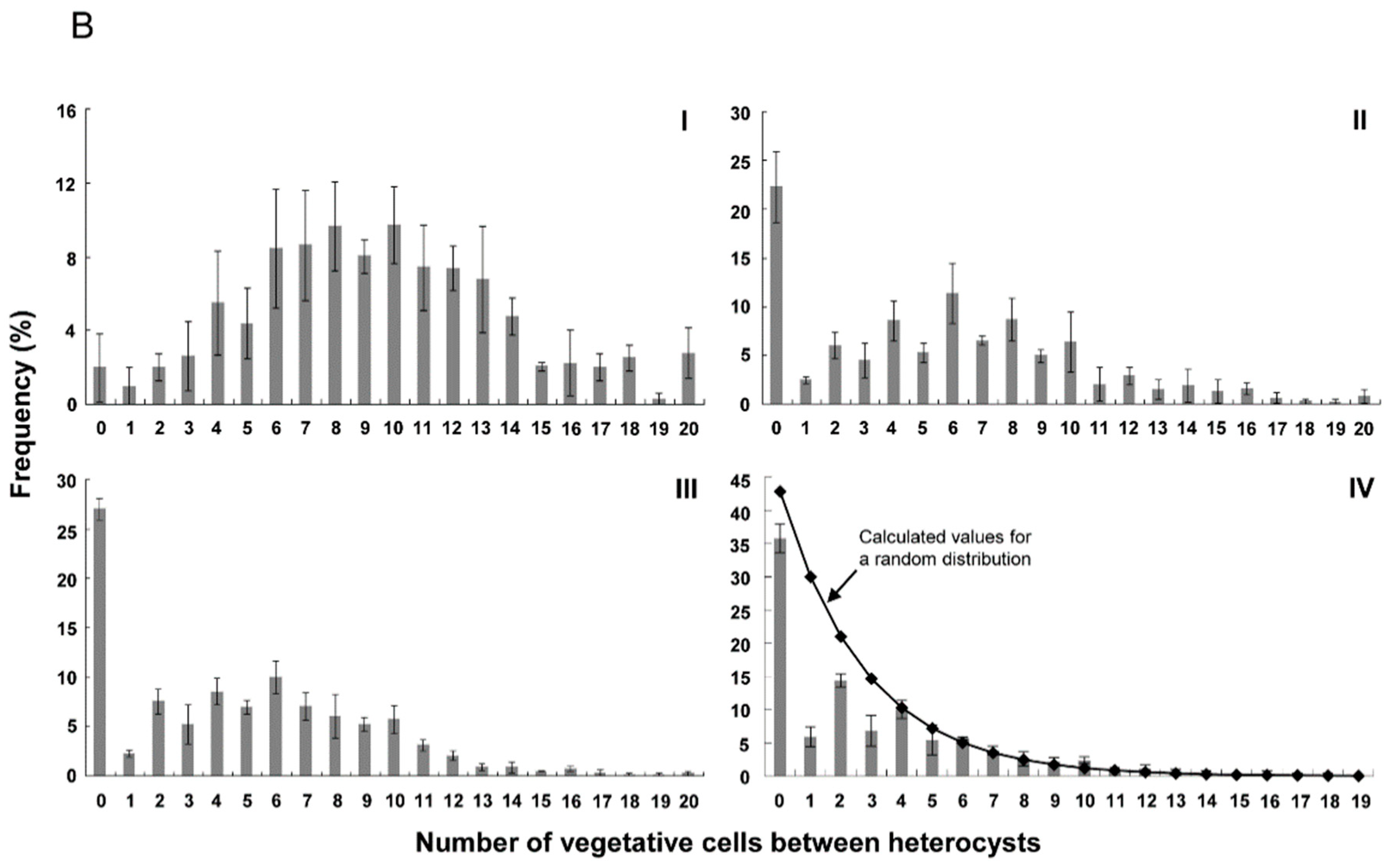
3.2. Distribution of Heterocysts along Filaments
3.3. Heterocyst Formation in a hetF Background
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. The paleobiological record of photosynthesis. Photosynth. Res. 2011, 107, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Wolk, C.P.; Ernst, A.; Elhai, J. Heterocyst metabolism and development. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 769–823. [Google Scholar]
- Castenholz, R.W. Oxygenic photosynthetic bacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2001; pp. 474–487. [Google Scholar]
- Tomitani, A.; Knoll, A.H.; Cavanaugh, C.M.; Ohno, T. The evolutionary diversification of cyanobacteria: Molecular-phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. USA 2006, 103, 5442–5447. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, H.; Gierer, A. Pattern formation by local self-activation and lateral inhibition. Bioessays 1991, 22, 753–760. [Google Scholar] [CrossRef]
- Kondo, S.; Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 2006, 329, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Buikema, W.J.; Haselkorn, R. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA 2001, 98, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cai, Y.; Hou, S.; Xu, X. Identification of the HetR-recognition sequence upstream of hetZ in Anabaena sp. strain PCC 7120. J. Bacteriol. 2012, 194, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhou, F.; Peng, S.; Gao, H.; Xu, X. The HetR-binding site that activates expression of patA in vegetative cells is required for normal heterocyst patterning in Anabaena sp. PCC 7120. Sci. Bull. 2015, 60, 192–201. [Google Scholar] [CrossRef]
- Hu, H.X.; Jiang, Y.L.; Zhao, M.X.; Cai, K.; Liu, S.; Wen, B.; Lv, P.; Zhang, Y.; Peng, J.; Zhong, H.; et al. Structural insights 249 into HetR-PatS interaction involved in cyanobacterial pattern formation. Sci. Rep. 2015, 5, 16470. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Callahan, S.M. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc. Natl. Acad. Sci. USA 2009, 106, 19884–19888. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Scappino, L.; Haselkorn, R. The patA gene product, which contains a region similar to CheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. USA 1992, 89, 5655–5659. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Koonin, E.V.; Haselkorn, R.; Galperin, M.Y. Cyanobacterial response regulator PatA contains a conserved N-terminal domain (PATAN) with an alpha-helical insertion. Bioinformatics 2006, 22, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Golden, J.W. Heterocyst pattern formation controlled by a diffusible peptide. Science 1998, 282, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Khudyakov, I. Ancient association of cyanobacterial multicellularity with the regulator HetR and an RGSGR pentapeptide-containing protein (PatX). Mol. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, J.; Vioque, A.; Haas, F.; Hess, W.R.; Muro-Pastor, A.M. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc. Natl. Acad. Sci. USA 2011, 108, 20130–20135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Cao, Z.; Zhao, J. Characterization of HetR protein turnover in Anabaena sp. PCC 7120. Arch. Microbiol. 1998, 169, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Black, T.A.; Cai, Y.; Wolk, C.P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 1993, 9, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.C.; Callahan, S.M. Ectopic expression of hetP can partially bypass the need for hetR in heterocyst differentiation by Anabaena sp. strain PCC 7120. Mol. Microbiol. 2010, 77, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Wang, Y.; Xu, X. Functional overlap of hetP and hetZ in regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J. Bacteriol. 2018, 200, e00707-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Li, C.; Gao, H.; Zhang, C.-C.; Xu, X. Three substrains of the cyanobacterium Anabaena sp. PCC 7120 display divergence in genomic sequences and hetC function. J. Bacteriol. 2018, 200, e00076-18. [Google Scholar] [CrossRef] [PubMed]
- Videau, P.; Rivers, O.S.; Tom, S.K.; Oshiro, R.T.; Ushijima, B.; Swenson, V.A.; Philmus, B.; Gaylor, M.O.; Cozy, L.M. The hetZ gene indirectly regulates heterocyst development at the level of pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 2018, 109, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Callahan, S.M. HetF and PatA control levels of HetR in Anabaena sp. strain PCC 7120. J. Bacteriol. 2008, 190, 7645–7654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Khudyakov, I.; Fan, Q.; Gao, H.; Ning, D.; Wolk, C.P.; Xu, X. A gene cluster that regulates both heterocyst differentiation and pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 2007, 66, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, P.B.; Curtis, S.E. Characterization of devH, a gene encoding a putative DNA binding protein required for heterocyst function in Anabaena sp. strain PCC 7120. J. Bacteriol. 2000, 182, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Hunt, H.D.; Ho, S.N.; Pullen, J.K.; Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene 1989, 77, 61–68. [Google Scholar] [CrossRef]
- Wolk, C.P.; Cai, Y.; Cardemil, L.; Flores, E.; Hohn, B.; Murry, M.; Schmetterer, G.; Schrautemeier, B.; Wilson, R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J. Bacteriol. 1988, 170, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Vepritskiy, A.; Muro-Pastor, A.M.; Flores, E.; Wolk, C.P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1997, 179, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Wolk, C.P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990, 9, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Videau, P.; Oshiro, R.T.; Cozy, L.M.; Callahan, S.M. Transcriptional dynamics of developmental genes assessed with an FMN-dependent fluorophore in mature heterocysts of Anabaena sp. strain PCC 7120. Microbiology 2014, 160, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J. Strong and regulated promoters in the cyanobacterium Anabaena PCC 7120. FEMS Microbiol. Lett. 1993, 114, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.Y.; Golden, J.W. Different functions of HetR, a master regulator of heterocyst differentiation in Anabaena sp. PCC 7120, can be separated by mutation. Proc. Natl. Acad. Sci. USA 2004, 101, 16040–16045. [Google Scholar] [CrossRef] [PubMed]



| Strains | Derivation/Relevant Characteristics a | Reference or Source |
|---|---|---|
| Anabaena 7120 | Wild Type (WT) | FACHB b |
| hetF::Tn5-1087b | Emr, Tn5-1087b inserted within hetF 310 bp from its 3′ terminus | [9] |
| hetR::C.CE2 | CmrEmr, C.CE2 (a chloramphenicol-resistance and erythromycin-resistance cassette) inserted into the ClaI site of hetR | [9] |
| hetR::C.CE2 (pHB4382) | CmrEmrNmr, pHB4382 bearing PrbcLPpetE-hetZ introduced into the hetR::C.CE2 mutant | This study |
| hetR::C.CE2 (pHB4409) | CmrEmrNmrSmrSpr, pHB4409 bearing PrbcLPpetE-hetP introduced into the hetR::C.CE2 mutant | This study |
| hetR::C.CE2 (pHB4551) | CmrEmrNmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into the hetR::C.CE2 mutant | This study |
| hetZ::C.K2 ΔhetP | CmrEmrNmr, hetZ hetP double mutant | [21] |
| hetZ::C.K2 ΔhetP (pHB1462) | CmrEmrNmrSmrSpr, pHB1462 bearing PhetZ-hetZ [25] introduced into the hetZ hetP double mutant | This study |
| hetZ::C.K2 ΔhetP (pHB4550) | CmrEmrNmrSmrSpr, pHB4550 bearing PhetP-hetP introduced into the hetZ hetP double mutant | This study |
| hetZ::C.K2 ΔhetP (pHB4551) | CmrEmrNmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into the hetZ hetP double mutant | This study |
| WT (pHB4551) | NmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into Anabaena 7120 | This study |
| Strains | Hours | Nitrogenase Activity (μmol C2H4 mg Chla−1 h−1) a | Heterocyst Frequency (%) b | Diazotrophic Growth | ||
|---|---|---|---|---|---|---|
| Anoxic | Aerobic | −RGSGR | +RGSGR c | |||
| WT d | 24 | 3.65 ± 0.62 | 2.83 ± 0.11 | 9.4 ± 0.4 | 0 | Good |
| 48 | 6.74 ± 0.63 | 3.50 ± 0.57 | 10.6 ± 0.7 | Not tested | ||
| WT + PrbcLPpetE-hetZ-PpetE-hetP e1 | 24 | Not measured | Not measured | 11.9 ± 1.2 | 11.4 ± 1.3 | Moderate |
| 48 | Not measured | Not measured | 15.8 ± 2.8 | Not tested | ||
| hetR::C.CE2 | 24 | 0 | 0 | 0 | Not tested | No |
| 48 | 0 | 0 | 0 | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetZ | 24 | 1.04 ± 0.14 | 0 | 2.9 ± 0.9 | 3.6 ± 0.4 | No |
| 48 | 1.20 ± 0.46 | 0.81 ± 0.13 | 3.5 ± 0.9 | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetP | 24 | 0.17 ± 0.10 | 0 | Not counted f | Not tested | No |
| 48 | 1.57 ± 0.26 | 0 | Not counted | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetZ-PpetE-hetP e2 | 24 | 4.00 ± 1.86 | 0.90 ± 0.07 | 11.5 ± 2.0 | 12.9 ± 0.9 | No |
| 48 | 4.12 ± 0.27 | 1.52 ± 0.78 | 17.5 ± 2.0 | Not tested | ||
| hetZ::C.K2 ΔhetP | 24 | 0 | 0 | 0 | Not tested | No |
| 48 | 0 | 0 | 0 | Not tested | ||
| hetZ::C.K2 ΔhetP + PrbcLPpetE-hetZ-PpetE-hetP e3 | 24 | Not measured | Not measured | 14.3 ± 1.2 | 15.7 ± 0.7 | Moderate |
| 48 | Not measured | Not measured | 17.6 ± 0.4 | Not tested | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, X. Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP. Life 2018, 8, 60. https://doi.org/10.3390/life8040060
Zhang H, Xu X. Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP. Life. 2018; 8(4):60. https://doi.org/10.3390/life8040060
Chicago/Turabian StyleZhang, He, and Xudong Xu. 2018. "Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP" Life 8, no. 4: 60. https://doi.org/10.3390/life8040060
APA StyleZhang, H., & Xu, X. (2018). Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP. Life, 8(4), 60. https://doi.org/10.3390/life8040060





